Abstract
Background
Patient-reported quality of life following salvage brachytherapy for radiorecurrent prostate cancer has not been well-characterized prospectively.
Methods
We examined 25 men who recurred after primary radiotherapy for prostate cancer and received MRI-guided salvage brachytherpy as part of a prospective phase II study. These patients prospectively received a validated patient-reported quality of life questionnaire to fill out at baseline, as well as 3, 15, and 27 months after re-irradiation to determine the degree of sexual, bowel, and urinary dysfunction (maximum dysfunction score = 100).
Results
On average, sexual function continued to decline with time, and patients had significantly worse sexual function scores at 27 months than baseline (p=0.01). However, while bowel and urinary symptoms worsened acutely at 3 or 15 months, they showed on average some improvement by 27 months, and there were no significant differences between baseline and 27 month urinary or bowel scores. An interval to re-irradiation less than 4.5 years and prior brachytherapy were each significantly associated with the largest decrements in bowel function (p=0.035).
Conclusion
Similar to the patterns seen in the de novo setting, patients who receive salvage brachytherapy report a worsening of bowel and urinary symptoms followed by some improvement by 27 months, while sexual function steadily declines over time. Interval to re-irradiation and type of prior radiation received may be used to counsel and optimize selection of men for salvage brachytherapy with regard to quality-of-life endpoints.
Keywords: Quality of Life, Salvage Therapy, Brachytherapy, Magnetic Resonance Imaging, Prostate Cancer, Prostate-Specific Antigen
INTRODUCTION
Following external beam radiation or interstitial brachytherapy for clinically-localized prostate cancer, approximately 20 to 50% of men will experience prostate-specific antigen (PSA) failure.1–3 At the time of failure, many such men will harbor clinically occult micrometastatic disease. However, a significant minority will have a local-only recurrence and therefore have a second chance at cure with definitive local therapy.
For those who receive salvage local therapy with either prostatectomy, cryotherapy, or brachytherapy, cancer control rates from single-institution series have been reported to range from 20% to 80%, and depend on the clinical characteristics of the patients selected.1, 4–6 Given that men with a PSA-only recurrence may be clinically asymptomatic for many years without treatment, and given that salvage local therapy offers a cure rate in the range of about 50%, it is crucial for patients and physicians to have a clear understanding of the potential quality-of-life tradeoff associated with pursuing salvage local therapy.
Currently, there is limited information on complications of salvage local therapy, and quality-of-life data from a patient perspective is non-existent. Therefore, we designed a prospective phase II study to evaluate efficacy and patient-reported quality of life after salvage MRI-guided brachytherapy using a validated questionnaire that was administered serially.7 Cancer control from this study was previously reported as 70% PSA failure-free survival at 4 years, and physician-rated complications have been previously reported Nguyen PL, et al as 30% grade 3 or 4 genitourinary/gastrointestinal toxicity at 4 years.8 The purpose of this report is to describe the patient-reported quality-of-life outcomes.
METHODS
Patient Eligibility
From October 2000 through October 2005, 25 men were enrolled on a prospective phase II study of salvage MRI-guided prostate brachytherapy. All of the patients had prior external beam radiation or interstitial brachytherapy for clinically localized prostate cancer and had experienced PSA failure based on the 1997 ASTRO consensus definition of 3 consecutive rises following a nadir.9
Requirements for eligibility included: biopsy-proven locally recurrent prostate cancer at least 2 years following initial radiation, no history of a transurethral resection of the prostate (TURP), biopsy Gleason score ≤ 7 prior to initial radiation, PSA<10 ng/ml within 3 months of registration, bone scan negative for distant metastases, pelvic CT or MRI negative for lymph node disease, ECOG performance status 0–2, age greater than 30, no history of uncontrolled diabetes mellitus, no contraindications to spinal or general anesthesia, and no indwelling pacemakers. In addition, enrollment required a cystoscopy showing absence of muscle invasive bladder cancer, no significant BPH causing ≥ 90% narrowing of the urethra, no urethral stricture requiring a TURP, and no bladder neck contracture requiring prior surgical correction. A pre-salvage PSA>10 following an invasive procedure such as prostate biopsy, colonoscopy, or cystoscopy did not exclude a patient from eligibility as long as a subsequent serum PSA level was noted to be <10 ng/ml within 3 months of registration. The pre-treatment baseline characteristics of the 25 trial participants are listed in Table 1.
TABLE 1.
