Abstract
Mice expressing lymphocytic choriomeningitis virus nucleoprotein (LCMV-NP) as a transgene in their β cells develop insulin-dependent diabetes mellitus (IDDM) only after LCMV infection. Inoculation of plasmid DNA encoding the insulin B chain reduced the incidence of IDDM by 50% in this model. The insulin B-chain DNA vaccination was effective through induction of regulatory CD4 lymphocytes that react with the insulin B chain, secrete IL-4, and locally reduce activity of LCMV-NP–autoreactive cytotoxic T lymphocytes in the pancreatic draining lymph node. In contrast, similar vaccination with plasmids expressing the LCMV viral (“self”) protein did not prevent IDDM, because no such regulatory cells were induced. Thus, DNA immunization with plasmids expressing self-antigens might constitute a novel and attractive therapeutic approach to prevent autoimmune diseases, if the antigens are carefully preelected for an ability to induce regulatory lymphocytes in vivo.
Introduction
DNA vaccination with plasmids expressing foreign microbial antigens is a well-established approach to inducing protective antiviral or bacterial immunity (1–3). After a single or repeated intramuscular or intradermal DNA injection(s), cellular and/or humoral immune responses to the encoded microbial protein are mounted, and long-lived memory lymphocytes are induced. However, in addition to their protective role during infections, lymphocytes can also have important regulatory functions. For example, when self-antigens are administered orally, self-reactive lymphocytes may be induced in the gut (4, 5); these cells may be able to suppress ongoing autoimmune destruction and prevent autoimmune disease when they home locally to a target organ under autoimmune attack, a process termed “oral immune tolerance” (4). In the well-established rat insulin promoter (RIP) lymphocytic choriomeningitis virus nucleoprotein (LCMV-NP) mouse model for virally induced insulin-dependent diabetes mellitus (IDDM), LCMV-NP is expressed as a “self” transgene in β cells (6, 7); following infection with LCMV, 90–100% of these mice develop diabetes mediated by CD4+ and CD8+ lymphocytes, which eliminate the viral infection and, at the same time, react with the LCMV viral “self” protein expressed in β cells (8). Two distinct advantages of this model are that the disease trigger (LCMV infection) can be precisely controlled, and the autoreactive (anti-NP) lymphocytes can be precisely tracked. We have shown previously that oral administration of insulin can prevent IDDM in this model by changing the cytokine profile in pancreatic islets from Th1 to Th2 (5). Here we evaluate the potential of DNA vaccination in control of autoimmune disease, using islet self-antigens to induce regulatory lymphocytes and prevent autoimmune diabetes. Such a therapy would constitute a safe and simple approach to protect at-risk prediabetic individuals.
Methods
DNA vaccine constructs, preparation, and injection.
The open reading frames encoding LCMV-NP or porcine insulin B chain were placed into cytomegalovirus promoter (pCMV), a plasmid described previously (25). DNA was prepared at a concentration of 1 mg/mL saline. After the mouse’s fur was shaved, 50 μL of the preparation was injected into the quadriceps femoris muscle of each mouse hindleg, under general anesthesia (using metophaneR). On each occasion, injection was made into both hindlegs (100 μL per mouse). Immunizations were administered according to the protocols given (see Figure 2) and were continued for a maximum of 4 weeks after LCMV infection (protocols 1 and 3).
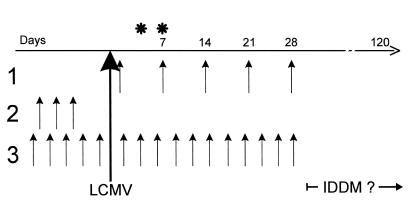
Figure 2.
DNA immunization protocols. Constructs were generated as described in Methods (see also reference 25), and plasmid injections were given intramuscularly into the quadriceps femoris muscle on each side (50 μg in 50 μL saline per injection), by 1 of the 3 protocols shown. For each protocol, the paired DNA injections are indicated by arrows; time between each set of injections was 7 days (protocol 1) or 3 days (protocols 2 and 3). The development of IDDM was followed for >3 months after LCMV infection. *Time at which LCMV-specific CTL responses were evaluated.
Transgenic mice.
