Abstract
The interaction between CD27 and its ligand CD70 has been implicated in regulating cellular immune responses to cancer. Here we report on the role of soluble CD27 (sCD27) in T-cell activation and its elevation in the serum of cancer patients after immunotherapy. In vitro, sCD27 is preferentially derived from activated CD4+ T cells. Adding sCD27 to stimulated peripheral blood mononuclear cells increases T-cell activation and proliferation, and is associated with the immunological synapse-related proteins myosin IIA, HMGB1, and the TCR Vβ chain. The pool of serum sCD27 is shown to be greater in healthy donors than in cancer patients. However, metastatic cancer patients treated with immunotherapy showed a significant increase in the serum sCD27-pool post-therapy (p < 0.0005); there was also an increased trend towards an association between enhanced sCD27-pool post-therapy and overall survival (p = 0.022). The identification of sCD27 as an immune modulator associated with enhanced human T-cell activation in vitro and in vivo provides a rationale for developing new immunotherapeutic strategies aimed at enhancing sCD27 for treating cancer and potentially other diseases.
Keywords: Soluble CD27 (sCD27), CD27/CD70, T-cell activation, soluble factors, cancer vaccine, prostate cancer, immunotherapy, ipilimumab (anti-CTLA4), PROSTVAC
Introduction
Previous studies have demonstrated that the interaction between CD27 and its ligand, CD70, plays a role in providing costimulation for prolonged lymphocyte survival, enhanced T-cell proliferation, and memory-cell formation (1, 2). CD27 is a 55-kD type I transmembrane protein and TNF receptor that is expressed on subsets of T, B, NK, and hematopoietic progenitor cells. CD27 controls the activity of these cells by engaging with CD70, which is transiently expressed by cells of the immune system upon activation (3-5). Data from murine studies indicate that CD27 costimulation induces Th1-cell formation. Studies in humans also show that CD27 signaling in naive CD4+ T cells promotes IL-12–induced Th1-cell development (6, 7). It was recently demonstrated that the expression of CD27 and CD70 on infused CD8+ tumor-infiltrating lymphocytes was associated with antitumor effects in an adoptive T-cell transfer clinical trial in melanoma (8, 9).
In the past two decades, studies of soluble receptors of cytokines and growth factors (10) have suggested that the majority of soluble receptors compete with their membrane-bound counterparts for ligands and thus act antagonistically; very few have been found to be agonistic (11). Currently, there is no definitive hypothesis regarding the immunological role of the soluble receptor CD27. However, another soluble molecule belonging to the same TNF family, soluble CD40L (sCD40L), has been previously investigated and has been shown to play a costimulatory role in immune activation and regulation (12, 13). A recent study showed that a soluble form of CD27 (sCD27) shed by Waldenström's macroglobulinemia (WM) lymphoplasmacytic cells mediated up-regulation of CD40L and a proliferation-inducing ligand (APRIL) on WM mast cells (14), suggesting that sCD27 may act agonistically in the process of immune activation. This agonistic effect may help to break immunological tolerance of tumors.
sCD27, a 32-kD protein identical to the extracellular domain of membrane-bound CD27, can be released after lymphocyte activation by differential splicing of the receptor protein or shedding from the cell surface by metalloproteinases (MMPs) (2, 15, 16). The production of sCD27 upon T-cell activation has been demonstrated previously by using anti-CD3 or a combination of anti-CD3 and anti-CD2 mAbs to stimulate human PBMCs in vitro (17). sCD27 has been detected in serum, plasma, and urine samples from healthy individuals, and increased levels have been documented in systemic lupus erythematosus, viral infections, and lymphoid malignancies (18-20). The level of sCD27 in plasma samples has been used as a marker of disease burden in patients with WM (20) and to monitor immune activation during antiretroviral therapy in patients with HIV (21). To our knowledge, there have been no reports on sCD27 in patients with solid tumors, or analyses of sCD27 pre- vs. post-immunotherapy.
In this study, we demonstrated for the first time that sCD27 is a functional protein directly involved in T-cell activation, and that an enhanced serum sCD27-pool showed a potential association with clinical outcome of cancer patients in an immunotherapy trial. In this report, the term “sCD27-pool” refers to the varying levels of sCD27 found in healthy donors and cancer patients. Generated from previous immune activation and maintained by additional activation of lymphocytes, this pool may fluctuate in response to immune activation such as immunotherapy. Further investigation of the immunological function of sCD27 may help to identify new strategies in cancer immunotherapy and potentially for the treatment of other diseases.
Materials and Methods
Serum samples from healthy donors were purchased from Innovative Research (Novi, MI) and Valley Biomedical (Winchester, VA). Cancer patients' sera were obtained from clinical trials conducted at the National Cancer Institute (http://clinicaltrials.gov). Pretreatment serum samples were obtained from the following trials: (1) Vaccine therapy in treating patients with stage D0 prostate cancer (NCT00514072), (2) prostate cancer patients with nonmetastatic castration-resistant prostate cancer (CRPC) (D0.5) (NCT00020254) (22), and (3) a second-generation poxviral vaccine (PSA-TRICOM) targeting prostate-specific antigen (PSA) in metastatic CRPC (NCT00060528) (22). Pre- and post-treatment serum samples were obtained from prostate cancer patients treated with ipilimumab (anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4)) in combination with PROSTVAC (NCT00113984). This study enrolled 30 patients to four sequential treatment cohorts. Patients in each cohort received a fixed dose of PROSTVAC vaccine plus 1, 3, 5, or 10 mg/kg of ipilimumab (23). Vaccine was given on days 1, 15, 43 and 71, with ipilimumab given concurrently starting with the second dose of vaccine. The latest updated overall survival information was obtained on August 2, 2012. For patients who were still alive, the date of the last follow-up was used in the data analysis. Healthy donors' PBMC samples were isolated from the Buffy coat by density gradient centrifugation (MP Biomedicals, Solon, OH). All patients signed a consent form approved by the Institutional Review Board of the National Cancer Institute (NCI). Studies were conducted in accordance with the Declaration of Helsinki.
Measurement of sCD27
Serum levels of sCD27 were determined by ELISA using kits from eBioscience (San Diego, CA) and Sanquin (Amsterdam, Netherlands).
Human T-cell activation
PBMCs (1×106/ml) from healthy donors were stimulated with anti-CD3/CD28 T-cell activation beads (Invitrogen, Carlsbad, CA) at a bead:cell ratio of 1:1, or 1:5 in some experiments, along with 10 U/ml of IL-2 for 15 days. The supernatant was collected on days 3, 7, and 15. PBMCs were also treated with 20 ng/ml PMA (Sigma-Aldrich, St. Louis, MO) and 1 μM ionomycin (Sigma) in the presence of 2 μM of the intracellular transport inhibitor monensin (Sigma) for 6 h. The supernatant was tested for sCD27 and cells were analyzed for surface markers and intracellular IFN-γ.