Clinical Characteristics of the Study Cohort Prior to Salvage Therapy With Salvage Treatment Details (N=25)
| Median Initial PSA in ng/ml (range) | 7.45 (4.2 to 18.4) |
| #PSA 0 to <4 (%) | 0 (0%) |
| #PSA 4 to <10 (%) | 23 (92%) |
| #PSA 10 to 20 (%) | 2 (8%) |
| Initial Gleason Score | |
| # Gleason 2+3=5 (%) | 1 (4%) |
| # Gleason 3+3=6 (%) | 18 (72%) |
| # Gleason 3+4=7 (%) | 6 (24%) |
| Initial Clinical T-Category | |
| #T1c (%) | 17 (68%) |
| #T2a (%) | 7 (28%) |
| #Unknown (%) | 1 (4%) |
| Initial Radiation Treatment Received | |
| #External Beam Radiation (66–70.2Gy) | 12 (48%) |
| #Brachytherapy (137 Gy, MRI-Guided) | 11 (44%) |
| #External Beam + Brachytherapy | 1 (4%) |
| #External beam + Hormones (4 months) | 1 (4%) |
| Median Pre-Salvage PSA Doubling Time (range) | 9.46 months (1.9 to 39.9) |
| #PSADT < 3 months (%) | 2 (8%) |
| #PSADT 3 to <6 months (%) | 5 (20%) |
| #PSADT 6 to <12 months (%) | 10 (40%) |
| #PSADT ≥12 months (%) | 8 (32%) |
| Median Interval Between Radiation Treatments | 5.2 years (2.5 to 12.8) |
| #<2 years (%) | 0 (0%) |
| #2 to <5 years (%) | 10 (40%) |
| #5 to <10 years (%) | 13 (52%) |
| #≥10 years (%) | 1 (4%) |
| Median Age in years at Salvage (range): | 65 years (56 to 82) |
| ECOG Performance Status | |
| 0 | 25 (100%) |
| 1 | 0 (0%) |
| 2 | 0 (0%) |
| Median PSA in ng/ml at Salvage (range) | 5.5 (1.4 to 11.6) |
| #PSA 0 to <4 (%) | 9 (36%) |
| #PSA 4 to 10 (%) | 15 (60%) |
| #PSA 10 to 20 (%) | 1 (4%) |
| Median number of salvage needles (range) | 20 (13–26) |
| Median total salvage activity (range) | 26.3 mCi (21.2–43.0) |
| Median salvage gland size (range) | 21 cc (12–66) |
| Median salvage activity/cc (range) | 1.18 (0.59–2.0) |
Treatment
No patient received neoadjuvant or adjuvant hormone therapy with their salvage implant. Patients were placed in the lithotomy position under general anesthesia. A foley catheter was inserted and clamped. An MR-compatible perineal template was secured to the MR table and placed against the patient’s perineum. A rectal obturator was placed to allow for the passage of intra-rectal gas. Axial, coronal, and sagittal images of the prostate were acquired at 5mm intervals using an MR pelvic coil in a 0.5 Tesla magnetic field (General Electric Medical Systems, Milwaukee, WI). The prostate gland, anterior rectal wall, and prostatic urethra were identified on each axial slice by an experienced genitourinary MRI radiologist. Based on the prostate gland and juxtaposed normal tissue volumes and the desired minimum prescription dose, an initial treatment plan and needle loading was determined as previously-described elsewhere.10
Needles were then loaded and placed under real-time MR image guidance with dosimetric feedback. As each catheter containing preloaded 125I sources was inserted, its position was identified in real time and compared to its planned location. The range of activity was 0.35 to 0.45 mCi/source with a median of 0.40 mCi/source. Adjustments to account for prostate motion, edema, or catheter divergence could be made before source deposition. The process was repeated in an iterative fashion for all planned catheters. The cumulative dose-volume histograms for the prostate gland, anterior rectal wall, and prostatic urethra were evaluated after each catheter insertion, which allowed for adjustments to the treatment plan intra-operatively if necessary. The prescribed minimum dose to the MRI-defined target volume (prostate only) was 137 Gray (Gy) calculated according to the formalism of AAPM Task Group 43, which is equivalent to the standard 160 Gy calculated by prior methods.11 Adjustments were made based on real-time dosimetric feedback to ensure that the intraoperative V100 (fractional volume of the target that receives at least 100% of the prescribed dose) was at least 100% and the D90 (dose that covers at least 90% of the target) was at least the full prescribed dose. The intraoperative constraint for the anterior rectal V100 (volume of the anterior rectum receiving at least 100% of the dose) was <10cc, and the constraint on the contoured urethra was that no point on the urethra could exceed 200Gy.) Treatment parameters of activity, number of needles, and prostate size are listed in Table 1.
Data Collection and Instruments
We asked participants to fill out a previously validated prostate-specific health related quality of life questionnaire prior to salvage therapy and at 3 months, 15 months, and 27 months after treatment. Telephone reminders were made to increase the response rate. The questionnaires were coded by a numerical identifier and were not reviewed or linked to patients until after the close of the study. The details about the development and validation of the questionnaire have been reported elsewhere.7
Focused questions were designed to assess symptomatology with the domains of sexual dysfunction, urinary incontinence, urinary obstruction/irritation, and bowel dysfunction. The urinary incontinence index assessed the degree of urinary control and the frequency and magnitude of leakage in men who had less than “complete control.” Five items assessed urinary obstruction and irritation: hesitancy, frequency of urination during the day, nocturia (urination at night), dysuria, and urgency. The items for bowel problems included diarrhea, urgency of bowel movements, pain, bleeding, passing mucus during bowel movements, abdominal cramping, and tenesmus. Sexual function items focused on the firmness of erections, difficulty in getting and keeping erections, and frequency of ejaculation and orgasm. Scores ranged between 0 (minimum) and 100 (maximum dysfunction). Tables 2–5 provide the range of possible answers to each question, and give sample scores that would be obtained if a patient gave a certain set of answers.
