Abstract
The surprising ability of thalidomude and its analogs to treat various hematologic malignancies is through the loss of two transcription factors.
The 55-year history of the drug thalidomide is Shakespearean in scope, awash in unintended consequences, tragedy, resilience, driven characters, and redemption. Indeed, the most notmious pharmaceutical of modern times comes replete with images of devastating birth defects still firmly embedded in the public consciousness. Less well known has been the resurgence in its use as a therapy to treat hematologic malignancy. On pages 305 and 30 I of this issue, Lu el al. (1) and Krönke et al. (2), respectively, report that thalidomide and derivative compounds have a toxic effect on multiple myeloma by causing the degradation of two transcription factors, Ikaros and Aiolos. This loss halts myeloma growth while simultaneously altering immune cell function.
In an archetypal example of drug repositioning, reports emerged 15 years ago that thalidomide could be profoundly effective in patients with multiple myeloma (3), a tumor of antibody-producing plasma cells in the bone marrow. The malignancy is characterized by anemia, bone fractures, kidney failure, and recurrent infection. Subsequently, the thalidomide analogs lenalidomide and pomalidomide (collectively called immune modulating drugs or IMiDs) were shown to be even more potent in treating multiple myeloma, and today these small molecules form a highly effective backbone of therapy for this increasingly treatable cancer and for other hematologic malignancies (4).
Many studies over the years have sought to explain the mechanism of teratogenicity of thalidomide. Thalidomide, lenalidomide, and pomalidomide were found to have wide-ranging and seemingly disparate cellular actions, including induction of oxidative stress and inhibition of angiogenesis, as well as multiple effects on the immune system—enhanced production of the cytokine interleukin-2 (IL-2) (which spurs T cell production), inhibition of the cytokine tumor necrosis factor (TNF), and the stimulation of natural killer cells (5). The property of antiangiogenesis inspired the suggestion that thalidomide might be useful as a last-gasp attempt to control drug-resistant multiple myeloma. A remarkable success story in controlling this cancer with thalidomide soon followed (3). Unfortunately, it did not ta ke long to show that although antiangin-genesis may he a ro!lsequencE' of thali domide therapy, it was not the mechanism of action that explained its ci inical effect.
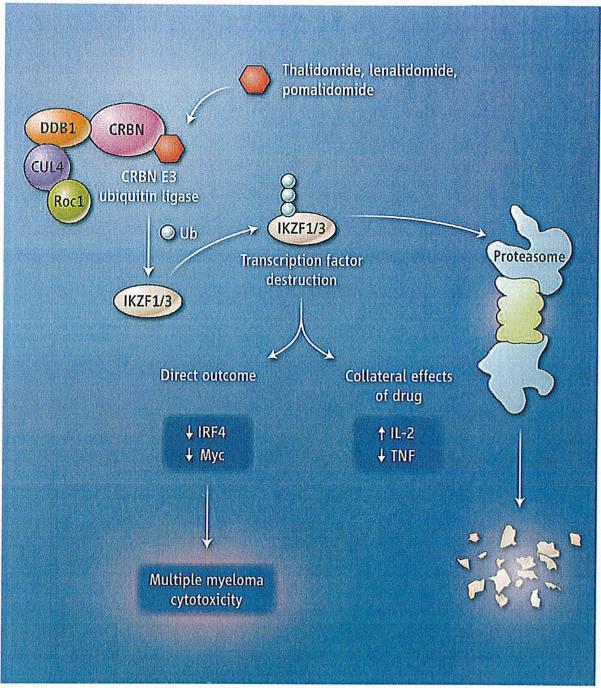
The seminal breakthrough emerged in 2010 when thalidomide was found to bind to the protein cereblon (6). Cereblon forms an E3 ubiquitin ligase complex with the proteins damaged DNA binding protein 1 (DDB 1), Cullin-4A (CUL4A), and regulator of cullins 1 (Roc 1). This complex tags specific proteins with ubiquitin, thereby targeting them for proteolysis. The drug-protein interaction disrupts the activity of the E3 ubiquitin ligase complex, which underpins the cyto-toxic and immune-modulating effects of IMiDs. Some of these effects were reversible by overexpressing the downstream proteins whose expression was reduced following IMiD treatment, such as the transcription factors interferon regulatory factor 4 (IRF4) and Myc (7-9). Although research into the clinical importance of this observation is still fresh , it seems clear that low amounts of cereblon in multiple myeloma cells correlate with clinical drug resistance and poor survival outcomes (10).