Antibodies and flow cytometry analysis
Percentages of CD8, CD3, CD25, CD27, and CD70 expression on the surface of PBMCs were determined by flow cytometry. Direct-staining mAbs were used to detect each of the cell-surface antigens. PerCP-Cy5.5 antihuman CD4 and CD8 (eBioscience) were used to detect the presence of cell-surface CD4 and CD8. PE mouse antihuman CD3 (BD Biosciences, San Jose, CA) and PE mouse antihuman CD70 (BD Biosciences) were used to detect CD3 and CD70, respectively. APC mouse antihuman CD25 (BD Biosciences) and APC-conjugated antihuman CD27 (eBioscience) were used to detect CD25 and CD27, respectively. Cell-surface expression was measured by flow cytometry (FACS Calibur, BD Biosciences) and the resulting data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
T-cell subsets isolation
Four T-cell subsets (CD4+ naïve, CD4+ memory, CD8+ naïve and CD8+ memory) were isolated from PBMCs of healthy donors using Miltenyi separation beads (Miltenyi Biotec, Auburn, CA). The purified cell subsets were tested by FACS analysis for surface markers: CD4+ naïve cells were CD4+CD45RA+, CD4+ memory cells were CD4+CD45RO+, CD8 naïve cells were CD8+CD56−CD57−CD45RO−, and CD8 memory cells were CD8+CD45RO+CD45RA−CD56−CD57−.
Fluorescence immnuohistochemistry staining
T cells were prepared on slides using cytospin, and fixed with acetone for 30 min, followed by a two-step staining method. The primary antibodies were mouse anti-human Myosin IIA (1:25 dilution) (BD Bioscience) and mouse anti-human 6× His-Tag biotin labeled antibody (1:50 dilution) (AnaSpec, Inc., Fremont, CA). The secondary antibodies were FITC Streptavidin (BD Bioscience) and goat anti-mouse Rhodamine (Invitrogen).
Cell proliferation assay
PBMCs (1×106/ml) were labeled with 5μM CFSE (Invitrogen) according to the manufacturer's instructions. The cells were then stimulated with anti-CD3/CD28 beads in the presence or absence of different amounts of sCD27. Four days after stimulation, FACS analysis was performed.
sCD27 depletion
To deplete sCD27, plates were coated with antihuman CD27 antibody or IgG control (20 μg/ml) (R&D Systems Inc., Minneapolis, MN) for 3 h at room temperature or overnight at 4°C. Plates were then washed three times with PBS. Serum derived from a healthy donor or purified recombinant sCD27 was added to the culture and incubated for 2 h. Following incubation, the serum or recombinant sCD27 was tested by ELISA to confirm depletion, and the supernatant of sCD27-depleted serum or recombinant human sCD27 was used for the coculture experiments.
Human sCD27 synthesis and purification
Recombinant human sCD27 protein was purified from the supernatant of HEK 293 cells that were transduced with a construct encoding the extracellular domain (aa 1 to 189) of CD27 protein (GENEART, Regensburg, Germany).
Immunoprecipitation and protein identification
Proteins associated with sCD27 were isolated using sCD27-his (6×) incubated with lysate of anti-CD3/CD28 bead-stimulated PBMCs derived from four healthy donors. ProFound™ Pull-down PolyHis Protein:Protein Interaction Kit was used for immunoprecipitation (Thermo Scientific, Rockford, IL). Purified proteins were separated by SDS-PAGE and stained using SimpleBlue (Invitrogen). Protein identification was performed using mass spectrometry as previously described (24).
Results
In vitro production of sCD27 from activated human PBMCs
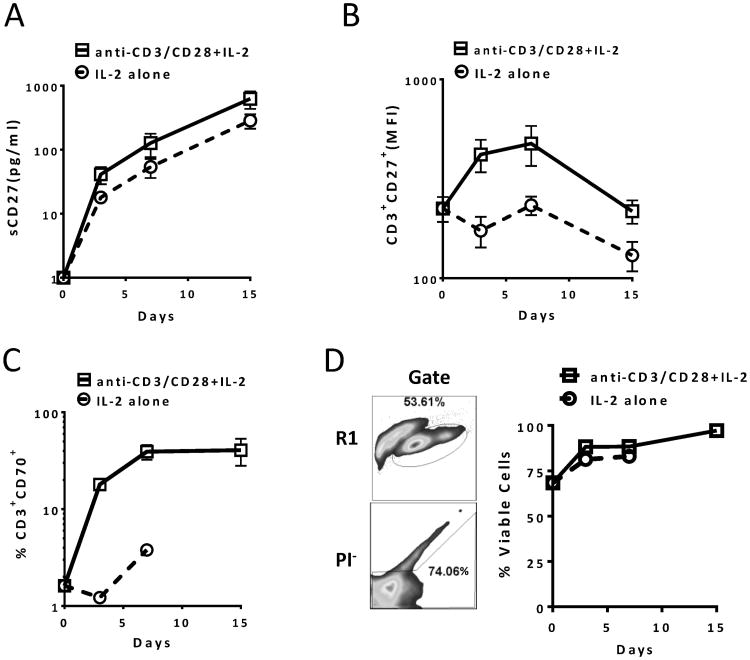
We first investigated sCD27 production upon T-cell stimulation and its association with surface expression of CD27 on T cells. Stimulating PBMCs derived from healthy donors with anti-CD3/CD28 beads plus IL-2 (20 U/ml) or IL-2 alone resulted in a linear increase in sCD27 production, and the highest level of sCD27 was seen in the supernatant on day 15. This could be the result of more T cells accumulated in the wells, and/or more sCD27 shed from each T cell (Fig. 1A). We analyzed CD27 expression on CD3+ T cells to determine the change in expression of membrane-bound CD27 during stimulation. Results suggested a transient increase followed by a decline in surface expression after anti-CD3/CD28 plus IL-2 stimulation (Fig. 1B). In addition, CD70-expressing T cells were enriched upon activation (Fig. 1C), and the production of sCD27 correlated linearly with the amount of CD70-expressing CD8+ T cells in the culture (Supplemental Fig. 1). Moreover, results from our (unpublished) data suggest that CD70-transduced T cells show enhanced anti-tumor activity and IL-2 production. We then evaluated the status of cell death after the T-cell specific stimulation. The results suggested that there was minimal cell death during the course of in vitro activation (Fig. 1D). These data demonstrated that T-cell activation increased sCD27 production, which more likely was the result of shedding from the T cells' surface, rather than activation-induced cell death of T cells, because >90% of the PBMCs were viable 15 days after the stimulation.