Table 2.
Sample Sexual Scores Based on Function Over Prior 4 weeks:
| Score | 0 | 28 | 56 | 72 | 100 |
|---|---|---|---|---|---|
| Erection strength | Full | Nearly full | Partial can penetrate | Partial can’t penetrate | No sexual activity |
| Difficulty getting erect | None | Some | A little | A lot | No sexual activity |
| Difficulty keeping erect | None | Some | A little | A lot | No sexual activity |
| Sensation of climax | Always | Sometimes | Not at all | Not at all | No sexual activity |
| Able to ejaculate | Always | Sometimes | Not at all | Not at all | No sexual activity |
Each worse answer raises score by about 5.6 points
Table 5.
Sample Incontinence Scores Based on Prior Week’s Symptoms
| Score | 0 | 30 | 60 | 80 | 100 |
|---|---|---|---|---|---|
| Control | Complete | Some leak | Mostly leak | Mostly Leak | No control |
| How often leak? | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| Usual Amount Leaked? | No leak | Few Drops | <1 TBSP | >1 TBSP | >1 TBSP |
Each worse answer raises score by 10 points. TBSP=Tablespoon
Statistical Analysis
We calculated the mean and standard deviation of the symptom scores at each time point and displayed them graphically to illustrate time trends. A paired t-test was used to determine whether there was a statistically significant change in the symptom scores from baseline to 27 months. Linear regression was used to determine whether there was an association between clinical parameters and each patient’s largest score change for a particular symptom. The largest change was determined by subtracting the baseline value for each symptom from the highest value that the patient had in follow-up. Due to the small number of patients, univariable regression was performed. The covariates examined were prostate size in grams (continuous), whether or not the patient had had prior interstitial brachytherapy, presence of vascular disease (including myocardial infarction or diabetes), prostate size (continuous), total activity in mCi (continuous), activity per cc of prostate (continuous), number of needles used (continuous), and whether the interval between initial radiation and salvage brachytherapy was less than 4.5 years, which has been previously identified as a predictor of increased physician-assessed gastrointestinal (GI) and genitourinary (GU) toxicity.8
Response Rate
Questionnaires were completed by all 25 (100%) participants in the study. However, one patient did not fill out a baseline questionnaire, and two patients had not yet reached the 27 month follow-up time by the time the study was closed. Therefore, there were 22 patients (88%) with both baseline and 27 month questionnaires. Twenty patients (80%) filled out all 4 questionnaires, and formed the basis for the analyses below. However, 2 patients who required a colostomy and urostomy for a rectourinary fistula prior to 27 months did not fill out their 27 month bowel and urinary dysfunction questions and could therefore not be included in the bowel and urinary symptom analyses. There were no significant differences in pretreatment clinical characteristics between the 20 patients who filled out all 4 questionnaires and the 5 patients who didn’t.
RESULTS
Changes in Dysfunction Scores Over Time
Sexual Dysfunction
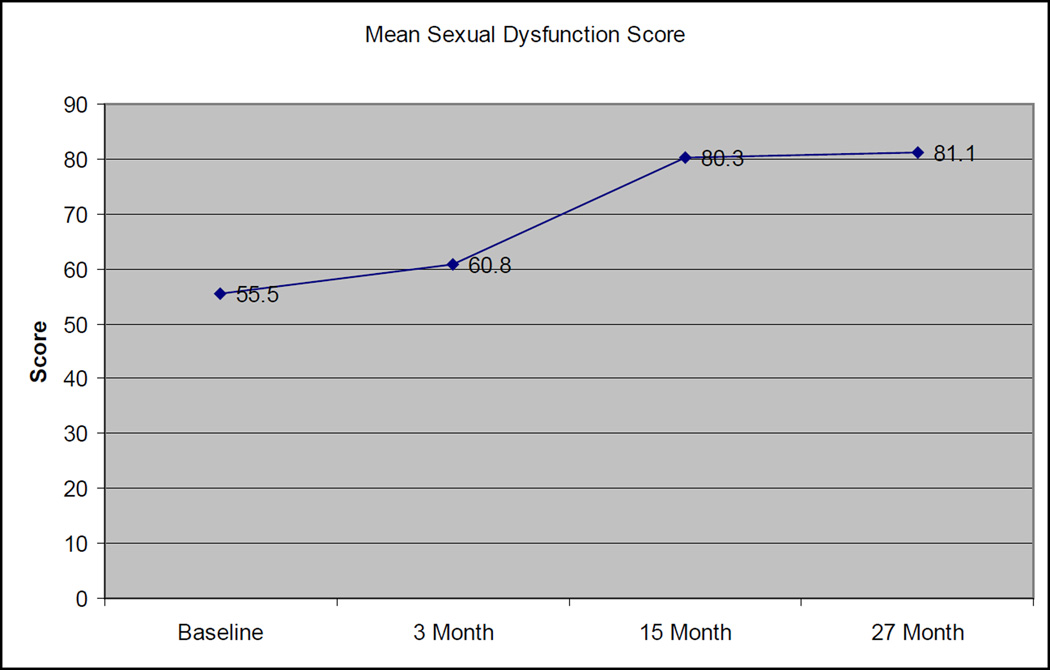
Among the 20 patients with evaluable sexual dysfunction scores at all 4 evaluation times, the mean sexual dysfunction score gradually worsened over time, with values of 55.5, 60.8, 80.3, and 81.1 at baseline, 3 months, 15 months, and 27 months, respectively (Figure 1). The increase in dysfunction scores from baseline to 27 months was statistically significant (p=0.01). To place these numbers into context, a patient could obtain a score of 56 if in the past four weeks, he had a “partial erection capable of penetration with manual assistance”, “a lot of difficulty” getting an erection, “a lot of difficulty” keeping an erection, was able to reach orgasm (sensation of climax) “some of the time”, and was able to ejaculate “some of the time”. A score of 78 could be obtained if in the past four weeks he had a “partial erection not capable of penetration even with manual assistance”, “a lot of difficulty” getting an erection”, “a lot of difficulty” keeping an erection, was not able to reach orgasm at all, and was not able to ejaculate at all (table 2).
