Abstract
The composite physiologic index(CPI) was derived to represent the extent of fibrosis on high resolution computed tomography, adjusting for emphysema in patients with idiopathic pulmonary fibrosis(IPF). We hypothesized longitudinal change in CPI would better predict mortality than forced expiratory volume in 1 second(FEV1), forced vital capacity(FVC), or diffusing capacity for carbon monoxide(DLCO) in all patients with IPF, and especially in those with combined pulmonary fibrosis and emphysema(CPFE).
Cox proportional hazard models were performed on pulmonary function data from IPF patients at baseline (n=321), 6 months (n=211) and 12 months (n=144). Presence of CPFE was determined by high resolution computed tomography.
A 5 point increase in CPI over 12 months predicted subsequent mortality (HR 2.1, p=0.004). At 12 months, a 10% relative decline in FVC, a 15% relative decline in DLCO or an absolute increase in CPI of 5 points all discriminated median survival by 2.1 to 2.2 years versus patients with lesser change. Half our cohort had CPFE. In patients with moderate/severe emphysema, only a 10% decline in FEV1 predicted mortality (HR 3.7, p=0.046).
In IPF, a 5 point increase in CPI over 12 months predicts mortality similarly to relative declines of 10% in FVC or 15% in DLCO. For CPFE patients, change in FEV1 was the best predictor of mortality.
Keywords: Chronic Obstructive Pulmonary Disease, Idiopathic Pulmonary Fibrosis, Prognosis, Pulmonary Function, Survival
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal diffuse parenchymal lung disease.(1–4) Progression of disease is heterogeneous. Some patients decline rapidly, others remain stable, and all appear at risk for developing acute exacerbations.(2, 3, 5) Methods to assess and monitor disease status and ultimately predict mortality and response to therapy are needed. A variety of variables including pulmonary function at time of diagnosis, hypoxemia at rest, desaturation during a 6-minute walk test, longitudinal changes in forced vital capacity (FVC), longitudinal changes in diffusing capacity for carbon monoxide (DLCO), and performance on cardiopulmonary exercise testing have been shown to have prognostic value. (6–12)
Smoking is a common risk factor for both emphysema and pulmonary fibrosis. (13–15) Therefore, patients with IPF may have combined pulmonary fibrosis and emphysema (CPFE). The presence of both pathologies could limit the ability to utilize FVC in the assessment and monitoring of disease course.(16, 17) The composite physiologic index (CPI) was developed to improve on previous prognostic measures in IPF by adjusting for emphysema and incorporating multiple measures of pulmonary function, namely forced expiratory volume in one second (FEV1), FVC and DLCO. (18) The CPI score at diagnosis more accurately predicted mortality than the individual pulmonary function tests alone in patients with concomitant emphysema. (18)
We hypothesized that longitudinal changes in CPI would more accurately predict mortality than previously published longitudinal declines in FVC of 10% and DLCO of 15% in all patients with IPF, and to a greater degree in patients with CPFE.(7–10) As such, we tested the CPI in a large cohort of patients diagnosed with IPF on biopsy or high resolution computed tomography (HRCT). We evaluated the magnitude of CPI change required to predict an increased risk of mortality, and compared the relevant longitudinal change in CPI to changes in individual pulmonary function tests. Finally, we evaluated if the presence/absence of emphysema impacted the ability of CPI or individual measures of pulmonary function to predict mortality.
Materials and Methods
Study Population
Patients with IPF were selected from the University of Michigan Interstitial Lung Disease Database. The diagnosis of IPF was made with either a surgical lung biopsy or HRCT scan diagnostic of usual interstitial pneumonia (UIP) using standard criteria.(1, 3, 4, 7) Patients were included if they had a pulmonary function test (PFT) performed at the University of Michigan within three months of diagnosis. Mortality data were confirmed through the Social Security Death Registry Index censured by three months to account for reporting lag. Follow-up time was calculated from date of baseline PFT to date of death or censure. IPF patients were eligible for the analysis of combined pulmonary fibrosis and emphysema if a HRCT had been performed at the University of Michigan within one year before or after diagnosis.
Methods
Patients with at least one additional PFT after baseline were eligible for longitudinal analyses. For the 6 month analysis, all PFTs from three to nine months after the baseline study were included to generate a regression line for each patient. An estimated 6 month PFT value was obtained from the regression. At least one PFT performed between nine and fifteen months after diagnosis was required to be included in the 12 month analysis. The same individual regression technique was used for 12 month data using all PFTs up to fifteen months after baseline. The CPI was calculated from the following formula: 91 − (0.65×%predicted DLCO) − (0.53×%predicted FVC) + (0.34×%predicted FEV1).(18) Relative changes in PFT values were calculated as the estimated 6 or 12 month value minus the baseline value, divided by the baseline value.
The index of concordance (IOC) was used to compare predictive ability of the longitudinal change in various PFTs and CPI.(19) The IOC analysis considers each combination of two patients in the dataset and measures how accurately the model predicts which patient will live longer. The higher the index of concordance, the more likely the variables in the model explain the outcome, in this case, mortality. Various cutpoints of relative and absolute change in CPI were studied to determine what change of CPI would yield the best index of concordance.