Figure 1.
sCD27 production is associated with T-cell activation in vitro. (A) Increased sCD27 in culture supernatant from PBMCs stimulated in vitro with anti-CD3/CD28 and IL-2. Eight PBMC samples (106 cells/ml) from healthy donors were stimulated with or without anti-CD3/CD28 beads (1:1 cell-to-bead ratio) in the presence of 10 U/ml of IL-2 for 15 days. Supernatant was collected on days 3, 7, and 15. On day 7, three of the activated PBMC samples were split and fresh medium was added due to cell overgrowth, and the dilution factor was considered when calculating the values. For day 0 sCD27 values, the analysis was done using culture medium without adding serum. The level of sCD27 was tested by ELISA, and for better data presentation, the values of sCD27 are shown in log10(y+1). A repeated measures ANOVA was performed on transformed data and orthogonal contrasts were used to test for linear trends on days 3-15 data only. (B) Expression of surface CD27 on T cells with or without stimulation. The cells described above were analyzed by FACS, and CD27 expression was measured by MFI of CD27 on CD3+ and propidium iodide negative (PI-) cell populations. The Y-axis is shown in log10(y). A repeated measures ANOVA was performed on transformed data and pair-wise p-values were adjusted using Holm's method. (C) CD70-expressing T-cell population in PBMCs with or without stimulation. FACS analysis was also done on days 0, 3, 7 and 15 for the cells described in (A) by examining the frequency of the CD3+CD70+ T-cell population in anti-CD3/CD28 and/or IL-2 stimulated PBMCs. A repeated measures ANOVA was performed on transformed data and pair-wise p-values were adjusted using Holm's method. The Y-axis is shown in log10(y). (D) No significant cell death after activation with anti-CD3/CD28 beads. The experiment described in (A) was also analyzed for viability after the activation by staining the cells with PI. An example of lymphocyte (R1) and PI negative cell gates is indicated on the left and % of PI negative cell population from 10 PBMCs is shown on the right. There were no data acquired for day 15 in the IL-2 alone group in (C) and (D). Data are presented as means ± SEM. MFI, mean fluorescence intensity.
Factors that influence sCD27 production
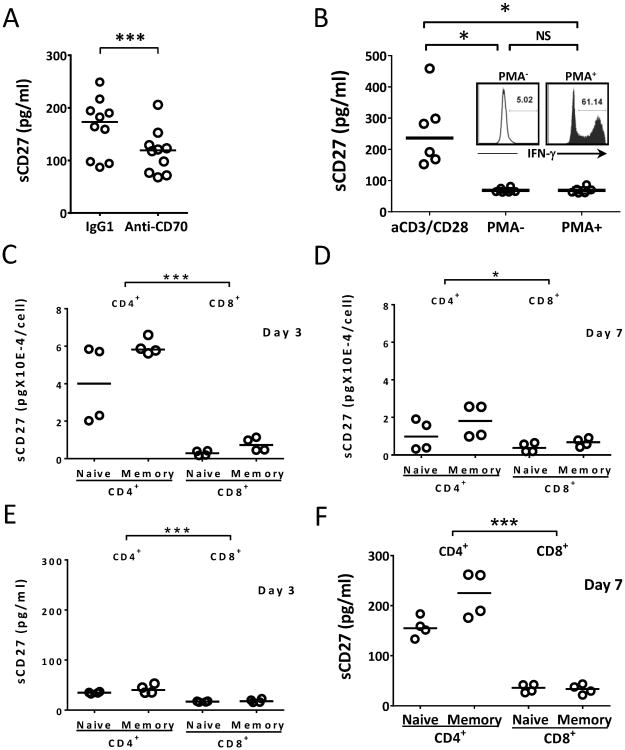
We proceeded to determine which factors can alter sCD27 production and which T-cell subsets are the major source for its production. In an experiment using an antibody that blocked CD70, there was a significant inhibition of sCD27 production (Fig. 2A), demonstrating that the shedding of CD27 from T cells is enhanced by ligation of CD27 with its ligand CD70, which has been shown to be expressed on activated T cells (8, 9). Next, we compared the production of sCD27 triggered by either T-cell receptor/CD3 plus CD28 or by PMA-ionomycin, which bypasses the TCR/CD3 signal. It has previously been reported that PMA-ionomycin stimulation suppresses CD27 surface expression on T cells (25). After stimulation with PMA-ionomycin, T cells produced increased amounts of IFN-γ, a sign of activation, but a minimal production of sCD27 was seen compared to that obtained by TCR/CD3/CD28 stimulation (Fig. 2B). These data suggest that TCR/CD3 engagement is necessary for the efficient production of sCD27. Finally, we tested the difference in sCD27 production among four T-cell subsets: naïve CD4+ and CD8+ and memory CD4+ and CD8+ T cells in sCD27 production 3 and 7 days after activation. The results illustrated that relatively more sCD27 was produced per CD4+ T cell (in particular, CD4+ memory T cell) on day 3 compared with CD8+ T cells, and this was also true for day 7, but with less significance between CD4+ and CD8+ T cells (Fig. 2C, 2D). Next, we evaluated the total sCD27 production in the supernatant during the T-cell stimulation. Relatively more sCD27 was accumulated in the culture supernatant on day 7 for the CD4+ T cells than the CD8+ T cells (Fig. 2E, 2F). Taken together, the data suggest that T-cell activation results in the production of sCD27, and that TCR/CD3 signaling and CD70 engagement are necessary for the optimal production of this soluble molecule. CD4+ T cells may be the predominant source for the sCD27 production upon T-cell activation.
Figure 2.
