Abstract
The relationship between clinicopathological features and long-term benefit to trastuzumab-based therapy was evaluated in 164 HER2-positive metastatic breast cancer patients. Treatment duration was associated with hormone receptor status and use of adjuvant trastuzumab; however, with low predictive value. With growing availability of targeted treatments for HER2-positive disease, a major clinical priority is identifying molecular predictors of benefit of anti-HER2 therapies.
Background
The magnitude of benefit of trastuzumab for the treatment of advanced HER2-positive breast cancer varies widely. In this retrospective study, we investigated the clinicopathological features associated with prolonged first-line trastuzumab-based treatment duration.
Patients and Methods
A total of 164 patients diagnosed with advanced HER2-positive breast cancer and treated with first-line trastuzumab-based therapy from 1999 to 2009 were identified. Duration of treatment was classified according to tertiles. Different logistic regression models including age, disease-free interval, number of metastatic sites, visceral disease, hormone receptor, and adjuvant trastuzumab were fitted to investigate associations with benefit of prolonged trastuzumab-based therapies. The predictive value of each model was assessed using C-statistics.
Results
At a median follow-up of 5.8 years (range, 0.7-22.1 years), patients in the short-, intermediate-, and long-term treatment duration groups were given first-line trastuzumab-based therapy for < 7.2 months, 7.2 to 14 months, and > 14 months, respectively. In the multivariate analysis, patients with long-term clinical benefit had a higher likelihood of having hormone receptor-positive tumors (odds ratio [OR]positive vs. negative = 2.39 [95% confidence interval (CI), 1.08-5.31]; P = .032); and a lower likelihood of having received adjuvant trastuzumab (ORadjuvant trastuzumab vs. no adjuvant trastuzumab = 0.30 [95% CI, 0.10-0.96]; P = .043]. C-statistics varied between 0.634 and 0.699.
Conclusion
Long-term benefit of trastuzumab-based therapy is associated with hormone receptor positivity and the absence of previous adjuvant trastuzumab. Nevertheless, clinicopathological features had a low predictive value for prolonged treatment duration. The validation of the current findings and the identification of molecular features associated the magnitude of trastuzumab benefit should be encouraged.
Keywords: HER2-positive, Outcomes, Treatment Duration
Introduction
Human epidermal growth factor-2 (HER2) protein over-expression or v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian) (ERBB2) gene amplification occurs in approximately 20% of primary breast carcinomas.1 Although traditionally, HER2-positive breast cancers were associated with a poor prognosis, the outcomes of this subtype of breast cancer have changed dramatically with the development and widespread use of trastuzumab. Though HER2-positivity is a predictor of benefit from trastuzumab therapy, there is significant interpatient variability in terms of duration of response, with a large range in median treatment duration across different studies.2-5 There are reports of cases with prolonged complete response to trastuzumab-based regimens in patients with HER2-positive metastatic breast cancer.6 In contrast, resistance to trastuzumab-based regimens is well recognized in clinical practice.7 This heterogeneity in treatment benefit can make treatment decisions difficult, and information on predictors of prolonged response to anti-HER2 therapies could help to guide clinical decisions.
In gene expression experiments, there is segregation of breast tumors according to hormone receptor (HR) before HER2 segregation, suggesting that HR status is the most important discriminator of breast cancer subtypes.8-16 Although trastuzumab is effective in HR-positive and HR-negative HER2-positive patients, there are accumulating data that suggest that the behavior of HER2-positive disease is influenced by HR status.17 For example, in an analysis of the National Comprehensive Cancer Network Breast Cancer Outcomes database, patients with HR-negative/HER2-positive disease were less likely to have recurrence in bone, and had a higher risk of death within 5 years of initial diagnosis compared with patients with HR-positive/HER2-positive disease.18
Another area of uncertainty is the effect of adjuvant trastuzumab on the efficacy of HER2-targeted therapies in the metastatic setting. Recent reports suggest that relapse after the use of adjuvant trastuzumab therapy might be associated with a lower rate of clinical benefit of HER2-targeted therapy.19
We aimed to describe the relationship between clinicopathological features and long-term clinical benefit of trastuzumab-based therapies. We hypothesized that patients with HR-positive breast tumors will have a longer duration of advanced first-line trastuzumab-based regimens, and that patients who have received adjuvant trastuzumab will have less benefit from an anti-HER2 regimen in the metastatic setting.
Patients and Methods
Data Source and Patient Selection
A retrospective consecutive series of 355 HER2-positive advanced breast cancer patients treated at the Dana-Farber Cancer Institute (DFCI) from January 1, 1999 to December 31, 2009 was identified using the DFCI clinical research information system. An end date was chosen to allow a minimum of 30 months of potential follow-up time. Follow-up information was available through July 1, 2012. The Dana-Farber/Harvard Cancer Center institutional review board approved the study and granted a waiver of signed informed consent.