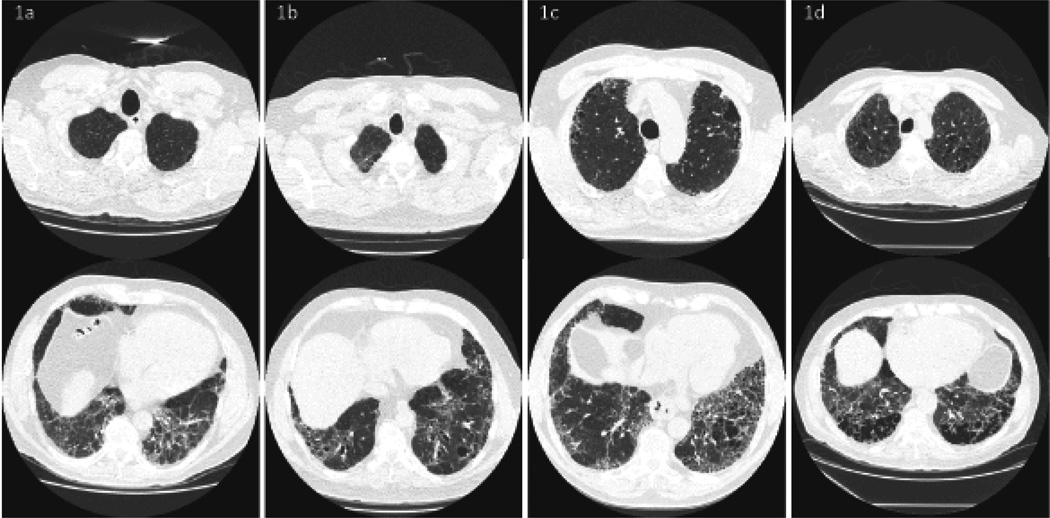
Emphysema was scored semi-quantitatively as none, mild (present but scant), moderate (notable or equivalent in extent to the fibrosis) or severe (the predominant pathology) by a thoracic radiologist experienced with HRCT scoring in prior clinical trials, blinded to tobacco history and patient outcome. Figure 1 contains representative images. Patients were grouped together for analysis as none/mild or moderate/severe emphysema.
Figure 1.
Examples of the high resolution computed tomography scan semi-quantitative scoring system. Each panel contains a characteristic upper and lower lobe slice of a patient scored as none (1a), mild (1b), moderate (1c) and severe (1d) emphysema.
Survival Analysis
Cox proportional hazards models adjusting for age at diagnosis, gender and smoking status were used to assess the relationship of FEV1, FVC, DLCOand CPI to mortality.(20) Longitudinal models included adjustment for baseline PFT value. Time zero for median survival calculations was the last pulmonary function test, either 6 or 12 months. Therefore, the survival only applies to patients able to provide a 6 or 12 month PFT. Longitudinal changes in CPI were compared to longitudinal changes in each of its PFT components, including the previously published relative declines in FVC (10%) and DLCO (15%).(7–10) We also evaluated changes in CPI and PFTs in patients stratified by amount of emphysema. All statistics were performed on SAS® 9.2 software, SAS Institute Inc., Cary, NC. Our institutional review board approved the study. Data on a subgroup of this patient cohort has been previously published.(7, 9) A portion of these results was presented at the 2010 American Thoracic Society International Conference.(21)
Results
Patient Population
We identified 396 patients with IPF from 1995 to 2007. Of these, 321 patients had a baseline PFT performed at the University of Michigan, 211 patients had 6 month data and 144 had 12 month data. Of the 99 who did not have any longitudinal data, 34 died. Fifteen of the 67 who had 6 but not 12 month data, died. The remainder returned to the community for continuity of care. HRCT performed at the University of Michigan was available for analysis in 169 and 118, from the 6 and 12 month cohorts respectively. Baseline demographics were not clinically different between patients participating in the baseline, 6 or 12 month analyses or between patients with or without a HRCT available for scoring of emphysema, Table 1.
Table 1.
Baseline Demographic and Pulmonary Function Data.
| Baseline n = 321 |
6 Month n = 211 |
6 Month HRCT n = 169 |
12 Month n = 144 |
12 Month HRCT n = 118 |
|
|---|---|---|---|---|---|
| Age at diagnosis, mean (SD) | 63.9 (9.7) | 63.2 (10.0) | 63.8 (9.8) | 62.3 (10.0) | 62.7 (10.0) |
| Male, n (%) | 217 (67.6) | 151 (71.6) | 127 (75.1) | 102 (70.8) | 88 (74.6) |
| Ever tobacco use, n (%) | 236 (73.5) | 162 (76.8) | 131 (77.5) | 109 (75.7) | 89 (75.4) |
| Tobacco pack-years, mean (SD) | 26.2 (27.5) | 27.2 (28.4) | 27.2 (28.0) | 26.6 (27.6) | 27.3 (28.1) |
| Surgical lung biopsy, n (%) | 245 (76.3) | 158 (74.9) | 118 (69.8) | 113 (78.5) | 88 (74.6) |
| Follow-up years, median (95%CI) | 5.2 (4.9–6.1) | 5.4 (5.1–7.0) | 5.1 (4.7–6.1) | 6.6 (5.3–7.7) | 5.4 (5.0–7.0) |
| Pulmonary Function | |||||
| FEV1 % predicted, mean (SD) | 79.2 (19.0) | 79.1 (17.2) | 79.1 (17.0) | 80.7 (18.6) | 80.9 (18.5) |
| FVC % predicted, mean (SD) | 67.6 (16.8) | 68.0 (15.8) | 67.7 (15.3) | 69.0 (16.4) | 69.0 (16.3) |
| DLCO % predicted, mean (SD) | 44.5 (16.2) | 46.2 (15.3) | 44.9 (14.8) | 48.0 (16.1) | 46.6 (15.6) |
| CPI, mean (SD) | 53.2 (12.4) | 51.8 (11.7) | 52.8 (11.2) | 50.7 (12.1) | 51.6 (11.7) |
Definition of abbreviations: n = number of patients, SD = standard deviation, CI = confidence interval, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity for carbon monoxide, CPI = composite physiologic index, HRCT = high resolution computed tomography.