Bypassing TCR engagement or CD70 blockage produced less sCD27; CD4+ T cells are the main source of sCD27 in vitro. (A) Blocking the interaction of CD27 with CD70 inhibited production of sCD27. Ten PBMC samples from healthy donors were stimulated with anti-CD3/CD28 beads and 20 μg/ml of IgG control or anti-CD70 antibody, and the supernatant was collected on day 4 post-stimulation. A two-way repeated measures ANOVA was performed on transformed data, by day. (B) PMA/ionomycin stimulation that bypasses TCR signaling produced minimal levels of sCD27 compared to anti-CD3/CD28 stimulation. Six PBMC samples (106 cells/ml) were incubated with or without PMA/ionomycin for 6 h. The supernatants were collected and analyzed by ELISA for sCD27 production, and T-cell activation was measured by intracellular production of IFN-γ in CD3+ T cells, as shown in the insert. For comparison, the same PBMCs were also stimulated with anti-CD3/CD28 and 10 U/ml of IL-2. After 3 days, the supernatants were collected and analyzed. A one-way repeated measures ANOVA was performed on transformed data. (C and D) CD4+ T cells appeared to produce a relatively greater amount of sCD27 per cell upon activation compared to CD8+ T cells. Four subsets of T cells (naïve CD4+, memory CD4+, naïve CD8+, and memory CD8+) were isolated from four PBMC samples using magnetic beads, and the subsets were stimulated with anti-CD3/CD28 in the presence of 10 U/ml of IL-2 for 7 days. The supernatants were collected on days 3 (panel C) and 7 (panel D) and evaluated for sCD27 by ELISA. Cell counts were also carried out. sCD27 production per cell was calculated (total amount of sCD27 divided by cell count). There was a highly significant difference between the CD4+ (naïve + memory) and CD8+ (naïve + memory) means on day 3 (p < 0.0001), and a trend of a difference on day 7 (p = 0.017). A three-factor factorial repeated measures ANOVA on the log10(y) transformed data was performed for (D) and (C). Note different scale in D. (E and F) CD4+ T cells appeared to produce a relatively greater amount of sCD27 upon activation compared to CD8+ T cells. There were highly significant differences between the CD4 and CD8 means on both days (p < 0.0001, pooled over naïve and memory cells); the difference was larger on day 7 than on day 3. Note different scale in F. The lines in the dot plots indicate the median values. *p < 0.05 (a trend); ***p < 0.001.
In vitro T-cell activation by recombinant sCD27
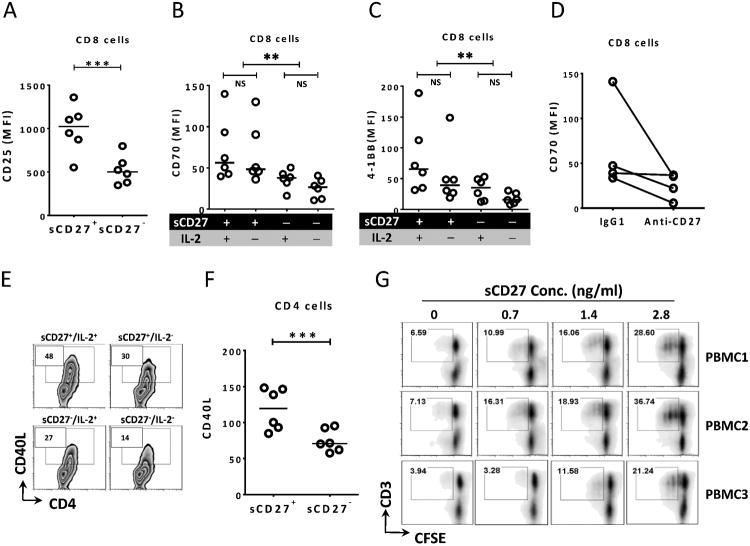
To further test our hypothesis that sCD27 is not only a by-product of T-cell activation but also a functional molecule, we used recombinant human sCD27 to evaluate its functionality. Analysis of T-cell activation markers by FACS analysis showed significant up-regulation of CD25, CD70, and 4-1BB (CD137) expression on CD8+ T cells 3 days after the addition of sCD27. These markers are important for CD8 cytotoxic function, and were only minimally expressed on CD8+ T cells before stimulation (Fig. 3A, 3B, 3C), suggesting that sCD27's capability for T-cell activation is similar to that of IL-2. Based on our analysis, the effect of sCD27 alone on up-regulation of these markers was highly significant (p < 0.0001 for CD70 and p < 0.005 for 4-1BB) (Fig. 3B, 3C). In order to ensure that this effect was specifically due to the presence of sCD27, a procedure using CD27-specific antibody to remove sCD27 was performed. The results showed that CD70 on T cells was decreased in three out of four samples, and CD25 expression on the T cells was also attenuated when sCD27 was depleted, suggesting potential involvement of sCD27 in T-cell activation (Fig. 3D and Supplemental Fig. 2A). In addition, there was a significant difference in CD25 expression on CD8+ T cells after culture in medium supplemented with serum that contained high (>1000 pg/ml) or low (<10 pg/ml) levels of sCD27 (Supplemental Fig. 2B). CD4+ T-cell activation was analyzed by testing CD40L expression on CD4+ T cells, a crucial surface marker for the CD4+ T-cell function. The data illustrated enhanced CD40L expression on CD4+ T cells with the addition of sCD27 (Fig. 3E, 3F). Finally, T-cell proliferation was enhanced in a dose-dependent manner with increasing amounts of recombinant sCD27 and stimulation with a low (1:5) CD3/CD28 bead to cell ratio. The rationale for using a minimum stimulus to the T cells is to mimic the insufficient antigenic stimulation of tumor cells (Fig. 3G). These results provide evidence that sCD27 could be a functional molecule involved in T-cell activation.
Figure 3.
sCD27 up-regulates the expression of activation markers on T cells and promotes T-cell proliferation in vitro. (A) sCD27 up-regulated surface expression of the activation marker CD25 on CD8+ T cells (p < 0.0005). Six PBMC samples were stimulated with anti-CD3/CD28 beads in the presence or absence of sCD27 (1.4 ng/ml). Three days after stimulation, FACS analysis was performed by analyzing CD25 on CD8+ T cells. A one-way repeated measures ANOVA was performed on the raw data. (B and C) sCD27 also up-regulated expression of the activation markers CD70 and 4-1BB. A two-factor factorial [sCD27 (+ or −), IL-2 (+ or −)] repeated measures ANOVA on log10(y) transformed data was performed, and Holm's methods were used to adjust p-values. When the effects of sCD27 and IL-2 on CD70 and 4-1BB expression were compared, the analysis showed that sCD27 enhanced CD70 and 4-1BB expression on CD8+ T cells (p < 0.001 and p < 0.005, respectively), pooled over IL-2. (D) Depletion of sCD27 showed a trend of decrease in T-cell activation by measuring CD70 expression on CD8+ T cells. Four PBMC samples were stimulated with anti-CD3/CD28 beads in the presence of supernatant in which sCD27 was not depleted (sCD27 value = 1.4 ng/ml, IgG1) or was depleted (sCD27 value = 0 pg/ml, anti-CD27) using anti-CD27 antibody. Four days later, surface expression of CD70 on CD8+ T cells was analyzed by FACS. A one-way repeated measures ANOVA was performed on transformed data, p = 0.093. (E and F) Cells from the experiment described in (A) were also evaluated for CD40 ligand (CD40L) surface expression on CD4+ T cells by FACS analysis. sCD27 enhanced CD40L expression on CD4+ T cells measured as the percent of positive cells (panel E) or MFI (panel F). A one-way repeated measures ANOVA was performed on the raw data. (G) sCD27 promotes T-cell proliferation in vitro. Six PBMC samples from healthy donors (106 cells/ml) were labeled with CFSE and then stimulated with anti-CD3/CD28 beads at a low (1:5) bead to cell ratio in the presence of different concentrations of purified recombinant sCD27 and IL-2 (10 U/ml). The CFSE dilution was analyzed by FACS on day 5 after stimulation, and CD3+CFSE− cells were gated. Results from three of six PMBC samples are shown. Lines in the dot plot graphs indicate the median values. **p < 0.01; ***p < 0.001. MFI, mean fluorescence intensity.