Figure 1. Change in Sexual Function Scores Over Time.
Standard deviations: 36.5 (Baseline), 30.9 (3 month), 24.3 (15 month), 29.4 (27 month)
At baseline, 14/22 (64%) had at least partial erections firm enough for penetration, but by 27 months, only 4 of 22 (18%) had erections firm enough for penetration (p=0.005, Fisher’s exact) without the use of oral agents such as sildenafil.
Obstruction/Irritation
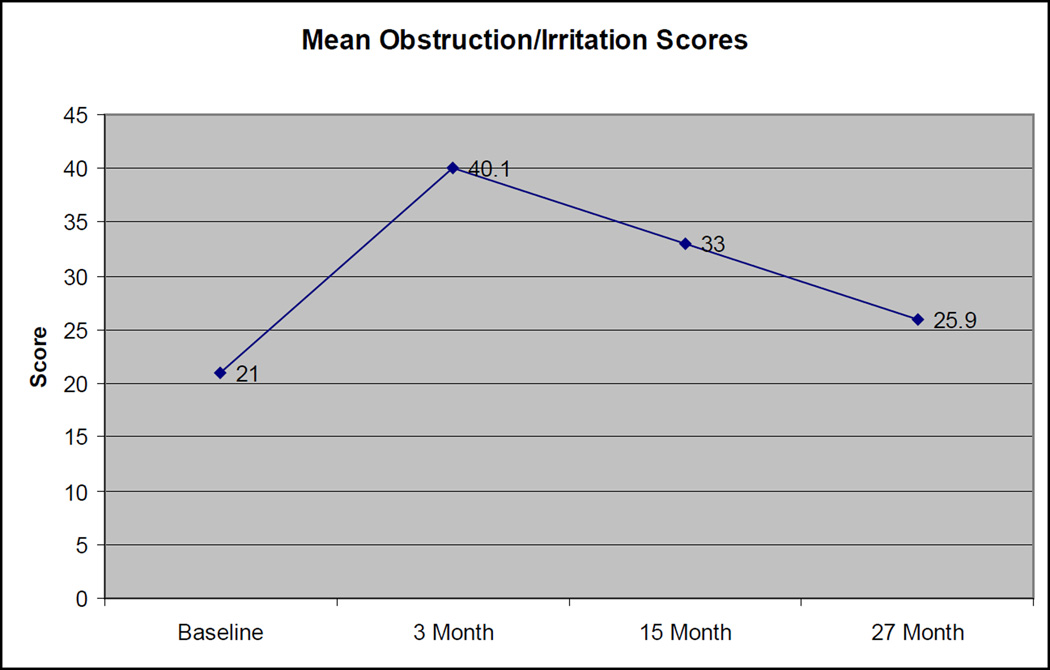
Among the 18 patients with evaluable obstruction/irritation scores at all 4 evaluation times, the mean obstruction/irritation scores initially worsened and then improved, with scores of 21, 40.1, 33, and 25.9, at baseline, 3 months, 15 months and 27 months, respectively (Figure 2). The pronounced increase in scores from baseline to 3 months was statistically significant (p=0.0004). To contextualize this, a score of 23 could represent a “fairly easy” urine flow, nocturia “once a night”. “<5” urinations per day, burning “1–2× per week”, and urgency “1–2× per week”, while a score of 40 might be the same features but with nocturia “2–3× per night” and urination occurring “5–8 times” per day (Table 3). The difference in scores from baseline to 27 months was not statistically significant (p=0.10). At baseline, 3/18 (17%) patients felt their urinary stream was slow, and at 27 months, 6 of 18 (33%) felt this way (p=0.44).