Comparison at Baseline
We assessed the impact of the baseline CPI and each individual component (FEV1, FVC, DLCO) on risk of subsequent mortality. Each component as well as the CPI was predictive of subsequent mortality with all measures showing increased risk of mortality with greater physiologic derangement (Table 2). A lower DLCO was associated with greater risk of mortality compared to similar degrees of dysfunction in FEV1 or FVC.
Table 2.
Hazard Ratios for Mortality Associated with Discrete Differences in Baseline Individual Pulmonary Function Tests and Composite Physiology Index.
| n = 321 | HR† | 95% CI | p-value |
|---|---|---|---|
| Difference in FEV1 | |||
| 5% less | 1.1 | 1.0 – 1.1 | 0.015 |
| 10% less | 1.1 | 1.0 – 1.2 | 0.015 |
| 15% less | 1.2 | 1.0 – 1.3 | 0.015 |
| Difference in FVC | |||
| 5% less | 1.1 | 1.0 – 1.1 | 0.003 |
| 10% less | 1.2 | 1.1 – 1.3 | 0.003 |
| 15% less | 1.2 | 1.1 – 1.4 | 0.003 |
| Difference in DLCO | |||
| 5% less | 1.1 | 1.1 – 1.2 | <0.001 |
| 10% less | 1.2 | 1.1 – 1.4 | <0.001 |
| 15% less | 1.4 | 1.2 – 1.6 | <0.001 |
| Difference in CPI | |||
| 5 points more | 1.2 | 1.1 – 1.2 | <0.001 |
| 10 points more | 1.3 | 1.2 – 1.5 | <0.001 |
| 15 points more | 1.6 | 1.3 – 1.9 | <0.001 |
Definition of abbreviations: HR = hazard ratio, CI = confidence interval, FEV1 = % predicted forced expiratory volume in 1 second, FVC = % predicted forced vital capacity, DLCO = % predicted diffusing capacity for carbon monoxide, CPI = composite physiologic index.
The hazard ratios are based on Cox models that assume a continuous change in each value. The hazards have been tabulated at clinically relevant differences. For example, a patient with a baseline FVC 10% less than another patient, has a 20% increased hazard (HR 1.2).
Identifying Relevant Longitudinal Change in CPI
We used index of concordance (IOC) to compare the ability to predict mortality from longitudinal changes in CPI and the individual measures of pulmonary function. An absolute increase of 5 points in the CPI at 6 months yielded the highest IOC of 0.664, therefore the model would correctly predict patients at an increased risk of mortality 66.4% of the time. At 12 months, an absolute increase of 15 had the highest IOC of 0.690 but only 6% of the cohort achieved this extent of worsening and an absolute increase in 5 points was within 1.2%, IOC of 0.678. Therefore we determined an absolute increase of 5 points to be a clinically meaningful longitudinal change for future analyses. By comparison, a relative decline in FVC of 10% and DLCO of 15% at 12 months yielded IOCs of 0.689 and 0.683 respectively, all roughly equivalent.
Longitudinal Change and Survival
Cox proportional hazards models of mortality with varying longitudinal changes in CPI, FEV1, FVC and DLCO over 6 and 12 months are shown in Table 3. At 6 months, only change in DLCO is consistently significant. The other PFTs and the CPI exhibited variable significance and in general were insignificant at greater perturbations from baseline. This may represent the inherent variability in these physiologic measures over short time periods. At 12 months, the models became more consistent as one would expect. The relative FVC decline of 10% and DLCO decline of 15% yielded similar hazard ratios at 2.4 and 2.3 respectively with p-values < 0.001. An increase in CPI of 5 was similar with a hazard ratio of 2.1. An increase in CPI of 20 yielded the highest hazard, at 5.2, but only applies to 2 patients. A CPI increase of 5 was still significant after removing those patients with an increase in CPI greater than 15, hazard ratio 1.9 (95%CI 1.1–3.2, p=0.017).
Table 3.