The transgenic RIP-LCMV-NP 25-3 H-2d mouse line used in this study expresses the NP of LCMV under control of the RIP in the pancreatic β cells and in the thymus, but not in any other tissues (8). BALB/c nontransgenic H-2d mice were used as controls in some experiments. The virus used for induction of IDDM was LCMV Armstrong (ARM) strain (clone 53b). Four- to 21-week-old RIP-NP 25-3 mice were inoculated intraperitoneally with 105 pfu LCMV ARM in a volume of 0.2 mL.
Oral antigens.
Porcine insulin was purified from pancreatic glands by Novo Nordisk (Bagsvaerd, Denmark). Insulin was solubilized in acid buffer, pH adjusted, and the solution was stored at –20°C until it was used. Peptides were synthesized on an automated peptide synthesizer (model 430A; Applied Biosystems, San Francisco, California, USA) by the solid-phase method using t-butoxyl or N-(9-fluorenyl)methoxycarbonyl (Fmoc) chemistry, and were then purified by high-pressure liquid chromatography on an RP300-C8 reverse-phase column (Fisher, Tustin, California, USA) and identified by fast atom bombardment of electrospray mass spectrometry. LCMV was grown, purified, and ultraviolet inactivated; NP peptides were synthesized by the Scripps Core facility. All oral antigens were administered using a blunt-end curved feeding tube inserted into the esophagus/stomach. RIP-NP mice were fed twice a week with 0.5 mL of an aqueous solution containing 1 mg/mL antigen. Feeding was started 1 week before infection with LCMV and discontinued after 8 weeks. Control groups received saline or BSA at a concentration of 1 mg/mL.
Glucose measurements.
Blood samples from RIP-LCMV mice were screened for diabetes twice a week, beginning at 10 weeks of age, by testing for hyperglycemia (Accucheck III; Boehringer Mannheim Biochemicals, Indianapolis, Indiana, USA). Diabetes was defined by 2 consecutive blood glucose analyses with values above 350 mg/dL.
CTL and precursor CTL assessments.
LCMV-specific cytotoxic T lymphocyte (CTL) activity in spleens harvested 7 days after intraperitoneal inoculation with 105 LCMV ARM was assessed in a standard 4- to 5-hour 51Cr-release assay on LCMV-infected and -uninfected, MHC-matched [BALB/c17(H-2d)] and -mismatched [MC57(H-2b)] target cells (7, 8). For determination of LCMV-specific precursor CTL (pCTL) frequency 7 days after infection, spleen cells from immunized mice were serial diluted and cultured in 96-well flat-bottom plates (12 wells per dilution; highest dilution: 16,000 cells per well) with LCMV-infected and irradiated (20 Gy) macrophages and irradiated spleen cells. After 8 days, cells from each well were split and tested on LCMV-infected and -uninfected BALB/cl7 targets in a 4- to 5-hour 51Cr-release assay. pCTL frequencies were assessed by plotting the fraction of negative cultures on a semilogarithmic scale against the number of splenocytes per culture. The pCTL frequencies are defined by the slope of the linear regression along at least 3 separate data points. Positive cultures were defined by a specific Cr51 release more than 3 SE above background lysis.
Cytokine ELISA assays.
Cytokines (IL-4, IFN-γ) produced by splenocytes were detected using ELISA (PharMingen, San Diego, California, USA). Briefly, 96-well Millititer HA plates (Millipore Corp., Bedford, Massachusetts, USA) were coated with the respective capture antibodies for IL-4 and IFN-γ, diluted to 2 μg/mL. After overnight incubation at 4°C, plates were washed 4 times with PBS/0.05% Tween-20 and preincubated with PBS/10% FCS for 1 hour at room temperature. Tissue culture supernatants and standards were added at various dilutions in PBS with 10% FBS and 0.05% Tween-20, and plates were incubated 2–4 hours at room temperature. Thereafter, plates were washed 4 times with PBS/Tween-20, and the respective detection antibodies for the cytokines were added at 1 μg/mL in PBS/Tween-20 containing 10% FCS. Plates were incubated at room temperature for 1 hour and washed 4 times in PBS/Tween-20 before streptavidin-peroxidase conjugate (Boehringer Mannheim Biochemicals) was added at 1:1,000 dilution. After a 30-minute incubation at room temperature, the color-substrate solution (ABTS) was added and left on the plates for 10–30 minutes. Plates were then counted in a ELISA reader at 490 nm.