Figure 2. Change in Obstruction/Irritation Scores Over Time.
Standard deviations: 13.4 (Baseline), 17.8 (3 month), 14.1 (15 month), 13.5 (27 month)
Table 3.
Sample Obstruction/Irritation Scores Based on Prior Week’s Symptoms
| Score | 0 | 28 | 56 | 72 | 100 |
|---|---|---|---|---|---|
| Urine Flow | Very easy | Fairly easy | Slow, no strain | Very Slow, some strain | Very slow, strains hard |
| Nocturia | Seldom | Once a night | 2–3× per night | 4+ per night | 4+ per night |
| Daily Urine Frequency | <5/day | 5–8/day | 9–12/day | 12+/day | 12+/day |
| Pain or Burning | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| Urgency | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
Each worse answer raises score by about 5.6 points
Bowel Dysfunction
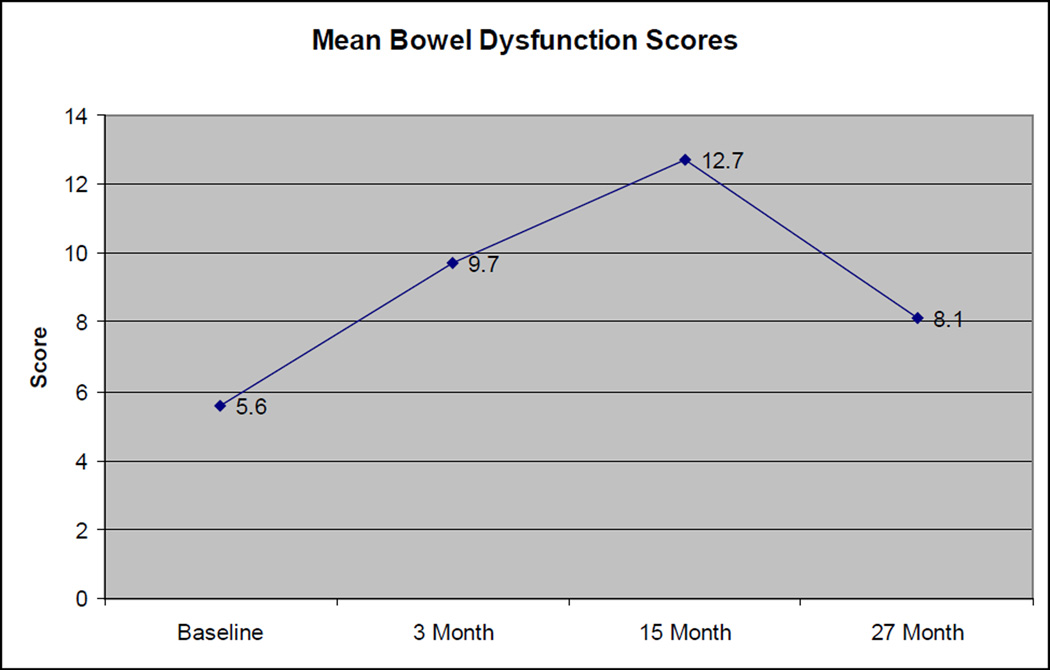
Among the 18 patients with evaluable bowel dysfunction scores at all 4 evaluation times, the mean bowel dysfunction score also had a worsening, then improving pattern, with mean scores of 5.6, 9.7, 12.7, and 8.1 at baseline, 3 months, 15 months, and 27 months, respectively (Figure 3). The increase in scores from baseline to 15 months was statistically significant (p=0.007), but the difference in scores from baseline to 27 months was not statistically significant (p=0.17). As noted in table 4, each successively worse answer raises a patient’s dysfunction score by 4.2 points, so a change from 5.6 to 12.7 at 15 months could be achieved by a two step increase in a single question (e.g. going from diarrhea 1–2× per week to once per day), or a one-step increase in 2 different questions (e.g. going from diarrhea 1–2× per week to 3+ times per week, and simultaneously going from having no urgency to having urgency 1–2× per week). There was no significant difference between the number of patients reporting occasional to fairly frequent bleeding with bowel movements at baseline (4/18) vs. at 27 months (5/18).
Figure 3. Change in Baseline Bowel Scores Over Time.
Standard deviations: 5.5 (Baseline), 7.6 (3 month), 10.8 (15 month), 6.6 (27 month)
Table 4.