Longitudinal Hazard Ratios for Mortality by Absolute Increase in Composite Physiologic Index and Relative Decrease in Individual Pulmonary Function Tests over 6 and 12 months.
| 6 Month Change n = 211 |
12 Month Change n = 144 |
|||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | HR | 95% CI | p-value | n (%) | HR | 95% CI | p-value | |
| Decline in FEV1 | ||||||||
| 5% | 81 (38) | 1.6 | 1.1–2.3 | 0.018 | 72 (50) | 2.3 | 1.4–3.7 | <0.001 |
| 10% | 44 (21) | 1.6 | 1.0–2.4 | 0.051 | 42 (29) | 2.2 | 1.3–3.5 | 0.002 |
| 15% | 24 (11) | 1.6 | 0.9–2.8 | 0.086 | 25 (17) | 2.1 | 1.3–3.6 | 0.005 |
| 20% | 8 (4) | 1.4 | 0.6–3.5 | 0.473 | 9 (6) | 3.6 | 1.6–8.1 | 0.001 |
| Decline in FVC | ||||||||
| 5% | 88 (42) | 1.8 | 1.2–2.7 | 0.002 | 75 (52) | 1.8 | 1.1–2.9 | 0.012 |
| 10% | 51 (24) | 1.4 | 0.9–2.1 | 0.122 | 51 (35) | 2.4 | 1.5–3.8 | <0.001 |
| 15% | 28 (13) | 1.1 | 0.6–1.8 | 0.857 | 26 (18) | 2.6 | 1.6–4.5 | <0.001 |
| 20% | 12 (6) | 2.0 | 1.0–4.0 | 0.051 | 15 (10) | 3.6 | 1.9–6.9 | <0.001 |
| Decline in DLCO | ||||||||
| 10% | 74 (35) | 1.7 | 1.1–2.5 | 0.011 | 68 (47) | 2.2 | 1.4–3.5 | 0.001 |
| 15% | 51 (24) | 1.6 | 1.1–2.5 | 0.029 | 57 (40) | 2.3 | 1.5–3.7 | <0.001 |
| 20% | 33 (16) | 1.8 | 1.1–3.0 | 0.030 | 37 (26) | 3.0 | 1.8–4.9 | <0.001 |
| 25% | 20 (9) | 2.3 | 1.2–4.2 | 0.010 | 26 (18) | 3.5 | 2.0–6.1 | <0.001 |
| Increase in CPI | ||||||||
| +5 | 51 (24) | 1.7 | 1.1–2.7 | 0.019 | 63 (44) | 2.1 | 1.3–3.5 | 0.004 |
| +10 | 20 (9) | 1.3 | 0.7–2.4 | 0.439 | 21 (15) | 2.3 | 1.2–4.2 | 0.011 |
| +15 | 4 (2) | 2.0 | 0.6–6.6 | 0.240 | 9 (6) | 3.3 | 1.4–7.7 | 0.007 |
| +20 | 2 (1) | 1.2 | 0.2–8.4 | 0.884 | 2 (1) | 5.2 | 1.1–23.8 | 0.036 |
Definition of abbreviations: n = number of patients, HR = hazard ratio, CI = confidence interval, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity for carbon monoxide, CPI = composite physiologic index.
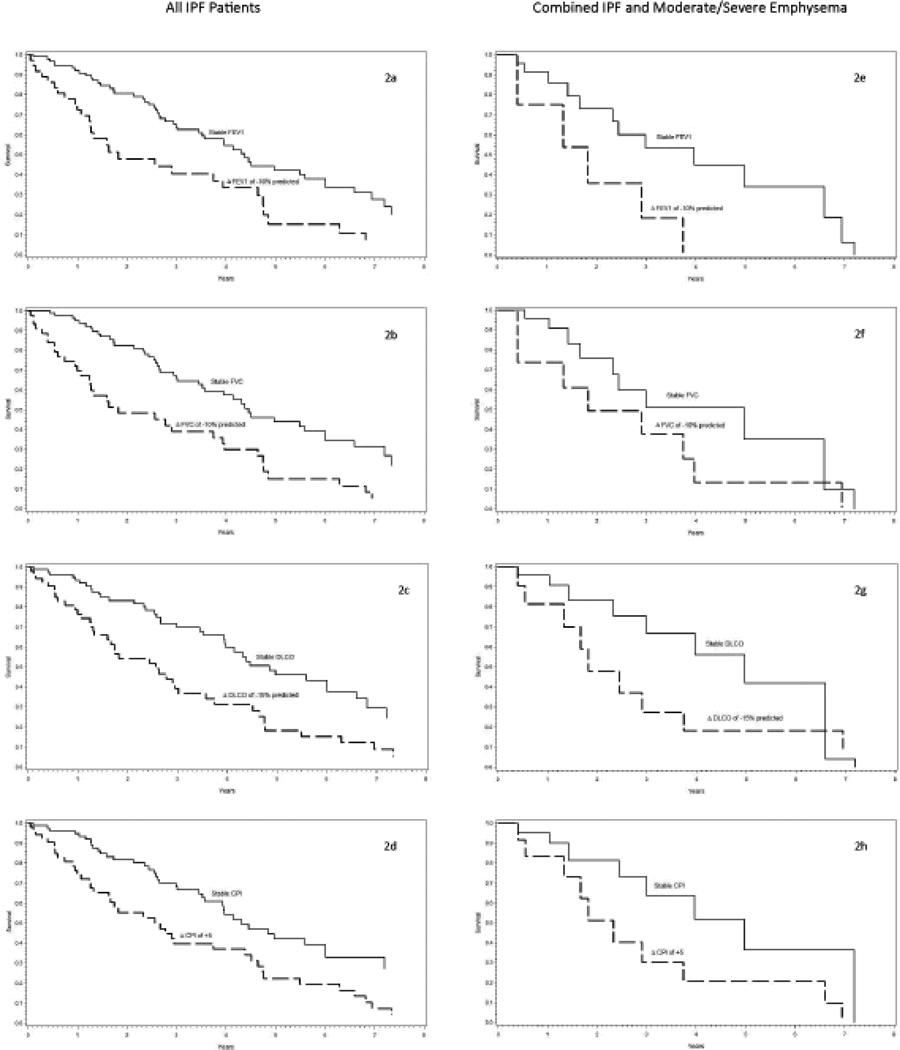
Survival curves based on 12 month PFT and CPI data are shown in Figure 2a–d. CPI performed similarly to its components. Median survival, from the date of the last PFT, for a 5 point increase in CPI over 12 months was 2.63 years (95%CI 1.63–3.93) compared to 4.75 years (3.53–6.81) for a less than 5 point change. This was similar to a 15% relative decline in DLCO, 2.58 years (1.63–3.57) versus 4.75 years (3.74–6.81). Longitudinal changes in all physiologic variables discriminated median survival in this patient population by 2 to 2.2 years.