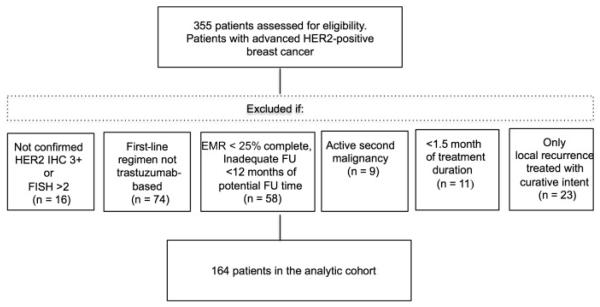
Patients were included in the analytic group if: (1) they had initial diagnosis of stage IV breast cancer or initial stage 0 to III breast cancer with evidence of subsequent local or distant recurrence; (2) they were treated with a first-line trastuzumab-based regimen in the metastatic setting; and (3) the pathology records of the primary or metastatic biopsy confirmed HER2-positive disease using an immunohistochemistry score of 3+ or a fluorescence in situ hybridization result ≥ 2.0. Patients were excluded if: (1) a second active malignancy was identified; (2) only local recurrence treated with curative intent was identified; (3) treatment duration was < 1.5 months; and (4) the electronic medical record was < 25% complete or there was inadequate follow-up of < 12 months from diagnosis of metastatic disease. A total of 164 patients were available for analysis (Figure 1).
Figure 1.
Patient Cohort
Abbreviations: EMR = electronic medical record; FISH = fluorescence in situ hybridization; FU = follow-up; IHC = immunohistochemistry.
Definition of Subgroups
Because an accurate evaluation of time to progression requires radiologic assessment of tumor size, duration of therapy was used as a surrogate of the regimens’ clinical benefit. Treatment duration was defined as time from start of first-line therapy to the first day of second-line therapy or death. If, at the cutoff date for analysis, patients were still using the first-line therapy, treatment duration was defined until the date the last cycle was received.
Modifications in treatment not related to disease progression or toxicity were not considered a change of treatment if they fulfilled certain prespecified criteria. For example, a switch to maintenance trastuzumab alone, or to trastuzumab plus endocrine therapy after achieving disease response or stabilization while taking a trastuzumab plus chemotherapy regimen was not considered a change of therapy. If a patient developed isolated central nervous system (CNS) progression but did not change systemic treatment, this was also not considered a change of therapy. However, if a patient switched therapy because of toxicity, this would be considered a change of therapy (10 patients had a change of treatment related to toxicity; 1 because of grade 4 palmar-plantar erythrodysesthesia, 1 because of the development of capillary leak syndrome, 1 because of interstitial pneumonitis, and 7 patients changed therapy because of cardiotoxicity).
Duration of treatment was classified in tertiles. Patients in the short-, intermediate-, and long-term treatment duration groups were using first-line trastuzumab-based therapy for < 7.2 months, 7.2 to 14 months, and > 14 months, respectively. An additional group of patients with extremely short-term duration of treatment was defined as being in the 10th percentile of treatment duration (≤ 2.8 months) and an additional group of patients with extremely long-term duration of treatment was defined as being in the 90th percentile of treatment duration (> 34.0 months).
Covariates of Interest
Tumor and Patient Characteristics
We used the following variables collected via chart review: date of diagnosis and recurrence, age at diagnosis, race, tumor size at diagnosis, nodal status at diagnosis, grade, estrogen receptor (ER) and progesterone receptor (PR) status, HER2 status, neo/adjuvant systemic therapies, sites of breast cancer recurrence, treatment types, treatment duration, clinical response, and date of last follow-up and/or death. HR-positive tumors were defined as being ER- and/or PR-positive, and HR-negative if they were ER- and PR-negative. Stage was assigned according to the version of the American Joint Committee on Cancer staging manual applicable at the time of diagnosis. Clinical response was categorized as noncomplete response vs. complete response, as abstracted from medical charts.
Statistical Analyses
The clinicopathological and treatment features were tabulated according to treatment duration subgroup and proportions were compared using Fisher exact test and Kruskal-Wallis test.
Univariate followed by multivariate logistic regression models were used to estimate the effect of HR status and use of adjuvant trastuzumab on the risk of long- vs. short-term clinical benefit of a trastuzumab-based regimen adjusting for known clinically relevant clinipathological characteristics. In the overall cohort, a model including HR (positive vs. negative), age at diagnosis (50 years or older vs. younger than 50 years), disease-free interval (DFI) (≥ 2 vs. < 2 years), number of sites at first recurrence and/or diagnosis of metastatic disease, visceral involvement (yes vs. no) was used. To examine the effect of adjuvant trastuzumab, a cohort restricted to patients with stage I to III breast cancer at diagnosis was used. In this model, HR status, adjuvant trastuzumab (yes vs. no), and number of sites at first recurrence and/or diagnosis of metastatic disease were included. The predictive value of each model was assessed using C-statistics.
Duration of therapy was also evaluated using Kaplan-Meier methods. Univariate analyses based on the log-rank test were performed.
All P values presented are 2-sided; a P value less than .05 was considered significant. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute).
Results
Description of the Study Cohort
A total of 164 patients were included in this analysis. The subgroup distribution was: 33% (n = 54) in the short-term treatment duration group, 33% (n = 55) in the intermediate treatment duration group, 33% (n = 55) in the long-term treatment duration group. Median follow-up time since diagnosis was 5.8 years (range, 0.7-22.1 years). The median age at diagnosis was 46.6 years (range, 22.0-83.3 years). Most (86.6%) patients were white (Table 1).