Sample Bowel Scores Based on Prior Week’s Symptoms
| Score | 0 | 25 | 50 | 75 | 100 |
|---|---|---|---|---|---|
| How often diarrhea | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| How often urgency | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| How often pain | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| How often bleeding | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| How often abd cramping | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
| How often tenesmus | Never | 1–2×/wk | 3+/wk | 1×/day | Multiple per day |
Each worse answer raises score by 4.2 points
Incontinence Scores
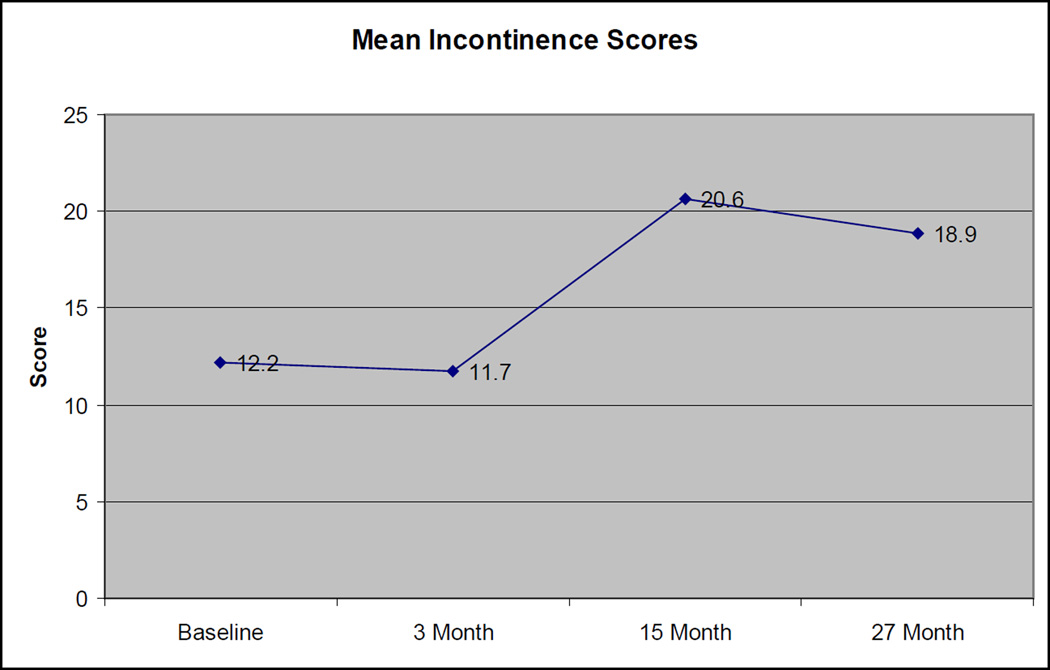
Among the 18 patients with evaluable incontinence scores at all 4 evaluation times, the mean incontinence score appeared to worsen and then slightly improve, with scores of 12.2, 11.7, 20.6, and 18.9 at baseline, 3 months, 15 months, and 27 months, respectively (Figure 4). The increase in scores from baseline to 27 months was not statistically significant (p=0.14). At baseline, 14 of 18 (78%) had complete urinary control (no leaking) at baseline, while at 27 months, only 10 of 18 (55%) had complete control (p=0.3, Fisher’s exact 2-sided).
Figure 4. Change in Mean Incontinence Scores Over Time.
Standard deviations: 18 (Baseline), 15.4 (3 month), 27.1 (15 month), 21.9 (27 month)
Predictors of Largest Increase in Bowel Dysfunction Score
Of the 18 patients who had evaluable bowel dysfunction scores at all 4 time intervals, the mean value of the largest increase in score for each patient was 12.5 (range: 0 to +29.2). An interval to reirradiation less than 4.5 years was significantly associated with the largest increase in bowel dysfunction score for each patient on linear regression (p=0.026, Coef=9.5, 95% CI= 1.3 to 17.7, R-squared = 0.274). For those with an interval to re-irradiation less than 4.5 years, the mean bowel dysfunction score was 16.1, compared to 7.7 for those with an interval greater than 4.5 years (p=0.035). In addition, in this subset of 18 patients, the patients who had an interval to re-irradiation less than 4.5 years were the same patients who had received prior brachytherapy, and therefore having received prior brachytherapy was also equally associated with the largest increase in bowel dysfunction. There was no association between the worst change in bowel score and any other variable, including cardiovascular disease, prostate size,.total activity, activity per cc of prostate tissue, or number of needles used. No association was found between any variable and the worst change in sexual function, obstruction/irritation, or incontinence.
DISCUSSION
In this prospective phase II study of men who underwent salvage brachytherapy for radiorecurrent prostate cancer, we performed an evaluation of patient-reported sexual, GI, and GU related quality of life at baseline, 3 months, 15 months, and 27 months after salvage therapy.
We observed two distinct patterns of symptoms. The first is a gradual worsening over time, as seen for the sexual dysfunction scores, which worsened at each time interval, and were statistically significantly worse at 27 months compared to baseline (p=0.01). The second type of pattern observed is an acute worsening at 3 to 15 months, followed by some gradual improvement by 27 months. This pattern was seen for the incontinence scores, urinary obstruction/irritation scores, and bowel dysfunction scores. For these scores there was often a significant acute worsening at 3 or 15 months, but by 27 months, there scores were on average not statistically different from the baseline scores (all p>0.1).