Figure 2.
Cox Model Survival† Estimates for Relevant 12 month Longitudinal Changes in Pulmonary Function Tests or Composite Physiologic Index in all patients with IPF (2a–d) and with Combined Pulmonary Fibrosis and Moderate/Severe Emphysema (2e–h). The uppermost figures, 2a and 2e, represent a relative decline in percent predicted FEV1 of greater than 10% (dashed line) versus less than 10% (solid line), followed by a relative decline in percent predicted FVC of greater than 10% (dashed line) versus less than 10% (solid line) in figures 2b and 2f. Figures 2c and 2g represent a relative decrease in percent predicted DLCO of 15% (dashed line) versus a less than 15% change (solid line). The bottom figures 2d and 2h represent an absolute CPI increase greater than 5 points (dashed line) versus less than 5 points (solid line).
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity for carbon monoxide, CPI = composite physiologic index, PFT = pulmonary function test.
† Survival is dated from the time of their last analyzed pulmonary function test, the 12 month study. Therefore, this survival applies to patients able to survive one year from their baseline study. The above physiologic cutpoints were chosen based on prior literature and the best prognostic fit according to index of concordance. All Cox models are adjusted for the baseline average PFT or CPI value, average age at diagnosis of 62, male gender and positive smoking history, with baseline hazards stratified by whether the patient was in the stable or change group.
Combined Pulmonary Fibrosis and Emphysema is Prevalent
Combined pulmonary fibrosis and emphysema was common in our cohort. Of the 169 patients with HRCT scans in the 6 month analysis, 86 (51%) had evidence of emphysema, 42 (25%) with moderate or severe emphysema. In the 12 month analysis, 55 of the 118 patients with HRCT (47%) had CPFE, 32 (27%) moderate or severe. The moderate/severe combined emphysema patients tended to have higher FEV1, FVC and lower CPI with equivocal DLco, than those with none/mild emphysema, Table 4. The mean FEV1/FVC ratios in the none/mild emphysema groups were 0.84 to 0.85, higher than those in the moderate/severe emphysema group, 0.78 to 0.79, yet all were greater than the global initiative for chronic obstructive lung disease (GOLD) criteria for obstruction, 0.70.(22)
Table 4.
Demographics of Patients Stratified by Presence and Severity of Combined Pulmonary Fibrosis and Emphysema.