Secondary cultures of insulin or LCMV-reactive lymphocytes.
Splenocytes were harvested from protected (pCMV-insB–treated) or nonprotected (pCMV-NP–treated) mice 28–45 days after infection with LCMV, and were cultivated in 24-well tissue culture plates in 7% RPMI–1640 containing antibiotics and glutamine. As indicated, irradiated LCMV-infected macrophages from syngeneic H-2d (BALB/c) mice or syngeneic splenocytes coated with insulin B chain or LCMV-NP peptide (RPQASGVYM) at 1 mg/mL, or infected with LCMV, were used as antigen-presenting cells (APCs). Cytokines were assayed in tissue culture supernatants 3 days and 1 week after culture, and cells were adoptively transferred intraperitoneally into syngeneic RIP-NP transgenic recipients after 8 days. Before the transfer, all cells were washed 3 times in PBS.
Immunization with insulin A or B chains.
Purified porcine insulin A and B chains were obtained from ZymoGenetics (Seattle, Washington, USA). In the immunizations, RIP-NP (H-2d) transgenic mice received 100 μg of B- or A-chain peptide in a 1:1 incomplete Freund’s adjuvant (IFA) emulsion. Injections were given twice, on days 2 and 8 after LCMV infection.
ELISPOT for IFN-γ production by LCMV-NP–specific CTLs.
ELISPOT assays for IFN-γ production were performed as described by us previously (5). In addition, to ensure an LCMV-NP–specific signal, each spleen or lymph node sample was incubated directly during the ELISPOT assay with or without LCMV-NP118–126 H-2d peptide for 36 hours at 37°C in 5% CO2. Background spots appearing in non–peptide-stimulated cultures (0–3 spots per well) were subtracted from those found in peptide-treated cultures (0–100 spots = readout range). Serial 5-fold dilutions were performed for each sample ranging from 2 × 105 to 2 × 103.
Results
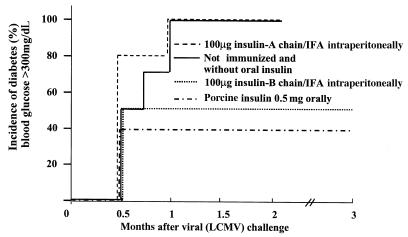
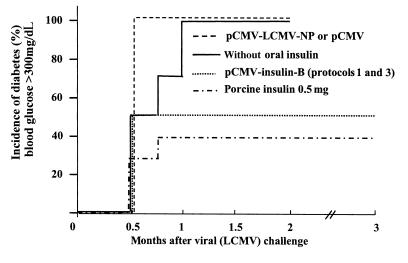
We had previously shown that whole porcine insulin, administered orally, could confer protection against IDDM in the RIP-LCMV-NP model system (5). To attempt to map protection to one or the other insulin chain, RIP-NP mice were immunized with insulin A or B chain in IFA; as shown in Figure 1, protection mapped to the insulin B chain. Because protein/IFA immunization is not permitted in humans, these results prompted us to use a plasmid expressing the insulin B chain for our next experiments. The immunization schemes used for the present study are displayed in Figure 2. Three protocols were applied in order to anticipate potentially different kinetic events after vaccination. The first delivered the plasmids only after viral infection; the second delivered them before viral infection; and the third before and after infection. When administered after LCMV infection, a plasmid expressing the porcine insulin B chain under control of the cytomegalovirus promoter (pCMV-insB) was effective in preventing IDDM in 50% of RIP-NP mice (Figure 3). DNA inoculations ceased 4 weeks after viral challenge, but IDDM was monitored over a 3-month observation period, during which no increase in blood glucose levels or pancreatic infiltration was noted. If pCMV-insB DNA was given only before the disease trigger (protocol 2), no protection was seen (data not shown). Others have suggested that expression of the encoded protein continues for some time after DNA inoculation. Although we have not directly addressed this question, the requirement for plasmid injections soon after the triggering event (protocols 1 and 3, but not 2, are effective) indicates that persistent insulin B-chain expression might be too limited (in time and/or quantity) to provide therapeutic benefit if given only before the trigger. However, the capacity of post-trigger DNA immunization to prevent IDDM extended well beyond the final injection, suggesting that its effect is relatively immediate and long-lived and that chronic immunization is unnecessary. In contrast to the dramatic protection conferred by B-chain DNA immunization, vaccination with pCMV-NP or pCMV failed to prevent IDDM, regardless of whether they were given before or after the triggering LCMV infection or given throughout the disease process (Figure 3). Thus, vaccination with a plasmid expressing a pancreatic self-antigen (pCMV-insB), but not with a plasmid expressing the viral (self) transgene, was effective in preventing IDDM.