Table 1.
Baseline Patient Demographic and Clinicopathological Characteristics
| Variable | Total (n = 164) |
Short-Term Treatment Duration (n = 54) |
Intermediate Treatment Duration (n = 55) |
Long-Term Treatment Duration (n = 55) |
P |
|---|---|---|---|---|---|
|
Follow-Up, Median
(Range), years |
5.8 (0.7- 22.1) |
4.1 (1.0-16.0) | 5.1 (0.7-16.7) | 7.0 (2.1-22.1) | .001 |
|
Age, Median (Range),
years |
46.6 (22.0- 83.3) |
48.6 (22.0-70.0) | 45.8 (29.4-75.3) | 47.0 (25.1-83.3) | .723 |
| Age Category, n (%) | .919 | ||||
| <50 years | 100 (61.0) | 32 (59.3) | 35 (63.6) | 33 (60.0) | |
| ≥50 years | 64 (39.0) | 22 (40.7) | 20 (36.4) | 22 (40.0) | |
| Race/Ethnicity, n (%) | .691 | ||||
| White | 142 (86.6) | 49 (90.7) | 46 (83.6) | 47 (85.5) | |
| Nonwhite | 17 (10.4) | 4 (7.4) | 6 (10.9) | 7 (12.7) | |
| Unknown | 5 (3.0) | 1 (1.9) | 3 (5.5) | 1 (1.8) | |
| T Stage, n (%) | .127 | ||||
| T1 | 60 (36.6) | 15 (27.8) | 21 (38.2) | 24 (43.6) | |
| T2 | 52 (31.7) | 22 (40.7) | 13 (23.6) | 17 (30.9) | |
| T3 | 8 (4.9) | 2 (3.7) | 3 (5.5) | 3 (5.5) | |
| T4 | 22 (13.4) | 9 (16.7) | 11 (20.0) | 2 (3.6) | |
| Unknown | 22 (13.4) | 6 (11.1) | 7 (12.3) | 9 (16.4) | |
| Nodal Status, n (%) | .596 | ||||
| Positive | 102 (62.2) | 35 (64.8) | 32 (58.2) | 35 (63.6) | |
| Negative | 45 (27.4) | 15 (27.8) | 18 (32.7) | 12 (21.8) | |
| Unknown | 17 (10.4) | 4 (7.4) | 5 (9.1) | 8 (14.6) | |
| AJCC Stage, n (%) | .241 | ||||
| I | 34 (20.7) | 8 (14.8) | 15 (27.3) | 11 (20.0) | |
| II | 50 (30.5) | 21 (38.9) | 11 (20.0) | 18 (32.7) | |
| III | 40 (24.4) | 13 (24.1) | 17 (30.9) | 10 (18.2) | |
| IV | 40 (24.4) | 12 (22.2) | 12 (21.8) | 16 (29.1) | |
| HR, n (%) | .136 | ||||
| Positive | 92 (56.1) | 25 (46.3) | 31 (56.4) | 36 (65.5) | |
| Negative | 72 (43.9) | 29 (53.7) | 24 (43.6) | 19 (34.6) | |
| Histologic Grade, n (%) | .515 | ||||
| Low/intermediate | 45 (27.4) | 18 (33.3) | 12 (21.8) | 15 (27.3) | |
| High | 102 (62.2) | 32 (59.3) | 38 (69.1) | 32 (58.2) | |
| Unknown | 17 (10.4) | 4 (7.4) | 5 (9.1) | 8 (14.6) | |
| Histology, n (%) | .160 | ||||
| Invasive ductal | 130 (79.3) | 42 (77.8) | 44 (80.0) | 44 (80.0) | |
| Invasive lobular component | 22 (13.4) | 11 (20.4) | 5 (9.1) | 6 (10.9) | |
| Other/unknown | 12 (7.3) | 1 (1.9) | 6 (10.9) | 5 (9.1) |
Abbreviations: AJCC = American Joint Committee on Cancer Staging; HR = hormone receptor.
Clinicopathological Characteristics and Patterns of Care
At baseline, most patients (75.6 %) presented with stage I to III disease. Most tumors were HR-positive (56.1%) and high-grade (62.2%). A higher proportion of patients within the subgroup with long-term treatment duration had HR-positive tumors compared with the short-term treatment duration group (65.5% vs. 46.3%; P = .136), though this difference was not statistically significant (Table 1). Most stage I to III patients received neoadjuvant or adjuvant systemic therapy; 26.6% received neoadjuvant or adjuvant trastuzumab. A smaller proportion of patients with long-term clinical benefit received neoadjuvant or adjuvant trastuzumab (15.4% vs. 33.3%; P = .146) nevertheless this was not a significant difference (Table 2).
Table 2.