These results in the salvage setting are consistent with the results of Sanda, et al of patient-reported quality of life among men who had not had any prior radiation and were undergoing primary brachytherapy for early-stage prostate cancer.12 These men were surveyed at baseline, 2, 6, 12, and 24 months, and it was found that while sexual function after brachytherapy appeared to get worse with time, the scores for incontinence, urinary obstruction/irritation, and bowel dysfunction appeared to acutely worsen in the first 2–12 months, and then gradually showed some improvement by 24 months of follow-up.
These results suggest that men who receive salvage brachytherapy for radiorecurrent disease can expect a pattern of symptoms similar to what is observed for men in the de novo setting. Specifically, sexual function will slowly get worse with time, but urinary and bowel symptoms will be tend to worsen acutely in the first few months, but may improve somewhat thereafter. The fact that there may be some improvement in the urinary and bowel symptoms with time is encouraging, as most re-irradiation series at other sites suggest that side effects are seen sooner and do not necessarily improve with time.13, 14
Another finding from this study is that an interval to re-irradiation less than 4.5 years, which was found in the initial report of this series to be a significant predictor of grade 3 or 4 physician-reported GI or GU toxicity, is also now significantly associated with worse patient-reported bowel dysfunction (p=0.035). Therefore, patients who have a short interval to re-irradiation may have a higher risk of adverse side effects and should be counseled about this potential risk. Physicians may consider limiting the dose to the anterior rectum when possible during salvage brachytherapy in patients who have a short interval to re-irradiation in order to try to reduce the risk of rectal side effects. Among patients for whom salvage brachytherapy or salvage prostatectomy may be equally feasible options, having a short interval to re-irradiation may be considered a relative contraindication to salvage brachytherapy and therefore more serious consideration given salvage prostatectomy, although this conclusion must be considered hypothesis generating, as the quality of life of salvage prostatectomy has not been carefully studied, and the impact of a short treatment interval between radiation and salvage prostatectomy is unknown.
Since prior brachytherapy was also found to be associated with the largest increase in bowel dysfunction, the same things could potentially apply to those with prior brachytherapy, although it was not possible in this study to separate the impact of prior brachytherapy from the impact of a short interval to re-irradiation on bowel dysfunction, as the patients in that subgroup analysis who had prior brachytherapy all had had an interval to re-irradiation less than 4.5 years. That prior brachytherapy would lead to worse bowel symptoms is plausible, as such patients may have received a higher rectal dose from their prior radiation treatment than those who had received external beam radiation.
A strength of this study is that it measured patient-reported quality of life outcomes, which are known to not always align with physician-judged toxicity, and which best reflect the actual experience of the patients. In addition, patients were treated uniformly on a prospective phase II protocol, and data was assessed prospectively in regular intervals with a high participation rate.
A potential weakness of this study is that analysis for each symptom axis was only performed on patients who answered the relevant questions at all 4 time points, which ensured that the same patients were being compared between time points, but which also led to the exclusion of two patients who developed rectourinary fistulas requiring a colostomy/urostomy, as they did not answer the questions related to urinary and bowel toxicity at 27 months. The questionnaires were not worded in a way that could adequately capture this type of dysfunction, and therefore we have likely somewhat underestimated the decrement in urinary and bowel function that patients will experience over time with this treatment. Of note, these two patients who developed fistulas in the follow-up period (11 and 12 months post-salvage) both had an interval to re-irradiation less than 4.5 years (1.5yrs and 4.1 yrs). One had a ratio of salvage activity per cc of tissue of 0.94 which was on the low end of the spectrum, but he had also received prior brachytherapy, which may have delivered a higher rectal dose than external beam radiation. The other patient had received external beam radiation to a dose of 70Gy, but his salvage ratio of activity per cc of tissue was 2.0, which was the highest for the cohort.
Another potential limitation of this study is that it only followed patients to a maximum of 27 months, and it is possible that late toxicities may develop even beyond that time. In particular, one patient in this series developed a rectourinary fistula requiring a colostomy/urostomy at 29 months, which was therefore not captured within the 27 month follow-up period. However, in most re-irradiation series toxicities generally occur sooner than when compared to a primary radiation series, and it is likely that most of the toxicities have been accounted for by 27 months.14 An additional potential weakness of this study is that we were not able to report exact intra-operative rectal and urethra dose parameters, which could potentially have been predictive of patient-reported quality of life. We instead used activity per cc of tissue as an approximation of exposure and could not find a correlation between this factor and outcome. Another potential weakness is that the number of patients in this study was small, and therefore it was likely not adequately powered to detect small differences in certain scores. For example, the differences in bowel and urinary dysfunction scores between baseline and 27 months were not significant in this study, but may have been significant if there were more patients in the study. A related consequence of small numbers is that it limits the precision of any particular point estimate. It should be noted though that there is an important distinction between what is clinically significant vs. statistically significant. Statistically significant differences seen in this study must still prove their relevance by showing themselves to be clinically significant. As described in the results, an average worsening in sexual dysfunction over 27 months from 55.5 to 80.1 likely represents some clinically significant changes in quality of life. On the other hand, the transient rise in bowel dysfunction at 15 months from 5.6 to 12.7 would likely be for most people a mild change at most. Of course, these numbers were taken in aggregate, and there may have been some patients who had very large quality-of-life decrements in this timeframe, so care must be taken when interpreting numbers solely in aggregate.