Figure 1.
Immunization with the insulin B chains, but not the insulin A chain, is effective in preventing IDDM in RIP-NP mice. RIP-NP transgenic mice were immunized with 100 μg of porcine insulin A or B chains in IFA (1:1) once 5 days after LCMV infection, or were fed oral whole-porcine insulin as a positive control (see Methods for feeding protocol). Diabetes was measured weekly by Accucheck (see Methods); the total observation period was 3 months. Group sizes were as follows: 5 mice received insulin A; 6 mice received insulin B; 10 mice received oral porcine insulin (5); 10 mice were untreated controls. Definition of diabetes is blood glucose consistently >350 mg/dL.
Figure 3.
Immunization with DNA plasmids expressing the insulin B chain, but not the LCMV-NP, is effective in preventing IDDM in RIP-NP mice. RIP-NP transgenic mice were treated with pCMV-NP (protocols 1, 2, or 3; Figure 2), with pCMV-insB (protocol 1; Figure 2), or were fed oral porcine insulin (see Methods). Diabetes was measured weekly by Accucheck (see Methods); the total observation period was 3 months. Group sizes were as follows: 10 mice received pCMV-insB; 10 mice received pCMV-NP; 10 mice received oral porcine insulin (5); 10 mice were untreated controls; 4 mice received pCMV. Definition of diabetes is blood glucose consistently >350 mg/dL. Note that injection of pCMV (protocol 3) did not lower diabetes incidence.
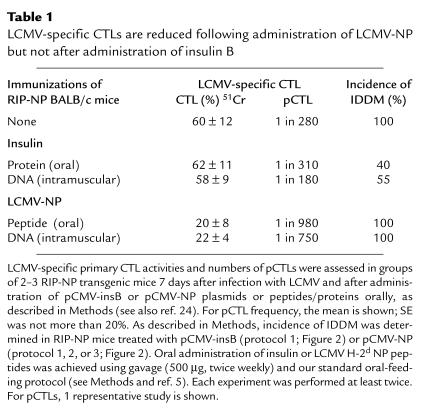
IDDM in this model system is triggered by NP-specific CD8+ T cells (8); a possible explanation for protection was a reduction in the frequency of these cells, although, conceptually, an antigen-specific effect on these cells appeared more likely with pCMV-NP than with pCMV-insB. We compared the LCMV-specific immune responses mounted after vaccination with pCMV-insB with those that followed pCMV-NP injection. As shown in Table 1, pCMV-NP immunization given before LCMV infection (protocol 2 or 3) resulted in a 3- to 4-fold reduction of LCMV-specific pCTLs; similarly, oral administration of NP peptide resulted in a transient 3-fold reduction of LCMV-pCTL responses. The reduction in CTL responses in pCMV-NP–immunized mice was temporary, because mice rechallenged with LCMV at least 21 days after the final DNA immunization mounted essentially normal CTL responses (data not shown). The affinity of these NP-specific cells was not altered by DNA immunization (Figure 4). However, despite these changes in NP-specific precursor frequencies, neither oral NP peptide nor injected NP DNA had any effect on the incidence of diabetes (Table 1). This finding is consistent with our previous observation that IDDM is abrogated only if pCTL numbers have been reduced to less than 1 in 5,000 (9). In contrast, 45–60% of mice fed insulin B chain or injected with pCMV-insB were protected from IDDM, although the NP-specific precursor frequencies were not significantly reduced (Table 1). Thus, the protective effect of pCMV-insB DNA immunization does not appear to be mediated by a reduction in NP-specific pCTLs.
Table 1.