Primary Treatment Characteristics for Stage I, II, and III Patients
| Variable | Total (n = 124) |
Short-Term Treatment Duration (n = 42) |
Intermediate Treatment Duration (n = 43) |
Long-Term Treatment Duration (n = 39) |
P |
|---|---|---|---|---|---|
|
Neo or Adjuvant
Chemotherapy, n (%) |
.105 | ||||
| No | 17 (13.7) |
4 (9.5) | 10 (23.3) | 3 (7.7) | |
| Yes | 107 (86.3) | 38 (90.5) | 33 (76.7) | 36 (92.3) | |
|
Neo or Adjuvant
Trastuzumab, n (%) |
.146 | ||||
| No | 91 (73.4) |
28 (66.7) | 30 (69.8) | 33 (84.6) | |
| Yes | 33 (26.6) |
14 (33.3) | 13 (30.2) | 6 (15.4) | |
|
Adjuvant Hormonal
Therapy, n (%) |
.238 | ||||
| No | 58 (46.8) |
24 (57.1) | 17 (39.5) | 17 (43.6) | |
| Yes | 66 (53.2) |
18 (42.9) | 26 (60.5) | 22 (56.4) |
The median DFI was 1.3 years (range, 0-14.3 years). The clinical and treatment characteristics at metastatic and/or recurrent disease are presented in Table 3. At time of metastatic and/or recurrent disease, the median number of metastatic sites was 2 (range, 1-5). Sixty-seven percent of patients had visceral disease. The median duration of therapy was 10.3 months (range, 1.5-110.8 months). Trastuzumab was given as monotherapy in 7.9% of cases, with endocrine therapy in 5.5% of cases, with chemotherapy in 82.3% of cases, and with concomitant anti-HER2 therapy in 4.3% of cases (36% of patients switched to trastuzumab monotherapy or trastuzumab and endocrine therapy after achieving a good clinical response to other combination regimens). Despite CNS progression, 23.8% of patients continued therapy. No significant differences in clinical or treatment characteristics between groups were observed. Only 2.4% of patients achieved a complete clinical response, as reported in the medical chart (Table 3).
Table 3.
Clinical and Treatment Characteristics for Patients at Time of Metastatic and/or Recurrent Disease
| Variable | Total (n = 164) |
Short-Term Treatment Duration (n = 54) |
Intermediate Treatment Duration (n = 55) |
Long-Term Treatment Duration (n = 55) |
P |
|---|---|---|---|---|---|
| DFI, years | .729 | ||||
| 1.3 (0- 14.3) |
1.3 (0-14.3) | 1.3 (0-12.7) | 1.2 (0-13.4) | ||
|
Sites at Initial Metastatic Diagnosis
and/or Recurrence |
.608 | ||||
| 2 (1-5) | 3 (1-5) | 2 (1-4) | 2 (1-5) | ||
|
Sites of Disease at Initial Metastatic
Diagnosis and/or Recurrence a |
|||||
| Soft tissueb | 85 (51.8) | 32 (59.3) | 25 (45.5) | 28 (50.9) | .368 |
| Bone | 86 (52.4) | 28 (51.8) | 29 (52.7) | 29 (52.7) | 1.0 |
| Liver | 82 (50.0) | 26 (48.2) | 27 (49.1) | 29 (52.7) | .923 |
| Lung | 59 (36.0) | 25 (46.3) | 17 (30.9) | 17 (30.9) | .164 |
| CNS | 12 (7.3) | 3 (5.6) | 7 (12.7) | 2 (3.6) | .212 |
| Other | 23 (14.0) | 9 (16.7) | 6 (10.9) | 8 (14.6) | .666 |
|
Duration of First Metastatic Anti-
HER2 Therapy, mo |
<.0001 | ||||
| 10.3 (1.5- 110.8) |
3.9 (1.5-7.1) | 10.3 (7.2-14.0) | 22.9 (14.1-105.7) | ||
|
Type of First Trastuzumab-Based
Regimen |
.116 | ||||
| Monotherapy | 13 (7.9) | 6 (11.1) | 2 (3.6) | 5 (9.1) | |
| Concomitant with endocrine therapy |
9 (5.5) | 0 (0) | 7 (12.7) | 2 (3.6) | |
| Concomitant with chemotherapy | 135 (82.3) |
46 (83.6) | 42 (76.4) | 47 (85.5) | |
| With taxane based regimen | 34 (25.2) | 11 (23.9) | 8 (19.1) | 15 (31.9) | |
| With vinorelbine | 87 (64.4) | 29 (63.0) | 29 (69.0) | 29 (61.7) | |
| With other agent | 14 (10.4) | 6 (13.1) | 5 (11.9) | 3 (6.4) | |
| Concomitant with other anti-HER2 target agent |
7 (4.3) | 2 (3.7) | 4 (7.3) | 1 (1.8) | |
|
Best Clinical Response During First-
Line Therapy |
.352 | ||||
| Complete response | 4 (2.4) | 0 (0) | 3 (5.5) | 1 (1.8) | |
|
CNS Progression During First-Line
Therapy (Non-CNS Disease Controlled) |
<.0001 | ||||
| 39 (23.8) | 3 (5.6) | 10 (18.2) | 26 (47.2) | ||
|
Switch to Trastuzumab Monotherapy
or Trastuzumab and Endocrine During First-Line Therapy |
<.0001 | ||||
| 59 (36.0) | 3 (5.5) | 19 (34.6) | 37 (67.3) |
Data are presented as median (range) or n (%).