Lu et al. and Krönke et al. demonstrate that the zinc finger-containing transcription factors Ikaros (IKZF 1) and Aiolos (IKZF3) are selectively bound by cereblon. After direct binding, IMiDs activate cereblon 's E3 ligase activity, resulting in the rapid ubiquiti-nation and degradation of Ikaros and Aiolos. lkaros and Aiolos are transcriptional regulators of B and T cell development (11), 12). Aiolos is required for normal plasma cell development in mice (13). The toxic effects on multiple myeloma cells resulting from the loss of these two transcription factors are reversed by deletion of critical cereblon-binding regions of lkaros prior to drug treatment. Under normal conditions, lkaros suppresses expression of the gene encoding IL-2 in T cells but conversely stimulates expression of IRF4 (a transcription factor that responds to infection). Thus, a decrease in Ikaros explains the perplexing question of how one drug can both activate the immune system (a boost in IL-2 production byT cells stimulates immune responses) and degrade B cell function (as the result of reduced IRF4 expression) simultaneously.
Analysis of the cereblon-Ikaros/Aiolos–IRF4/Myc signaling pathway now opens doors to developing of more precise and effective therapeutics and biomarkers for drug response while raising some more issues for biologists and clinicians. For example, how can Ikaros depletion simultaneously be an effective anticancer target but also act as a tumor suppressor underpinning the development of acute lymphoblastic leukemia, an early B cell malignancy (14)? Presumably, different Ikaros isoforms function as regulators of gene expression in different cellular contexts. A logical progression might be that deletion of Ikaros by the IMiDs, under the wrong circumstances, could simultaneously kill multiple myeloma cells but promote pre–B cell leukemogenesis. Indeed, clinical experience demonstrates a slightly higher risk of leukemias and B cell malignancies in patients treated with immune modulatory drugs, albeit only after exposure to a second genotoxic DNA-damaging agent, such as Melphalan, a commonly used multiple myeloma treatment (15). There are other clinical dilemmas that remain unexplained, such as why only one-third of relapsed patients respond to a single-agent immune modulatory drug and why patients lose cereblon or find alternative pathways to become resistant to these drugs.
Another perplex ing clinical dilemma is that the action of IMiD drugs seems to absolutely require effective proteasomal degradation of lkaros and Aiolos—a finding that flies in the face of clinical experience, which seems to support that combining an immune modulator with a protea-some inhibitor as a highly effective strategy for treating multiple myeloma (4). Evidently, the scientific saga of thalidomide and its analogs is a story still not fully told.
It is tempting to update the mythic tale of the Greek god Ikaros flying too close to the Sun, only to have his waxed wings melt, to the genomic-era equivalent as the namesake Ikaros transcription factor comes too close to its molecular sun—namely, cereblon that is bound to an immune modulatory drug, with its inevitable degradation (see the figure). A final but speculative conclusion of the studies by Lu et al. and Kronke et al. is that small molecules that enhance the ubiquitination and degradation of specific target proteins may represent a new class of therapeutics for manipulating proteins that were previously viewed as undruggable.
Immune modulators and myeloma.
The small-molecule drugs thalidomide, lenalidomide, and pomalidomide bind to the protein cereblon (CRBN), which activates the enzymatic activity of the CRBN E3 ubiquitin ligase complex. The transcription factors lkaros (IKZF1) and Aiolos (IKZF3) are modified with ubiquitin (Ub) molecules, targeting them for proteolysis. This alters the function ofT cells and B cells, with a toxic outcome for multiple myeloma cells.
References
- 1.Lu G, et al. Science. 2014;343:305. doi: 10.1126/science.1244917. 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krönke J, et al. Science. 2014;343:301. doi: 10.1126/science.1244851. 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhal S, et al. N. Engl. J. Med. 1999;341:1565. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 4.Stewart AK, Richardson PG, San-Miguel JF. Blood. 2009;114:5436. doi: 10.1182/blood-2009-07-204651. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YX, Kortuem KM, Stewart AK. Leuk. Lymphoma. 2013;54:683. doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito T, et al. Science. 2010;327:1345. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YX, et al. Blood. 2011;118:4771. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffer AL, et al. Nature. 2008;454:226. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LH, et al. Br. J. Hoemotol. 2013;160:487. [Google Scholar]
- 10.Schuster SR, et al. Leuk. Res. 2014;38:23. doi: 10.1016/j.leukres.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan B, et al. EMBO J. 1997;16:2004. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgopoulos K, et al. Cell. 1994;79:143. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 13.Cortés M, Georgopoulos K. J. Exp. Med. 2004;199:209. doi: 10.1084/jem.20031571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullighan CG, et al. Nature. 2008;453:110. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig H, et al. Blood. 2012;119:3003. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]