LCMV-specific CTLs are reduced following administration of LCMV-NP but not after administration of insulin B
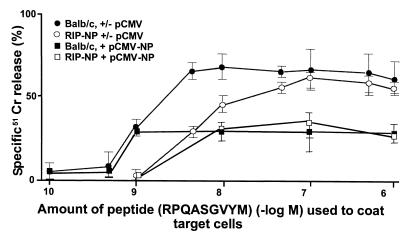
Figure 4.
Affinities of NP-specific CTL following DNA immunization. LCMV-NP CTLs were determined by using serial log dilutions of LCMV-NP H-2d peptide on syngeneic BALB/c targets in a 5-hour 51Cr-release assay. The overall plateau release was decreased in pCMV-NP–treated groups compared with pCMV-treated controls; however, the fall-off of the curve was not shifted, indicating that there were no significant affinity differences. Decrease in CTL numbers was confirmed by pCTL analysis (Table 1). Three mice per group were injected with pCMV or pCMV-NP, according to protocol 2 in Figure 2.
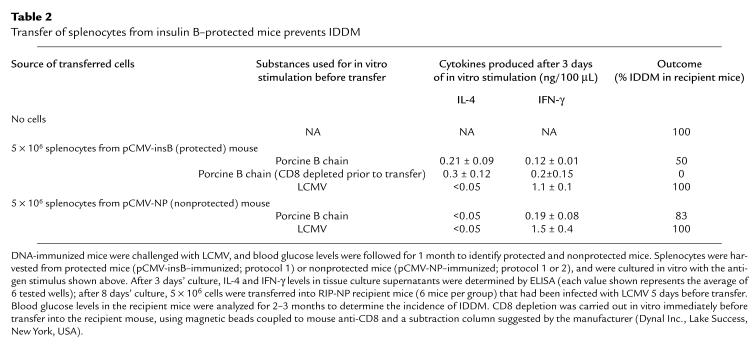
To identify the cell population involved in protection, adoptive transfer studies were carried out. Splenocytes were isolated from mice immunized with pCMV-NP or pCMV-insB, and were cultivated in the presence of insulin B chain or LCMV viral antigen. Augmented IL-4 production was seen only in B chain–stimulated cultures derived from protected mice (Table 2), whereas an increase in IFN-γ production was observed in LCMV-stimulated cultures. Upon adoptive transfer into RIP-NP recipients 5 days after LCMV infection, only lymphocytes from protected mice were able to prevent IDDM (50%; Table 2). Furthermore, CD8 depletion of the protective population before transfer did not prevent protection (Table 2), indicating that the protective T-cell type is not CD8+; we suggest that it is most probably of the CD4+ subset. Thus, regulatory cells producing IL-4 were present after vaccination with pCMV-insB, but not after administration of plasmids expressing an LCMV protein.
Table 2.
Transfer of splenocytes from insulin B–protected mice prevents IDDM
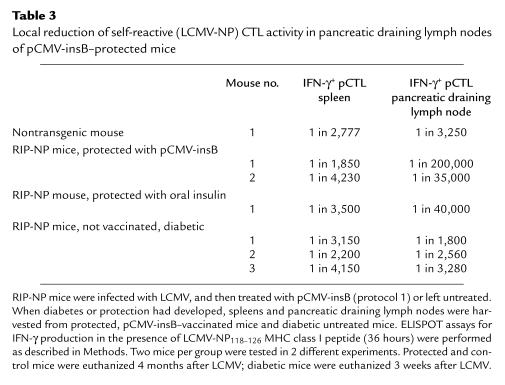
Lastly, experiments were carried out to identify the mechanism by which these regulatory lymphocytes acted. Spleens and pancreatic draining lymph nodes from pCMV-insB–protected mice and untreated diabetic mice were harvested, and the number of IFN-γ–producing LCMV-NP CTLs was determined by ELISPOT (Table 3). Protected mice exhibited ∼50-fold lower LCMV-NP precursor numbers locally in their pancreatic lymph nodes compared with diabetic mice, whereas systemic pCTL frequencies were similar in both groups. This finding points toward the ability of pCMV-insB–induced regulatory cells to act as “bystander suppressors” on LCMV-NP–autoreactive CTLs locally in the pancreatic draining lymph node. The absence of a systemic effect fits well with the concept that, after β-cell destruction, insulin is presented by “professional” APCs only in the pancreatic lymphoid organs and not systemically in the spleen or other site.
Table 3.