Abbreviations: CNS = central nervous system; DFI = disease-free interval.
Proportion of patients does not add up to 100% because patients could have more than 1 site of recurrence.
Soft tissue includes: chest wall, breast, local and/or distant lymph nodes, or skin.
Effect of HR Status and Adjuvant Trastuzumab on Treatment Duration
Using univariate analysis, patients with HR-positive tumors were more likely to experience long-term clinical benefit using an advanced first-line trastuzumab-based regimen (odds ratio [OR], 2.20; 95% confidence interval [CI], 1.02-4.75; P =.045). After multivariable adjustment according to age at diagnosis, DFI, number of sites involved, and evidence of visceral disease, this difference persisted (OR, 2.39; 95% CI, 1.08-5.31; P = .032); C-statistics: 0.634 (Table 4).
Table 4.
Results of Logistic Regression for Long-Term vs. Short-Term Clinical Benefit
| Univariate Analysis, OR (95% CI) |
P | Multivariate Analysis, OR (95% CI) |
P | |
|---|---|---|---|---|
| HR (Positive vs. Negative) | 2.20 (1.02-4.75) | .045 | 2.39 (1.08-5.31) | .032 |
|
Age Category (50 years and older vs.
younger than 50 years) |
0.97 (0.45-2.08) | .937 | 1.06 (0.48-2.35) | .890 |
| DFI Category (≥2 vs. <2 years) | 1.04 (0.49-2.20) | .924 | 0.86 (0.39-1.90) | .707 |
|
Sites at Initial Metastatic Diagnosis
and/or Recurrence (Continuous) |
0.92 (0.68-1.26) | .612 | 0.83 (0.59-1.18) | .300 |
| Visceral Involvement (Yes vs. No) a | 1.45 (0.64-3.27) | .374 | 1.82 (0.74-4.49) | .193 |
Abbreviations: DFI = disease-free interval; HR = hormone receptor.
Visceral involvement included liver and lung.
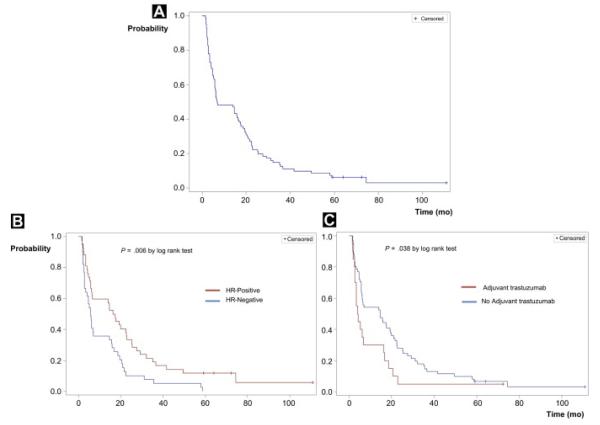
In the cohort restricted to those who presented with initial stage I to III disease, HR status continued to be associated with treatment duration (univariate analysis: OR, 2.63; 95% CI, 1.07-6.45; P =.035; multivariate analysis: OR, 3.10; 95% CI, 1.18-8.11; P = .021). In the multivariate model, there was a lower likelihood of long-term clinical benefit among patients who had received adjuvant trastuzumab, adjusting for HR status and sites of initial metastatic diagnosis (multivariate OR, 0.30; 95% CI, 0.10-0.96; P = .043), C-statistics: 0.699 (Table 5, Figure 2).
Table 5.
Results of Logistic Regression for Long-Term vs. Short-Term Clinical Benefit Among Initial Stage I to III Patients
| Univariate Analysis, OR (95% CI) |
P | Multivariate Analysis, OR (95% CI) |
P | |
|---|---|---|---|---|
| HR (Positive vs. Negative) | 2.63 (1.07-6.45) | .035 | 3.10 (1.18-8.11) | .021 |
| Adjuvant Trastuzumab (Yes vs. No) | 0.36 (0.12-1.07) | .067 | 0.30 (0.10-0.96) | .043 |
|
Sites at Initial Metastatic Diagnosis
and/or Recurrence (Continuous) |
0.80 (0.55-1.17) | .244 | 0.69 (0.45-1.06) | .094 |
Abbreviation: HR = hormone receptor.
Figure 2.
Duration of First-Line Trastuzumab-Based Treatment for Stage I to III Patients (Short-Term and Long-Term Treatment Duration Groups) (A) Overall Time to Treatment Change. (B) Time to Treatment Change According to Hormone Receptor Status. (C) Time to Treatment Change According to Adjuvant Therapy
Abbreviation: HR = hormone receptor.