| 6 Month Analysis | 12 Month Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| None/Mild | Mod/Severe | None/Mild | Mod/Severe | |||||
| n | 127 | 42 | 86 | 32 | ||||
| Age at diagnosis, µ(SD) | 63.8 | (9.9) | 64.0 | (9.6) | 62.8 | (10.1) | 62.6 | (9.8) |
| Male, n (%) | 94 | (74.0) | 33 | (78.6) | 65 | (75.6) | 23 | (71.9) |
| Ever tobacco use, n (%) | 89 | (70.1) | 42 | (100) | 57 | (66.3) | 32 | (100) |
| Tobacco pack-yrs, mean (SD) | 21.0 | (25.0) | 46.4 | (28.4) | 20.8 | (25.8) | 45.4 | (26.5) |
| Surgical lung biopsy, n (%) | 98 | (77.2) | 20 | (47.6) | 71 | (82.6) | 17 | (53.1) |
| Follow-up years, median (95%CI) | 5.1 | (4.7–6.1) | 5.3 | (3.3–7.9) | 5.3 | (5.0–7.0) | 6.0 | (2.5–6.8) |
| HRCT_Type | ||||||||
| Volumetric, n (%) | 35 | (27.6) | 16 | (38.1) | 17 | (19.8) | 9 | (28.1) |
| Incremental, n (%) | 91 | (71.1) | 26 | (61.9) | 69 | (80.2) | 23 | (71.9) |
| Emphysema | ||||||||
| None, n (%) | 83 | (65.4) | - | 63 | (73.3) | - | ||
| Mild, n (%) | 44 | (34.6) | - | 23 | (26.7) | - | ||
| Moderate, n (%) | - | 32 | (76.2) | - | 27 | (84.4) | ||
| Severe, n (%) | - | 10 | (23.8) | - | 5 | (15.6) | ||
| Pulmonary Function | ||||||||
| FEV1 % predicted, mean (SD) | 77.4 | (16.9) | 84.0 | (16.2) | 78.1 | (17.4) | 88.5 | (19.6) |
| FVC % predicted, mean (SD) | 64.8 | (14.3) | 76.2 | (15.2) | 65.3 | (14.8) | 79.2 | (16.1) |
| FEV1/FVC Ratio, mean (SD) | 0.84 | (0.06) | 0.78 | (0.07) | 0.85 | (0.05) | 0.79 | (0.07) |
| DLCO % predicted, mean (SD) | 45.8 | (14.3) | 42.2 | (16.0) | 46.7 | (15.8) | 46.3 | (15.3) |
| CPI, mean (SD) | 53.2 | (10.8) | 51.7 | (12.5) | 52.6 | (11.7) | 49.1 | (11.5) |
Definition of abbreviations: n = number of patients, Mod = moderate, HRCT = high resolution computed tomography, CI = confidence interval, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity for carbon monoxide, CPI = composite physiologic index.
CPFE and Survival
A longitudinal decline in FEV1 was the strongest and most consistent predictor of mortality for patients with moderate to severe emphysema on HRCT, Table 5. In contrast, change in FVC, DLCO, and CPI were not predictive at 12 months follow-up and only FVC was predictive at 6 months. Further, the hazard ratio associated with a 10% decline in FEV1 increased for increasing levels of emphysema on HRCT. The hazard ratio increased from 1.8, 95% CI [0.8–4.1], to 2.5 [1.2–5.1] to 3.7 [1.0–13.7] in patients with no emphysema, none/mild emphysema, and moderate/severe emphysema respectively. Cox survival curves are shown in Figure 2e–h.
Table 5.
Longitudinal Hazard Ratios for Mortality Associated with Absolute Increases in Composite Physiologic Index and Relative Decreases in Individual Pulmonary Function Tests over 6 and 12 months in Patients with Combined Pulmonary Fibrosis and Emphysema.
| 6 Month Change | 12 Month Change | |||||||
|---|---|---|---|---|---|---|---|---|
| None to Mild Emphysema | n = 127 | n = 86 | ||||||
| n (%) | HR | 95% CI | p-value | n (%) | HR | 95% CI | p-value | |
| 10% Decline FEV1 | 31 (24.4) | 1.4 | 0.8–2.6 | 0.268 | 26 (30.2) | 2.5 | 1.2–5.1 | 0.012 |
| 10% Decline FVC | 36 (28.3) | 1.4 | 0.8–2.6 | 0.209 | 29 (33.7) | 2.8 | 1.4–5.9 | 0.005 |
| 15% Decline DLCO | 31 (24.4) | 2.4 | 1.3–4.4 | 0.005 | 33 (38.4) | 2.9 | 1.4–5.7 | 0.003 |
| +5 Increase CPI | 33 (26.0) | 2.4 | 1.3–4.4 | 0.003 | 34 (39.5) | 3.6 | 1.7–7.7 | 0.001 |
| Moderate to Severe Emphysema | n = 42 | n = 32 | ||||||
| n (%) | HR | 95% CI | p-value | n (%) | HR | 95% CI | p-value | |
| 10% Decline FEV1 | 4 (9.5) | 8.4 | 1.9–37.8 | 0.006 | 6 (18.8) | 3.7 | 1.0–13.7 | 0.046 |
| 10% Decline FVC | 5 (11.9) | 4.1 | 1.2–14.0 | 0.025 | 9 (28.1) | 2.1 | 0.8–6.0 | 0.154 |
| 15% Decline DLCO | 13 (31.0) | 2.1 | 0.8–5.5 | 0.140 | 12 (37.5) | 2.1 | 0.7–6.0 | 0.174 |
| +5 Increase CPI | 11 (26.2) | 1.9 | 0.6–5.7 | 0.279 | 15 (46.8) | 2.4 | 0.8–7.9 | 0.135 |
Definition of abbreviations: n = number of patients, HR = hazard ratio, CI = confidence interval, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity for carbon monoxide, CPI = composite physiologic index.
In the none/mild emphysema group, a 5 point increase in the CPI appeared to be the best predictor with the highest hazard at 12 months: 3.6 (95%CI 1.7–7.7), p=0.001, table 5. Twelve month relative declines in DLco and FVC performed similarly with hazards of 2.9 and 2.8 respectively. In a separate 12 month analysis of patients with no emphysema, a 5 point increase in CPI outperformed the other measures with a hazard ratio of 4.4 (1.8–10.7) compared to relative declines in DLco 2.8 (1.3–4.4) and FVC 2.6 (0.8–2.6).