Proteins potentially associated with sCD27
We showed above that sCD27 is a potential functional protein. In addition, previous reports have shown that soluble receptors shed from the cell surface may have different binding patterns or function (due to refolding) than their membrane-bound counterparts. To find molecules that could interact with sCD27, we used recombinant sCD27 his-tag as a bait protein to identify binding partners in a lysate of activated PBMCs. Three immunological synapse-related proteins, myosin IIA, high-mobility group box 1 (HMGB1), and TCR Vβ chain, were identified as potential binding partners for sCD27. No peptide sequence of CD70 was identified in the assay, since there was no CD70 expression on any cell type in the PBMCs (8, 9), and the bait protein sCD27 was seen only in the positive lane. Among these three proteins, myosin IIA and HMGB1 showed some background in the control lane, but none was seen for the TCR Vβ chain (Table I). In addition, we performed an experiment using immunohistochemistry to confirm these observations. Confocal microscopy showed that sCD27 and myosin IIA were co-localized in activated T cells (Supplemental Fig. 3), which confirms our previous findings. These data further show that sCD27 may be associated with the T-cell synapse during the process of T-cell activation.
Table I. Proteins with potential to interact with sCD27 after T-cell activation.
| Protein¶ | TPC* in Lane 2 (positive) | TPC in Lane 4 (background) | Gene | Accession No. |
|---|---|---|---|---|
| Myosin IIA | 79 | 14 | MYH9 | P35579 |
| High mobility group protein B1 | 44 | 5 | HMGB1 | P09429 |
| TCR-Vβ chain | 25 | 0 | TCRBV | N/A |
| CD27§ | 13 | 0 | TNFRSF7 | P26842 |
Proteins associated with sCD27 were isolated using sCD27-his incubated with a lysate of anti-CD3/CD28 bead-stimulated PBMCs derived from four healthy donors. The ProFound™ Pull-down PolyHis Protein: Protein Interaction Kit was used for immunoprecipitation. Purified proteins were separated by SDS-PAGE and stained using SimpleBlue. Protein identification was performed using mass spectrometry. Each protein was identified based on the sequence and amount of peptides that eluted from the positive and control lanes. The total peptide count in the positive lane had to be 5-fold higher than the control.
In descending order, these proteins showed the most potential for interaction with sCD27, based on total peptides eluted from the positive lane.
Peptides derived from bait protein CD27 were detected only in the positive lane.
TPC, total peptide count.
Healthy donors have a relatively larger serum pool of sCD27 than patients with cancer, and immunotherapy with PROSTVAC plus ipilimumab elevated the pool in these patients
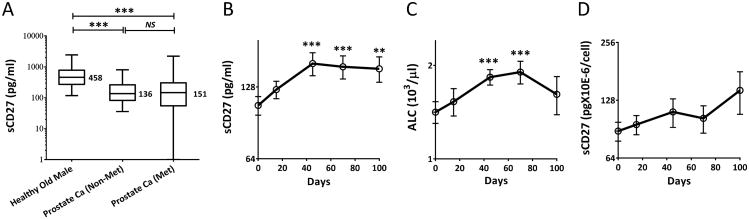
Serum samples from age and gender matched healthy donors and patients with prostate cancer were evaluated for sCD27. Here, we use “sCD27-pool,” which refers to the varying levels of sCD27 in serum, and we found that this pool was significantly larger in healthy donors than in prostate cancer patients (and other types of cancers, data not shown) with either metastatic or nonmetastatic disease (p < 0.0001; Fig. 4A). This observed difference was not due to the quality of the serum samples, since the samples were also tested for another soluble protein, sCD40L, which appeared in reverse proportions to sCD27 in healthy donors and cancer patients (13). In addition, we compared PBMCs derived from healthy donors and cancer patients for the production of sCD27 upon in vitro stimulation (Supplemental Fig. 4). There were no differences in the frequency of CD3+CD27+ T cells between healthy donors and cancer patients, and no differences in the amounts of sCD27 in supernatants after 3-15 days of in vitro culture. Results of a recently completed clinical trial of PROSTVAC vaccine in combination with ipilimumab (anti-CTLA4) in patients with metastatic CRPC offer insights into a possible association between increased serum sCD27 and clinical outcomes (23). PROSTVAC is a poxviral-based vaccine targeting prostate-specific antigen (PSA) and containing transgenes for three costimulatory molecules (B7.1, ICAM-1 and LFA-3, designated TRICOM). A randomized, placebo-controlled, multi-center trial with this vaccine demonstrated improved patient survival (26). Ipilimumab, a fully human mAb against a negative immune modulator, CTLA-4, has been used as monotherapy or in combination therapy and has been demonstrated to induce durable objective responses in patients with advanced melanoma (27, 28). This study enrolled 30 patients to four sequential treatment cohorts. Patients in each cohort received PROSTVAC plus 1, 3, 5, or 10 mg/kg of ipilimumab. Overall, there was no clear trend observed between distributions of serum sCD27 and the dose of ipilimumab. It should be noted, however, that 3/3 patients in the 1 mg/kg cohort and 3/6 patients in the 3 mg/kg cohort had received prior chemotherapy. A comparison of pre- and post-treatment levels of sCD27 in these patients found a significant increase in serum sCD27-pool as early as 45 days, which was 4 weeks after the first dose of ipilimumab (p < 0.001, Fig. 4B). These increases in sCD27 levels were in accordance with the slight increases in absolute lymphocyte count (Fig. 4C). In addition, there was a discrete (but statistically non-significant, p > 0.40) increase in sCD27 production per lymphocyte after treatment (Fig. 4D).