Clinicopathological Characteristics and Patterns of Care of Patients With Extremely Long-Term Treatment Duration
The clinical and treatment characteristics of patients with extremely short- and long-term treatment duration are presented in Table 6. Seventeen patients received a first-line trastuzumab-based regimen for more than 34 months (median, 49.5; range, 35.2-110.8 months) and 17 patients received trastuzumab for less than 2.8 months (median, 2.1; range, 1.5-2.8 months). Because of the small number of patients, we did not formally compare groups. Numerically, in the group of patients with extremely long-term treatment duration, more patients had stage IV cancer at diagnosis, HR-positive tumors, and bone disease at metastatic diagnosis; fewer patients received neo/adjuvant trastuzumab.
Table 6.
Clinical and Treatment Characteristics for Patients With Extremely Short- and Long-Term Treatment Duration
| Variable | Extremely Short-Term Treatment Duration (n = 17) |
Extremely Long-Term Treatment Duration (n = 17) |
|---|---|---|
| Age, years | 45.6 (30.6-61.7) | 52.0 (25.2-83.3) |
| Stage at Initial Diagnosis | ||
| Stage IV | 1 (5.9) | 5 (29.4) |
| Stage I-III | 16 (94.1) | 12 (70.6) |
| Neo or Adjuvant Trastuzumab a | 5 (31.3) | 1 (8.3) |
| HR | ||
| Positive | 6 (35.3) | 12 (70.6) |
| Negative | 11 (64.7) | 5 (29.4) |
| DFI, years | 1.5 (0.0-10.3) | 0.5 (0-13.4) |
|
Sites at Initial Metastatic Diagnosis and/or
Recurrence |
2 (1-5) | 2 (1-4) |
|
Sites of Disease at Initial Metastatic Diagnosis and/or
Recurrence b |
||
| Soft tissuec | 9 (52.9) | 10 (58.8) |
| Bone | 6 (35.3) | 11 (64.7) |
| Liver | 9 (52.9) | 6 (35.3) |
| Lung | 10 (58.8) | 5 (29.4) |
| CNS | 0 (0) | 0 (0) |
| Other | 4 (23.5) | 1 (5.9) |
| Duration of First Metastatic Anti-HER2 Therapy, mo | 2.1 (1.5-2.8) | 49.5 (35.2-110.8) |
| Type of First Trastuzumab-Based Regimen | ||
| Monotherapy | 1 (5.9) | 2 (11.8) |
| Concomitant with endocrine therapy | 0 (0) | 1 (5.9) |
| Concomitant with chemotherapy | 16 (94.1) | 14 (82.4) |
| Concomitant with other anti-HER2 target agent | 0 (0) | 0 (0) |
| Best Clinical Response During First-Line Therapy | ||
| Complete response | 0 (0) | 1 (5.9) |
|
CNS Progression During First-Line Therapy (Non-
CNS Disease Controlled) |
0 (0) | 10 (58.8) |
|
Switch to Trastuzumab in Monotherapy or
Trastuzumab and Endocrine Furing First-Line Therapy |
0 (0) | 12 (70.6) |
Data are presented as median (range) or n (%).
Abbreviations: CNS = central nervous system; DFI = disease-free interval.
Proportion of stage I to III patients who received neo or adjuvant trastuzumab.
Proportion of patients does not add up to 100% because patients could have more than 1 site of recurrence.
Soft tissue includes: chest wall, breast, local and/or distant lymph nodes, or skin.
In the group of patients with extremely long-term clinical benefit of first-line trastuzumab-based regimens, 11 patients (65%) had CNS progression over time. The CNS progression occurred at the first year after diagnosis in 1 patient, at the second year in 5 patients, at the third year in 4 patients, and at the fourth year in 1 patient. Ninety-one percent of these patients continued the same therapy after CNS progression (median duration, 2.0 years; range, 0.4-4.2 years). As of August 1, 2012, 9 patients (52.9%) in the group of patients with extremely long-term clinical benefit were alive and 4 of these (23.5%) had not yet progressed for the duration of their first-line regimen. In the group of patients with extremely short-term clinical benefit, just 1 patient was alive, and was receiving fourth-line treatment.
Discussion
In this cohort of advanced HER2-positive breast cancer patients treated in the first-line setting with a trastuzumab-based regimen, we found significant associations between HR status and adjuvant trastuzumab and clinical benefit of treatment. Patients with HR-positive tumors were more likely to achieve a long-term clinical benefit to a first-line trastuzumab-based regimen. Our results also suggested a lower likelihood of long treatment duration in patients who relapse after adjuvant trastuzumab treatment. Nevertheless, the analytic model based on classic clinicopathological features that we used, appears to have a low predictive value to characterize a subset of patients with prolonged first line trastuzumab-based treatment duration. Moreover, we identified a subgroup of patients with extremely prolonged clinical benefit to trastuzumab-based regimens (> 34.0 months), 2 of them to trastuzumab monotherapy.
Trastuzumab has been widely used for treatment of patients with HER2-positive metastatic breast cancer since its approval by the Food and Drug Administration in 1998. Improvements in overall response, time to progression, and survival have been extensively reported.2-5 However, limited information is available to identify the subset of patients most likely to experience long-term benefit to trastuzumab therapy in the advanced setting.