Local reduction of self-reactive (LCMV-NP) CTL activity in pancreatic draining lymph nodes of pCMV-insB–protected mice
Discussion
We have shown that DNA vaccination with a plasmid expressing the insulin B chain can effectively reduce autoimmune diabetes when given during the prediabetic phase. The protection is mediated by insulin B–reactive, IL-4–producing (Th2) lymphocytes — most likely of the CD4+ lineage — and does not result from a generalized/systemic reduction in the diabetogenic NP-specific effector cells, the activity and number of which remain unchanged (Table 1). We find that protection instead results from “bystander suppression,” induced in the islets or pancreatic draining node by the insulin B–specific CD4+ T cells, which leads to a significant local reduction in NP-specific autoreactive T cells (Table 3). We suggest that immediately following LCMV infection, NP-specific CD8+ cells begin to attack the islets; the insulin released by dying islet cells is presented by local APCs, which stimulate the pCMV-insB–induced CD4+ cells to secrete IL-4. This process modifies the local APCs, diminishing their ability to appropriately stimulate the NP-specific CD8+ T cells. Thus, local pCTL frequency is diminished, islet destruction stops at an early stage, and the mouse does not develop IDDM. The local failure to activate, expand, or maintain activation of NP-specific T cells following LCMV challenge does not prevent their systemic activation outside the islets; the resulting virus-specific CTL response in insulin B–treated mice, when measured systemically, appears normal and is capable of clearing viral infection (data not shown).
The fact that pCMV-insB is effective only when used to inoculate after the triggering event suggests that insulin B–reactive cells are activated quickly by DNA immunization but remain functional for only a brief period, during which time they presumably encounter antigen locally in the pancreatic islets or draining lymph node. If such antigen recognition takes place after either DNA immunization or adoptive transfer of primed lymphocytes, the result is long-term protection against IDDM due to permanent reduction of autoreactive CTLs in the pancreatic draining node. In contrast, vaccination with a viral self-antigen does not confer protection, despite a marked but transient reduction in the number of viral-specific lymphocytes. Why do T cells reactive to 1 self-antigen (insulin) differentiate into protective Th2 lymphocytes, whereas T cells to another self-antigen (NP) fail to do so? Likely both antigens can be presented equally well by dendritic cells (10). It is possible that the difference reflects different cell numbers and/or affinities. Both antigens are expressed in the thymus (8, 11); therefore, both self-reactive repertoires undergo negative selection, potentially to different degrees, depending on their affinity to the MHC/peptide complex (12). In RIP-LCMV-NP mice, only low-affinity LCMV-NP–specific CD8+ lymphocytes can reach the periphery (Figure 4 and ref. 8). After vaccination with pCMV-NP, their affinities appear unaltered (Figure 4), but their numbers are reduced (Table 1). Similar to recent observations in the experimental allergic encephalomyelitis model (13), this might be due to deletion- or activation-induced cell death. In contrast, CD8+ T cell–mediated lytic responses to insulin could not be detected in insulin B–treated mice (pCTL <1:500,000; data not shown), and proliferation to insulin was difficult to quantitate, indicating very low cell numbers and perhaps more efficient negative selection. These findings suggest that the insulin-specific T-cell repertoire is very limited and may comprise only low numbers of CD4+ T cells prone to differentiate into the Th2 phenotype. Alternatively, the ubiquitous presence of insulin in the periphery, as it fulfills its hormonal functions, might predispose insulin-reactive T cells to be regulatory, as has been found in a model of autoimmune thyroiditis (14). However the LCMV-NP–specific CD4+ and CD8+ lymphocytes also undergo thymic negative selection; why do they develop into Th1/CTLs? We suggest that the viral infection itself constitutes a very strong inflammatory stimulus that may drive the response down the Th1/TC1 path. Therefore, we suggest that it may be important to select self-antigens that are not involved in an ongoing Th1-type immune response during the time of DNA vaccination. This would explain why systemic injections/vaccinations with certain self-antigens (e.g., NP in the studies reported here and insulin/glutamate decarboxylase in NOD mice) sometimes do not result in Th2 deviation (15–21) or require external addition of IL-4, because these antigens are part of an ongoing autoreactive Th1 process. For this reason, oral antigen administration (4) or certain adjuvants (22) or immunomodulators (23) are needed to achieve Th2 lineage commitment.