Figure 4.
Serum sCD27-pools were larger in healthy donors than in cancer patients, and PROSTVAC plus ipilimumab significantly elevated the pool in these patients. (A) sCD27 levels (analyzed by ELISA) in sera from healthy donors (n = 54) were compared with pre-treatment sera from prostate cancer patients (n = 50) with rising PSA but no radiographic evidence of metastases (NCT00514072 and NCT00020254), and from patients with metastatic prostate cancer (n = 120) (NCT00060528 and NCT00113984). Healthy donors were age and gender matched with prostate cancer patients. Box-and-whisker plots are shown. The Y-axis is shown log10(y+1) and median values are indicated. Comparison was performed using one-way repeated measures ANOVA on transformed data and Holm's method was used to adjust p-values. (B) There was a significant elevation of serum sCD27-pool after administration of PROSTVAC plus ipilimumab. Pre- and post-treatment serum samples from 29 out of 30 patients enrolled in the trial were available for evaluation. sCD27 serum values were analyzed by ELISA, and comparisons were performed between mean values of each post-treatment blood draw: days 15 (n = 29), 45 (n = 29), 70 (n = 24), and 100–120 (n = 26) and the mean baseline (day 0) values for all patients. The data were calculated using the mean of four blinded tests and the Y-axis is shown in log2(y). (C) An increase in peripheral blood absolute lymphocyte count (ALC) after the treatment. Analysis similar to that described in (B) was also done for ALC. (D) Values of sCD27 in serum per lymphocyte of the patients. A calculation of sCD27 per cell at each time point of blood draw was carried out by using the total amount of sCD27 in serum of each patient divided by their ALC value on the same day. The Y-axis is shown in log2(y). For the statistical analysis, we performed a one-way repeated measures ANOVA on the transformed data. We compared the mean of the day 0 data vs. the means of the other 4 days and adjusted the p-values using Dunnett's method. **p < 0.01; ***p < 0.001. Figures show means ± SEM.
sCD27-pool level elevation associated with clinical outcomes for patients treated with PROSTVAC plus ipilimumab
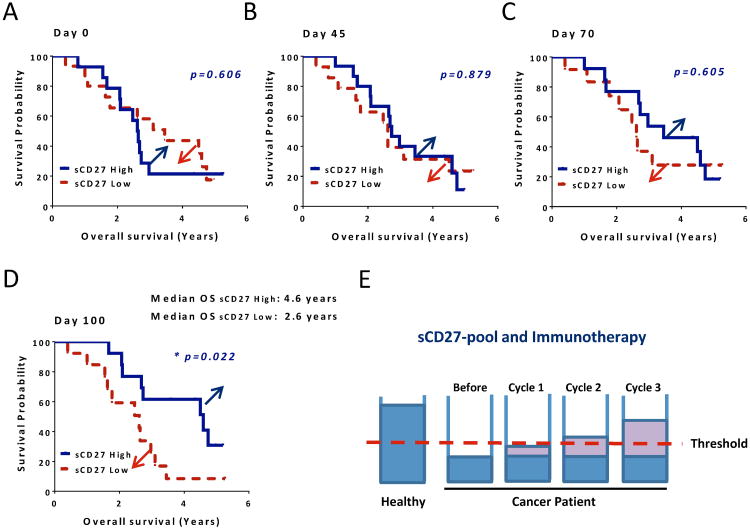
The sCD27-pool in cancer patients can be significantly enlarged by immunotherapy. Next we wanted to determine whether this sCD27 increase can bring a positive effect on clinical outcome for these patients. First we separated patients into two groups using median values of sCD27 on day 0, and increased sCD27 (differences from day 0) for the post-treatment days and then evaluated the association between sCD27 and overall survival. No differences were seen between the two groups on day 0 (Fig. 5A, p = 0.606) and day 45 (Fig. 5B, p = 0.879). However, a gap emerged (as indicated in blue and red arrows) between groups on day 70, which was 4 weeks after the second dose of ipilimumab (Fig. 5C, p = 0.605), and a strong trend was observed at 100 days (Fig. 5D, p = 0.022, a trend). The median overall survival was 4.6 years in the sCD27 high group, which was 2 years longer than for patients in the sCD27 low group, suggesting that patients who had a gain of sCD27-pool after the treatment may have a better chance of surviving longer. In order to clearly describe our interpretation and hypothesis, a schema is shown in Fig. 5E. We assume that there is a putative threshold (red dashed line) for the pool of sCD27, which is a minimum level that is required for proper anti-tumor immunity in humans. The data shown above indicated that immunotherapy can help some patients to raise their sCD27-pool levels, which could bring a positive clinical outcome for these patients. Once again, the data we show here illustrate that sCD27 may contribute to tumor immunity.
Figure 5.
Association between elevation of sCD27-pool and clinical outcome after treatment with PROSTVAC plus ipilimumab. (A) Association between baseline CD27-pool in serum and overall survival. Separating patients into two groups, sCD27 high and sCD27 low using median values of sCD27 for day 0, Kaplan-Meier survival curves are shown for the association between sCD27 at baseline for these two groups and overall survival. (B, C and D) Association between CD27-pool enlargement in patients and overall survival after the therapy. Patients were separated into two groups: sCD27 high and sCD27 low using median increased sCD27 values (differences from day 0 baseline) for 45, 70 or 100-120 days post-treatments. The association between sCD27-pool and overall survival is shown using Kaplan-Meier survival curves, which were plotted for patients from the date of the first administration of PROSTVAC plus ipilimumab to the date of death, or the date of the last follow-up for patients who were still alive. The graphs of days 45, 70 and 100-120 after the treatment are shown. Strata were compared using the log-rank test. The values of sCD27 used for this evaluation were from an average of four blinded ELISA assays. (E) A schematic depiction of the sCD27-pool in healthy donors and cancer patients, and its elevation after immunotherapy. The level of the sCD27-pool is >3 fold higher in healthy individuals than in cancer patients before immunotherapy. After the therapy, the pool was cumulatively refilled as more treatment cycles were given, and some patients reached above a putative threshold (a red dashed line), which is the level required for proper immune function. * p = 0.022 (a trend).