Overall, grouping patients by emphysema status increased each pulmonary function model’s index of concordance, therefore knowing emphysema status provided the best mortality prediction.
Discussion
In a large cohort of patients with IPF we describe that 1) baseline individual pulmonary function parameters (FEV1, FVC, or DLCO) and the CPI were predictive of subsequent mortality; 2) an increase in CPI of at least 5 points over 6 or 12 months significantly predicted mortality and was therefore clinically relevant; 3) longitudinal changes in DLCO and CPI are more predictive than FVC and FEV1, and comparable to each other; 4) combined pulmonary fibrosis and emphysema (CPFE) is common in patients diagnosed with IPF; 5) longitudinal change in FEV1 was most predictive of mortality in patients with CPFE; 6) CPI may be the best predictor in patients without emphysema. These data confirm the value of longitudinal physiological monitoring in IPF patients but extend previous results to highlight the importance of accounting for the presence and severity of emphysema in choosing the optimal longitudinal measure of physiological derangement to predict outcome.
Comparison of CPI and Individual Measures of Pulmonary Function
Without accounting for emphysema, baseline PFT measurements and CPI were all predictive of mortality. The baseline DLCO and CPI were the best predictors, and similar, which is not entirely consistent with the original article describing CPI.(18) In Wells and a follow-up article by Latsi which incorporated longitudinal changes in patients with UIP and NSIP, the baseline CPI was reported to be a better predictor for mortality than baseline DLCO.(10) These differences could be due to differences in patient population or sample size with more than 200 additional cases of IPF in this dataset.
To compare CPI as a longitudinal predictor, we identified the magnitude of CPI change that increases risk of subsequent mortality: a CPI increase of at least 5 points at 6 or 12 months follow-up. Although extreme worsening of CPI was associated with an even higher risk of mortality, the small numbers of patients with these severe changes made those cut points impractical. Importantly, a recent report from the IFIGENIA study group demonstrated a difference of 5.47 in CPI in placebo treated patients compared to a change of 0.509 in NAC treated patients.(23) This finding suggests broad applicability of the cut point of 5; however, there were too few deaths in the study to examine the relationship of this increase on mortality. An increase in CPI of at least 5 points remained a significant predictor of mortality even when patients whose CPI worsened greater than 15 were removed highlighting that an increase of 5 in CPI is significant and not just driven by the subset of patients with extreme worsening.
Throughout our study the DLCO and CPI were comparable predictors both at baseline and longitudinally. The FVC was as strong as DLCO at 12 months. Median survival discrimination between these three predictors was comparable. In sum, these analyses suggest that contrary to our hypothesis, in all-comers with IPF, longitudinal change in either FVC or DLCO appear equivalent to changes in CPI. However when patients with emphysema are removed, CPI appears to be a better predictor.
Impact of CPFE
Prior studies highlight that longitudinal changes in FVC and DLCOare important, but imperfect predictors of subsequent mortality.(2, 7, 8, 10, 24) This imperfection could be due to concomitant emphysema. In our cohort, combined moderate to severe emphysema was seen in one-quarter of our IPF patients with HRCT, any emphysema was seen in half. Grouping patients by emphysema status improved the ability of the models to predict mortality by index of concordance analysis. These results argue that CPFE is common, and identifying CPFE is important in clarifying the appropriate prognostic measure. However, identifying patients with CPFE does not appear to be feasible with spirometry alone. Our patients with moderate/severe emphysema did not have FEV1/FVC ratios consistent with obstruction per the GOLD criteria.(22) Less than half of CPFE patients from one series, 42.6%, met GOLD criteria for COPD.(25) Therefore, accurately diagnosing CPFE may require HRCT.
The differential ability of individual measures of pulmonary function to predict mortality based on the quantity of emphysema is a novel and important finding of our study. Our hypothesis predicted that CPI would be the strongest predictor of mortality in patients with CPFE. Yet, our data highlight that change in FEV1 appeared to be the best surrogate for predicting subsequent mortality in IPF patients with moderate to severe emphysema. Interestingly, the hazard ratios associated with declining FEV1 increased with increasing emphysema on HRCT scan in a dose-dependent like fashion. FEV1was not a significant predictor in the patients without emphysema just as changes in FVC, DLCO, or CPI at 12 months were not consistently predictive of subsequent mortality in patients with moderate/severe emphysema.
In our dataset, patients who had a decline in FVC over time almost always had a decline in FEV1 as well. This fact underscores why CPI may not be an effective longitudinal measure in CPFE since FEV1 and FVC have opposite effects on the CPI: a lower FEV1 decreases the CPI, a lower FVC increases it. Therefore the CPI may remain balanced in the face of progressive obstruction and restriction. More surprising, the DLCO did not statistically predict mortality in the emphysema patients which may be due to selection bias as more severely ill patients may not be able to perform a DLCO test or increased survivorship in a small number of patients. The Cox survival curves show good separation for 4 to 5 years for both CPI and DLCO, with more separation for DLCO, but come back together with increased survivorship in a small sample.