Hormone receptor status has been linked to differences in benefit of anti-HER2 therapies among patients diagnosed with early-stage HER2-positive breast cancer, particularly in those treated with neoadjuvant therapy, and with differences in patterns of recurrence.17 In the advanced setting, the effect of HR on response to trastuzumab is less clear. Trastuzumab given either alone or in combination with chemotherapy in the first-, second-, or third-line treatment for advanced HER2-positive breast cancer have shown similar magnitude of benefit regardless of HR status.20-23
In this study, we evaluated how HR status influenced the length of response to trastuzumab therapy in the first-line treatment of advanced breast cancer. Patients with prolonged first-line treatment duration had a higher likelihood of having HR-positive tumors (multivariate OR, 2.39; 95% CI, 1.08-5.31; P = .032) than patients with short-term duration of treatment. This is consistent with findings within other cohorts. Namely, in registHER, a prospective observational study of metastatic HER2-positive breast cancer patients treated with trastuzumab-based chemotherapy regimens, HR-positive status has also been identified as prognostic factor for longer progression-free survival (median progression-free survival, 11.2 months vs. 8.3 months, for HR-positive vs. HR-negative; P = .010, respectively).24
Recently there have been reports of the effect of adjuvant trastuzumab on outcomes of HER2-positive breast cancer treated with anti–HER2-targeted therapy in the metastatic setting. Murthy et al showed that patients with HER2-positive metastatic breast cancer who were trastuzumab-naive, had a higher response rate to HER2–targeted-based therapy than patients who received trastuzumab as adjuvant therapy.19 Our results are consistent with these results. A smaller proportion of patients with long-term clinical benefit had received neoadjuvant or adjuvant trastuzumab (patients with long-term vs. short-term clinical benefit, 15.4% vs. 33.3%, multivariate OR, 0.30; 95% CI, 0.10-0.96; P = .043). The same trend was observed in patients with extremely long-term vs. short-term treatment duration (8.3% vs. 31.3%).
Although these findings add evidence to the existence of heterogeneity within HER2-positive disease driven by some clinico-pathological characteristics, such as HR and the adjuvant use of trastuzumab, the analytic models used in our study have an overall low predictive value to define a group with long-term clinical benefit (overall cohort, C-statistic, 0.634; cohort of stage I to III patients at diagnosis, C-statistic, 0.699). Therefore, although informative, they are not sufficient per se to guide treatment decisions.
Nine percent of patients with the long-term treatment duration in our cohort received trastuzumab monotherapy. In trials of trastuzumab monotherapy, the accurate assessment of median duration of response was not possible because of censuring, however, it was clear that some of the responding patients had very prolonged responses.4,25 Consistent with these results, there have been reports of a durable clinical response to trastuzumab-based regimens, including trastuzumab monotherapy.6 Furthermore, 5.5% of patients received trastuzumab concomitant with endocrine therapy, none with short-term treatment duration. Because of the small number of patients in these subgroups, we cannot draw definitive conclusions, however, in an era of “abundance of riches,” in which multidrug combination regimens are increasing as the norm,26,27 it would be of particular interest to better characterize these patients, which probably can do well with less.
Finally, we identified a cohort of patients with extremely prolonged clinical benefit using trastuzumab-based regimens (> 34 months). The clinicopathological characteristics of this group of patients were similar to the group defined as long-term responders. In our study, most patients with extremely long-term benefit of anti-HER2 therapies experienced CNS progression (58.8%), though they continued to respond systemically to trastuzumab-based therapies. As described in other studies, CNS recurrence was widely dispersed over time in this group of patients.28 Moreover, most of these patients had long periods of systemic stability after their initial CNS event (median, 2.0 years; range, 0.4-4.2 years). Previous studies showed that brain metastases are common in HER2-positive advanced breast cancer treated with trastuzumab.29,30 Furthermore, other reports showed that a high proportion of patients responding to trastuzumab treatment developed CNS disease.31 Our findings highlight the clinical relevance of CNS disease in the subgroup patients with good response to anti–HER2-based therapy. Therefore, our findings add evidence for the need for new treatment strategies for patients with brain metastases as systemic therapies for extracranial disease continue to improve.
We acknowledge the limitations of our study. This was a single institution, retrospective study with a relatively small sample size; however, it allowed examining the effect of treatment duration of the most relevant biologic segregator of breast cancer, HR, and of the use of adjuvant trastuzumab and adjust for clinically relevant confounders. Furthermore, this was a single academic institution study and we cannot rule out a potential referral bias. However, it is unlikely that the clinicopathological characteristics that influence anti-HER2 therapy duration would be different in a population-based sample. Finally, we did not conduct a pathologic review of the tumor samples, which could be relevant in a study on the effect of HR in HER2-positive disease. However, specimens were reviewed by academic pathologists as part of routine clinical care at DFCI.
Nevertheless, our results are not meant to draw definitive conclusions, but are intended to be hypothesis-generating. The validation of our findings in an independent and larger cohort is planned. However, it is unlikely that the use of classic clinico-pathological markers alone will be sufficient in identifying long-term responders to trastuzumab-based therapy. Therefore, it is a major research priority to identify molecular predictors of benefit of anti-HER2 therapy.