Discussion
Previous studies have found that sCD27 is elevated in certain diseases associated with immune-cell activation, such as autoimmune diseases and lymphomas (18-20). However, the immunological function of sCD27 has not been studied in depth, and reports in the literature have shown conflicting results. One study using recombinant human sCD27 in vitro suggested that sCD27 could block CD27-mediated signaling and thereby have an inhibitory effect (29). In contrast, other studies showed that sCD27 shed by WM lymphoplasmacytic cells induced up-regulation of CD40L and APRIL on WM mast cells (14), and it also induced IgG production through the activation of antigen-primed B cells (30), suggesting that sCD27 may act agonistically in the process of immune activation. CD27 is expressed only on subsets of T, B, NK, and hematopoietic progenitor cells, and some subsets, such as late-stage T effector cells, lose CD27 expression (31). Cancer patients may have an exhausted T-cell pool with more CD27− cells (8, 9). These data underscore the potential role of sCD27 in immune activity and clinical response in cancer patients.
Various models describe several possible mechanisms of soluble receptors in immunologic regulation (11). The studies reported here show that production of sCD27 requires ligation of CD27 with CD70. T-cell activation that bypassed the TCR produced minimal sCD27, indicating that TCR engagement is an important factor in the shedding of sCD27. IL-2 alone can also induce a similar amount of sCD27 without significant CD70 expression on T cells, compared with anti-CD3/CD28 plus IL-2, suggesting a distinct mechanism may be involved in its induction of sCD27. This finding is consistent with a previous study illustrating that IL-2 can dramatically decrease CD27 expression on the T-cell surface (9). The examination of four T-cell subsets for sCD27 production upon in vitro stimulation showed that all subsets produced sCD27, and CD4+ memory T cells produced relatively more sCD27 per cell. While these subsets may not be equally responsive to TCR/CD3/CD28 stimulation (memory CD4+ T cells may be activated earlier than other subsets), and in vitro stimulation may not be equivalent to the in vivo response, nevertheless, this in vitro result suggests another potential role of memory CD4+ T cells in immune regulation.
To test the functionality of sCD27, PBMCs were stimulated in vitro with varying combinations of purified recombinant sCD27 protein and IL-2. Stimulation with sCD27 provided a strong proliferative signal and induced increased lymphocyte proliferation compared with IL-2. A previous study showed that IL-2 modulates CD27 and CD70 expression on T cells, indicating that the immunological action of IL-2 may be through the CD27-CD70 signaling pathway (9). More importantly, markers of T-cell activation were significantly up-regulated after the addition of sCD27. These activation markers have been shown to be critical to the cytotoxic activity of CD8+ T cells (9, 14, 32, 33), and were expressed minimally or not at all on CD8+ T cells prior to stimulation. Furthermore, CD4+ T cells in PBMCs showed enhanced CD40L surface expression after stimulation, an effect that may facilitate B-cell function as well as dendritic-cell maturation and CD8+ T-cell priming (1).
The in vitro data indicating that sCD27 can help to up-regulate CD70 expression on T cells, in addition to the fact that sCD27 pre-exists at a high level in healthy individuals, suggest that sCD27 may be involved in the induction of CD70 expression on T cells upon activation, a signal that is crucial for potent T-cell activation (9). Finally, our search for proteins that could potentially be associated with sCD27 revealed a protein complex composed of three protein sequences: myosin-IIA, HMGB1, and the TCR Vβ chain. Myosin IIA, the most abundant protein in cells, has been shown to play a central role in the formation and persistence of the immunological synapse and T-cell signaling (34). Inhibition of myosin IIA decreases the association of active ZAP-70 with TCR (35). Our data showed that sCD27 is co-localized with myosin IIA in activated T cells (Supplemental Fig. 3), implying that sCD27 may be associated with myosin IIA directly or via other proteins in the complex. In addition, a previous study illustrated a direct association between myosin IIA and TCR (34), and the finding of the TCR Vβ chain in the complex of sCD27 pulling down further indicates that sCD27 is closely related to the T-cell synapse. To date, CD70 is the only ligand found to bind to the extracellular domain of membrane-bound CD27. The intracellular domain of CD27 was found to bind to SIVA, a pro-apoptotic protein (36). In our study, CD70 was not pulled down by sCD27. Two possible explanations may be proposed: First, when receptors are shed from the cell surface, their structure and functionality may alter from the membrane-bound version, which means they may lose the binding site to their ligand. Second, there was minimal or no expression of CD70 on the cell subsets within the PBMCs after overnight stimulation with anti-CD3/CD28 (data not shown). Further studies are required to elucidate the interaction of these proteins with sCD27.
The finding that levels of serum sCD27 are significantly higher in healthy donors than in cancer patients suggests that cancer patients may produce less sCD27, potentially due to (a) a lack of stimulation due to immunosuppression, which attenuates the shedding of this molecule; (b) immune senescence and exhaustion, which results in the accumulation of more CD27 negative, late stage effector cells; (c) intrinsic defects in sCD27 production. However, no difference was found in sCD27 production during in vitro stimulation, when we compared PBMCs derived from healthy donors and cancer patients (Supplemental Fig. 4), which led us to rule out the possibility of an intrinsic defect in the shedding of CD27 in cancer patients.
Our findings demonstrated that there was no difference in overall survival between patients who had higher levels of sCD27-pool than another group of patients that had lower levels of sCD27-pool before the therapy. We then evaluated the enlargement of the sCD27-pool in serum after each treatment cycle and its association with overall survival. Interestingly, as more treatment cycles were given, a greater association was seen. After three treatment cycles, a statistical significance was revealed for the correlation between the sCD27-pool level elevation and overall survival. Although there was an elevation in absolute lymphocyte count (ALC) after treatment, no correlation between ALC and overall survival was found. Based on our result that healthy donors have a larger sCD27-pool, we had speculated that patients who possessed a relatively larger pool before the treatment should have a better clinical outcome. However, the results were surprising; the pre-value was not associated with clinical outcome, but the increased value after three cycles of treatment showed a trend of correlation with overall survival. We found that with increased treatment cycles, there was a clear separation in overall survival between patients who had an enlargement of the sCD27-pool vs. patients who had a minimum change of this pool. In order to clearly illustrate our data interpretation, a hypothetical schema was drawn (Fig. 5E). In general, the levels of sCD27-pool in cancer patients are very low; even for patients who have relatively higher levels of sCD27, the average level may still be below the threshold that is necessary for proper immune function. However, after multiple treatments of immunotherapy, the pool can be cumulatively refilled for some patients, and when the elevation has reached or crossed the threshold, the benefit of sCD27 may begin to show. In the clinical trial, 34 immune-related adverse events (irAEs) grades 2-4 occurred during 40% of the cycles (23). Of the 21 patients who experienced an irAE, 13 had elevated sCD27 following treatment. However, no clear trend was observed between values of serum sCD27 and the dose of ipilimumab, or in the correlation between irAE occurrence/grade and elevation of sCD27 in serum (data not shown).