As the ideal scenario for patients who initially present with favorable risk disease is to avoid a local recurrence and the need for salvage therapy altogether, it is worth exploring why these failures may have occurred. Those that received external beam radiation as the sole initial treatment (n=12) all received a dose of 70.2Gy or less, and it is now known that dose escalation beyond this level could have reduced their risk of recurrence15, 16. Those that received brachytherapy alone as initial treatment (n=11) had all received an MRI-guided implant which attempts to reduce dose to portions of the anterior prostate where cancer is rarely found but which can cause significant urinary toxicity. While PSA control with this technique was found to be equivalent to surgery in a matched cohort (95% at 5 years),17 it is certainly conceivable that these patients had harbored some microscopic disease in the anterior prostate, although with good patient selection, the chance of this happening is extremely small.
In conclusion, despite these potential limitations, it appears that patients who undergo salvage brachytherapy for radio-recurrent prostate cancer can expect to have a gradual worsening of sexual function, and can expect to have urinary and bowel symptoms that acutely worsen, but then show some improvement by 27 months, in a pattern that is similar to those treated in the de novo setting. Those with an interval to re-irradiation less than 4.5 years may have a greater risk of both physician-assessed toxicity and patient-reported bowel dysfunction, and this factor should therefore be taken into consideration in counseling patients on their salvage treatment options and in the selection of rectal dose distributions during salvage brachytherapy. Caution may also be needed in patients who have received prior brachytherapy.
Acknowledgement
We would like to thank Dr. Mark Hurwitz and Dr. Clair Beard for their expertise and suggestions regarding the management of men facing local failure following radiation therapy. We also wish to acknowledge (in alphabetical order) Lynn Lopes, RN, Allison Taylor, NP and Kristin Valentine, BS for their outstanding clinical and research efforts and support of the men in this study. Without their sentinel contributions, this study would not have been possible.
REFERENCES
- 1.Nguyen PL, D’Amico AV, Lee AK, et al. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer. 2007;110:1417–1428. doi: 10.1002/cncr.22941. [DOI] [PubMed] [Google Scholar]
- 2.Shipley WU, Thames HD, Sandler HM, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. Jama. 1999;281:1598–1604. doi: 10.1001/jama.281.17.1598. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Izawa JI, Madsen LT, Scott SM, et al. Salvage cryotherapy for recurrent prostate cancer after radiotherapy: variables affecting patient outcome. J Clin Oncol. 2002;20:2664–2671. doi: 10.1200/JCO.2002.06.086. [DOI] [PubMed] [Google Scholar]
- 5.Ward JF, Sebo TJ, Blute ML, et al. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol. 2005;173:1156–1160. doi: 10.1097/01.ju.0000155534.54711.60. [DOI] [PubMed] [Google Scholar]
- 6.Grado GL, Collins JM, Kriegshauser JS, et al. Salvage brachytherapy for localized prostate cancer after radiotherapy failure. Urology. 1999;53:2–10. doi: 10.1016/s0090-4295(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 7.Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care. 2001;39:1118–1130. doi: 10.1097/00005650-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen PL, Chen MH, D’Amico AV, et al. Magnetic resonance image-guided salvage brachytherapy after radiation in select men who initially presented with favorable-risk prostate cancer: a prospective phase 2 study. Cancer. 2007;110:1485–1492. doi: 10.1002/cncr.22934. [DOI] [PubMed] [Google Scholar]
- 9.ASTRO. Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 10.D’Amico AV, Cormack R, Tempany CM, et al. Real-time magnetic resonance image-guided interstitial brachytherapy in the treatment of select patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:507–515. doi: 10.1016/s0360-3016(98)00271-5. [DOI] [PubMed] [Google Scholar]
- 11.Nath R, Anderson LL, Luxton G, et al. Dosimetry of Interstitial Brachytherapy Sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 12.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 13.Indelicato DJ, Meadows K, Gibbs CP, Jr, et al. Effectiveness and Morbidity Associated with Reirradiation in Conservative Salvage Management of Recurrent Soft-Tissue Sarcoma. Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Kasperts N, Slotman B, Leemans CR, et al. A review on re-irradiation for recurrent and second primary head and neck cancer. Oral Oncol. 2005;41:225–243. doi: 10.1016/j.oraloncology.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Zietman AL. Correction: Inaccurate analysis and results in a study of radiation therapy in adenocarcinoma of the prostate. Jama. 2008;299:898–899. doi: 10.1001/jama.299.8.898-c. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico AV, Tempany CM, Schultz D, et al. Comparing PSA outcome after radical prostatectomy or magnetic resonance imaging-guided partial prostatic irradiation in select patients with clinically localized adenocarcinoma of the prostate. Urology. 2003;62:1063–1067. doi: 10.1016/s0090-4295(03)00772-6. [DOI] [PubMed] [Google Scholar]