Conclusion
Our study supports other observations that suggested that HER2-positive disease is a heterogeneous entity. Although our data suggest that HR is a major driver of the HER2 clinical phenotype, influencing treatment duration, and therefore, clinical benefit of anti-HER2 therapy, and that probably the use of adjuvant trastuzumab also influences clinical benefit of anti–HER2-targeted treatments; they also suggest that classical clinicopathological markers are suboptimal for predicton of long-term response to anti–HER2-targeted therapy. We believe that these findings reinforce the need to identify molecular predictors of benefit and resistance to anti–HER2-based therapies in the near future.
Clinical Practice Points
There is significant heterogeneity in the clinicopathological features among HER2-positive patients and particularly in patients that have prolonged benefit of advanced first-line trastuzumab-based regimens.
Patients with HR-positive disease had a higher likelihood of a prolonged clinical benefit of trastuzumab-based treatments.
Recurrence after the use of trastuzumab in the adjuvant setting is associated with a lower likelihood of prolonged trastuzumab-based regimen duration.
There is a subset of patients who have a prolonged benefit of anti–HER2-based regimens, including trastuzumab monotherapy.
It is unlikely that classic clinicopathological markers alone will be sufficient to identify patients with prolonged responses to anti-HER2 therapy.
It will be critical to identify molecular predictors of benefit of and resistance to anti–HER2-based therapies.
A significant proportion of patients with long-term benefit from anti-HER2 therapy develop CNS disease in the setting of systemic response. The identification of treatment strategies in this subset of patients is warranted.
Acknowledgments
I. Vaz-Luis is supported by the Scholars in Clinical Science Program of Harvard Catalyst, The Harvard Clinical and Translational Science Center (Award No. UL1 RR025758) and contributions from Harvard University and its affiliated academic health care centers.
The study was supported by the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer (NIH P50 CA089393), the National Comprehensive Cancer Network, Breast Cancer Research Foundation (to N.U. Lin), Nancy and Randy Berry Junior Faculty Award (to N.U. Lin), the Karen Webster and David Evans Research Fund, and Fundacao para a Ciencia e Tecnologia (HMSP-ICS/0004/2011, Career development award to I. Vaz-Luis).
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Disclosure Dr. E.P. Winer has received research funding from Genentech, Dr. I.E. Krop has received research funding from Genentech, and Dr. N.U. Lin has received research funding from Genentech and GlaxoSmithKline. All other authors have no conflicts of interest.
References
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 5.Burstein HJ, Harris LN, Marcom PK, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol. 2003;21:2889–95. doi: 10.1200/JCO.2003.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Gullo G, Zuradelli M, Sclafani F, et al. Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol. 2012;23:2204–5. doi: 10.1093/annonc/mds221. [DOI] [PubMed] [Google Scholar]
- 7.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–24. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Perreard L, Fan C, Quackenbush JF, et al. Classification and risk stratification of invasive breast carcinomas using a real-time quantitative RT-PCR assay. Breast Cancer Res. 2006;8:R23. doi: 10.1186/bcr1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2010;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol. 2013;24:283–91. doi: 10.1093/annonc/mds286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14:R129. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy RK, Varma A, Mishra P, et al. Impact of adjuvant trastuzumab on outcomes of HER2-positive breast cancer patients treated with HER2-targeted therapy in the metastatic setting. ASCO Meeting Abstracts. 2012;30(suppl):527. [Google Scholar]
- 20.Bayo-Calero JL, Mayordomo JI, Sanchez-Rovira P, et al. A phase II study of weekly vinorelbine and trastuzumab in patients with HER2-positive metastatic breast cancer. Clin Breast Cancer. 2008;8:264–8. doi: 10.3816/CBC.2008.n.030. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Nakagami K, Mizutani M, et al. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res Treat. 2010;119:127–36. doi: 10.1007/s10549-009-0498-7. [DOI] [PubMed] [Google Scholar]
- 22.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 23.Yardley DA, Burris HA, 3rd, Hanson S, et al. Weekly gemcitabine and trastuzumab in the treatment of patients with HER2-overexpressing metastatic breast cancer. Clin Breast Cancer. 2009;9:178–83. doi: 10.3816/CBC.2009.n.029. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman PA, Mayer M, Paik S, et al. registHER: trastuzumab-based taxane or vinorelbine treatment selection in patients with HER2/neu-positive metastatic breast cancer: patient characteristics and preliminary outcomes (abstract) San Antonio Breast Cancer Symposium. 2006:a2066. [Google Scholar]
- 25.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. New Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gradishar WJ. HER2 Therapy–an abundance of riches. New Engl J Med. 2012;366:176–8. doi: 10.1056/NEJMe1113641. [DOI] [PubMed] [Google Scholar]
- 28.Olson EM, Najita JS, Burstein HJ, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013 Jan 23; doi: 10.1016/j.breast.2012.12.006. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yau T, Swanton C, Chua S, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45:196–201. doi: 10.1080/02841860500486630. [DOI] [PubMed] [Google Scholar]
- 30.Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. 2007;12:766–73. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 31.Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004;40:379–82. doi: 10.1016/j.ejca.2003.09.018. [DOI] [PubMed] [Google Scholar]