The studies reported here suggest that sCD27 is a functional protein rather than a mere by-product of T-cell activation, and that it acts as an agonist rather than an antagonist in the process of T-cell activation. sCD27's potential associations with proteins closely related to T-cell activation, effector activity, TCR rearrangement, and synapse formation suggest that sCD27 may be critical to facilitating T-cell activation. In addition, soluble receptors, like cytokines, can function as systemic immune mediators. It is important to note that levels of sCD27 were determined after two to three monthly treatments and compared with 2- to 3-year or even longer survival. Therefore, assessing the size of sCD27-pool that was elevated by immunotherapy at early time points could potentially provide insightful information for planning therapeutic strategies for cancer patients. In the future, larger trials in a range of human cancers, in addition to prostate cancer, will be needed to validate the hypothesis-generating findings reported here.
Supplementary Material
Acknowledgments
The authors thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript; Sandra Doren for her help with clinical samples; and Chiara Intrivici and Catherine Jamis for their technical assistance.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations used in the article
- ALC
absolute lymphocyte count
- anti-CTLA4
anti-cytotoxic T-lymphocyte antigen 4
- CD40L
CD40 ligand
- CRPC
castration-resistant prostate cancer
- HMGB1
high-mobility group box 1
- irAE
immune-related adverse events
- MFI
mean fluorescence intensity
- MMP
metalloproteinase
- NCI
National Cancer Institute
- PI
propidium iodide
- PSA
prostate-specific antigen
- RAGE
receptor for advanced glycation end-products
- sCD27
soluble CD27
- sCD40L
soluble CD40L
- TPC
total peptide count
- WM
Waldenström's macroglobulinemia
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 2.Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 3.Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA. CD70 represents the human ligand for CD27. Int Immunol. 1994;6:477–480. doi: 10.1093/intimm/6.3.477. [DOI] [PubMed] [Google Scholar]
- 4.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J Immunol. 1997;159:4959–4965. [PubMed] [Google Scholar]
- 5.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 6.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RA. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 7.Vardouli L, Lindqvist C, Vlahou K, Loskog AS, Eliopoulos AG. Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther. 2009;16:848–860. doi: 10.1038/cgt.2009.31. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. 2008;283:14177–14181. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64:135–146. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, Ghalie R, Caron DA, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY, Madan RA, Gulley JL, Schlom J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030–3038. doi: 10.1182/blood-2012-05-427799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glouchkova L, Ackermann B, Zibert A, Meisel R, Siepermann M, Janka-Schaub GE, Goebel U, Troeger A, Dilloo D. The CD70/CD27 pathway is critical for stimulation of an effective cytotoxic T cell response against B cell precursor acute lymphoblastic leukemia. J Immunol. 2009;182:718–725. doi: 10.4049/jimmunol.182.1.718. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Chu P, Takahashi S, Hamada H, Kipps TJ. Metalloprotease inhibitors block release of soluble CD27 and enhance the immune stimulatory activity of chronic lymphocytic leukemia cells. Exp Hematol. 2007;35:434–442. doi: 10.1016/j.exphem.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Loenen WA, De Vries E, Gravestein LA, Hintzen RQ, Van Lier RA, Borst J. The CD27 membrane receptor, a lymphocyte-specific member of the nerve growth factor receptor family, gives rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur J Immunol. 1992;22:447–455. doi: 10.1002/eji.1830220224. [DOI] [PubMed] [Google Scholar]
- 17.Hintzen RQ, de Jong R, Hack CE, Chamuleau M, de Vries EF, ten Berge IJ, Borst J, van Lier RA. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 18.Font J, Pallares L, Martorell J, Martinez E, Gaya A, Vives J, Ingelmo M. Elevated soluble CD27 levels in serum of patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1996;81:239–243. doi: 10.1006/clin.1996.0184. [DOI] [PubMed] [Google Scholar]
- 19.van Oers MH, Pals ST, Evers LM, van der Schoot CE, Koopman G, Bonfrer JM, Hintzen RQ, von dem Borne AE, van Lier RA. Expression and release of CD27 in human B-cell malignancies. Blood. 1993;82:3430–3436. [PubMed] [Google Scholar]
- 20.Ciccarelli BT, Yang G, Hatjiharissi E, Ioakimidis L, Patterson CJ, Manning RJ, Xu L, Liu X, Tseng H, Gong P, Sun J, Zhou Y, Treon SP. Soluble CD27 is a faithful marker of disease burden and is unaffected by the rituximab-induced IgM flare, as well as by plasmapheresis, in patients with Waldenstrom's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9:56–58. doi: 10.3816/CLM.2009.n.014. [DOI] [PubMed] [Google Scholar]
- 21.De Milito A, Aleman S, Marenzi R, Sonnerborg A, Fuchs D, Zazzi M, Chiodi F. Plasma levels of soluble CD27: a simple marker to monitor immune activation during potent antiretroviral therapy in HIV-1-infected subjects. Clin Exp Immunol. 2002;127:486–494. doi: 10.1046/j.1365-2249.2002.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, Arlen PM. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zofall M, Fischer T, Zhang K, Zhou M, Cui B, Veenstra TD, Grewal SIS. Histone H2A.Z cooperates with RNAi and heterochromatin factors to suppress antisense RNAs. Nature. 2009;461:419–422. doi: 10.1038/nature08321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jong R, Loenen WA, Brouwer M, van Emmerik L, de Vries EF, Borst J, van Lier RA. Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. J Immunol. 1991;146:2488–2494. [PubMed] [Google Scholar]
- 26.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agematsu K, Kobata T, Sugita K, Freeman GJ, Beckmann MP, Schlossman SF, Morimoto C. Role of CD27 in T cell immune response. Analysis by recombinant soluble CD27. J Immunol. 1994;153:1421–1429. [PubMed] [Google Scholar]
- 30.Dang LVP, Nilsson A, Ingelman-Sundberg H, Cagigi A, Gelinck LBS, Titanji K, De Milito A, Grutzmeier S, Hedlund J, Kroon FP, Chiodi F. Soluble CD27 induces IgG production through activation of antigen-primed B cells. J Intern Med. 2012;271:282–293. doi: 10.1111/j.1365-2796.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Fay NC, Smoligovets AA, Wu HJ, Groves JT. Myosin IIA modulates t cell receptor transport and CasL phosphorylation during early immunological synapse formation. PLoS ONE. 2012;7:e30704. doi: 10.1371/journal.pone.0030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad KVS, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A. 1997;94:6346–6351. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.