Limitations
Important limitations of this study include the possibility of selection or referral bias, the absence of data to assess for concomitant pulmonary hypertension, and the lack of prospectively defined treatments which precludes our ability to evaluate any potential impact of treatment on outcome. The lack of available HRCT in all patients could lead to selection bias. However, our subset of patients with HRCT scans had similar clinical characteristics to the remainder of the cohort, theoretically lessening the chance that only patients with emphysema had HRCT scans available for analysis. Also, our CPFE patient population is similar to cohorts previously described in terms of baseline FEV1 and DLCO. Our patients tended to have a lower FVC and a higher percentage of women.(25–27) Another limitation is the lack of a computer generated quantitative score for the HRCT scans. The available HRCT scans spanned many years during which significant advancements were made in HRCT technology. Further prospective, quantitative HRCT information on IPF patients could provide refinement to this analysis and extend these findings. Other limitations include referral bias as complicated cases with dual diagnoses may be more likely to be referred. We do not have systematic data in the CPFE cohort to control for pulmonary hypertension or treatments received in the survival analyses. Therefore we cannot reflect on the significance that known pulmonary hypertension or a particular COPD phenotype may have in an individual patient. Also, the most consistent longitudinal findings occur at 12 month follow-up pulmonary function testing. Therefore applying our results would require that a patient be alive and able to perform the testing at 12 months.
In a large, well characterized cohort of patients with IPF an increase in CPI of at least 5 points is a meaningful predictor of subsequent mortality. Longitudinal changes in CPI were comparable to changes in FVC and DLCO unless emphysema was absent wherein CPI was superior. Combined pulmonary fibrosis and emphysema is common in patients diagnosed with IPF. FEV1 appears to be the best physiologic predictor of mortality in CPFE.
Acknowledgements
With great appreciation, we thank the patients and physicians of the University of Michigan Fibrotic Lung Disease Network. Funding for this manuscript was provided by the National Institutes for Health/National Heart, Lung and Blood Institute in the form of: T32 HL07749-17, U10 HL080371, RO1 HL091743-01, K23 HL68713-01, P50 HL56402-06, K24 HL004212 and K23 HL93351.
References
- 1.American Thoracic Society, European Respiratory Society. American thoracic society/european respiratory society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty K, Toews G, Travis W, Colby T, Kazerooni E, Gross B, Jain A, Strawderman R, III, Paine R, III, Flint A, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002;19:275–283. doi: 10.1183/09031936.02.00182002. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, King TE., Jr Demystifying idiopathic interstitial pneumonia. Arch Intern Med. 2003;163:17–29. doi: 10.1001/archinte.163.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty K, Mumford J, Murray S, Kazerooni E, Gross B, Colby T, Travis W, Flint A, Toews G, Lynch J, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 8.Collard H, King T, Bartelson B, Vourlekis J, Schwarz M, Brown K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: Prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: The prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 11.Lama V, Flaherty K, Toews G, Colby T, Travis W, Long Q, Murray S, Kazerooni E, Gross B, Lynch J, III, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 12.Fell CD, Liu LX, Motika C, Kazerooni EA, Gross BH, Travis WD, Colby TV, Murray S, Toews GB, Martinez FJ, et al. The prognostic value of cardiopulmonary exercise testing in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:402–407. doi: 10.1164/rccm.200802-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: A risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases: A concise review. Eur Respir J. 2001;17:122–132. doi: 10.1183/09031936.01.17101220. [DOI] [PubMed] [Google Scholar]
- 15.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz DA, Merchant RK, Helmers RA, Gilbert SR, Dayton CS, Hunninghake GW. The influence of cigarette smoking on lung function in patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;144:504–506. doi: 10.1164/ajrccm/144.3_Pt_1.504. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty K, Brown K, Colby T, Costabel U, Flint A, Franks TJ, Gross B, Hansell D, Hunninghake G, Kazerooni E, et al. Smoking related interstitial lung disease - an international working group report. Eur Resir J. 2006;28(supplement 50):382s. [Google Scholar]
- 18.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FEJ, KL L, DB M. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Cox D. Regression models and life tables (with discussion) J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 21.Schmidt SL, Sundaram B, Tayob N, Han MK, Nambiar A, Gross BH, Kazerooni EA, Chughtai A, Flint A, Myers J, et al. Longitudinal changes in the composite physiologic index and pulmonary function in patients with idiopathic pulmonary fibrosis and emphysema. Am J Respir Crit Care Med. 2010;181:A1122. [Google Scholar]
- 22.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Nhlbi/who global initiative for chronic obstructive lung disease (gold) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 23.Behr J, Demedts M, Buhl R, Costabel U, Dekhuijzen RP, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. Lung function in idiopathic pulmonary fibrosis--extended analyses of the ifigenia trial. Respir Res. 2009;10:101. doi: 10.1186/1465-9921-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King T, Safrin S, Starko K, Brown K, Noble P, Raghu G, Schwartz D. Analyses of efficacy end points in a controlled trial of interferon-gamma1b for idiopathic pulmonary fibrosis. Chest. 2005;127:171–177. doi: 10.1378/chest.127.1.171. [DOI] [PubMed] [Google Scholar]
- 25.Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 15:265–271. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- 26.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur Respir J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 27.Cottin V, Le Pavec J, Prevot G, Mal H, Humbert M, Simonneau G, Cordier JF. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 35:105–111. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]