In conclusion, careful selection of the appropriate self-antigen appears vital to the successful modulation of autoimmune disease, and DNA vaccination is a promising means of delivery. Indeed, coadministration of appropriate plasmid-encoded immune modulators may allow the design of vaccines that skew toward the Th2 phenotype. Achieving induction of autoreactive regulatory cells appears more attractive than the deletion of autoreactive inflammatory cells, for which all initiating self-antigens involved in a given disease would have to be known. In contrast, for immune regulation, only 1 self-antigen released under attack must be targeted.
Acknowledgments
Studies were supported by grants from the National Institutes of Health (NIH) (1RO1 AI-44451 and 1R29 DK-51091) M. von Herrath was supported by a Career Development Award from the Juvenile Diabetes Foundation International (296120) and J.L. Whitton was supported by NIH grant AI-27028. This is manuscript no. 12140-NP from the Scripps Research Institute. The authors thank Diana Frye for assistance with the manuscript.
References
- 1.Davis HL. Plasmid DNA expression systems for the purpose of immunization. Curr Opin Biotechnol. 1997;8:635–640. doi: 10.1016/s0958-1669(97)80041-9. [DOI] [PubMed] [Google Scholar]
- 2.Hassett DE, Whitton JL. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 3.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 4.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 5.von Herrath MG, Dyrberg T, Oldstone MBA. Oral insulin treatment suppresses virus-induced antigen-specific destruction of beta cells and prevents autoimmune diabetes in transgenic mice. J Clin Invest. 1996;98:1324–1331. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohashi PS, et al. Ablation of tolerance and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–318. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 7.Oldstone MBA, Nerenberg M, Southern PJ, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 8.von Herrath MG, Dockter J, Oldstone MBA. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 9.von Herrath MG, et al. In vivo treatment with a MHC class I-restricted blocking peptide can prevent virus-induced autoimmune diabetes. J Immunol. 1998;161:5087–5096. [PubMed] [Google Scholar]
- 10.Akbari O, et al. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–178. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997;9:1355–1365. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- 12.Ashton-Rickardt PG, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 13.Lobell A, et al. Vaccination with DNA encoding an immunodominant myelin basic protein peptide targeted to Fc of immunoglobulin G suppresses experimental autoimmune encephalomyelitis. J Exp Med. 1998;187:1543–1548. doi: 10.1084/jem.187.9.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liblau R, Tisch R, Bercovici N, McDevitt HO. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol Today. 1997;18:599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 16.Vaysburd M, Lock C, McDevitt H. Prevention of insulin-dependent diabetes mellitus in nonobese diabetic mice by immunogenic but not by tolerated peptides. J Exp Med. 1995;182:897–902. doi: 10.1084/jem.182.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JW, Ihm SH, Kim KW. Viruses as a triggering factor of type 1 diabetes and genetic markers related to the susceptibility to the virus-associated diabetes. Diabetes Res Clin Pract. 1989;7(Suppl. 1):S47–S58. doi: 10.1016/0168-8227(89)90088-0. [DOI] [PubMed] [Google Scholar]
- 18.Han HS, Jun HS, Utsugi T, Yoon JW. Molecular role of TGF-β, secreted from a new type of CD4+ suppressor T cell, NY4.2, in the prevention of autoimmune IDDM in NOD mice. J Autoimmun. 1997;10:299–307. doi: 10.1006/jaut.1997.0137. [DOI] [PubMed] [Google Scholar]
- 19.King C, et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity. 1998;8:601–613. doi: 10.1016/s1074-7613(00)80565-8. [DOI] [PubMed] [Google Scholar]
- 20.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianani R, Sarvetnick N. Viruses, cytokines, antigens, and autoimmunity. Proc Natl Acad Sci USA. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995;7:793–798. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 23.Smith JA, Bluestone JA. T cell inactivation and cytokine deviation promoted by anti-CD3 mAbs. Curr Opin Immunol. 1997;9:648–654. doi: 10.1016/s0952-7915(97)80044-1. [DOI] [PubMed] [Google Scholar]
- 24.von Herrath MG, Yokoyama M, Dockter J, Oldstone MBA, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama M, Zhang J, Whitton JL. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]