Abstract
The past decade has seen an increasing shift in animal communication towards more studies that incorporate aspects of signaling in multiple modalities. Although nonhuman primates are an excellent group for studying the extent to which different aspects of condition may be signaled in different modalities, and how such information may be integrated during mate choice, very few studies of primate species have incorporated such analyses. Here, we present data from free-ranging male rhesus macaques on sex skin coloration (modeled to receiver perception), bark vocal signals, androgen levels, morphometric variables, dominance status, and female mate choice. We show that, consistent with data on females, most intra- and interindividual variation in sex skin appearance occurs in luminance rather than color. Sex skin luminance was significantly correlated across different skin regions. Sex skin luminance did not correlate with the majority of bark parameters, suggesting the potential for the two signals to convey different information. Sex skin appearance was not related to androgen levels although we found some evidence for links between androgen levels and bark parameters, several of which were also related to morphometric variables. We found no evidence that either signal was related to male dominance rank or used in female mate choice, though more direct measures of female proceptive behavior are needed. Overall, the function of male sex skin coloration in this species remains unclear. Our study is among the first nonhuman primate studies to incorporate measurements of multiple signals in multiple modalities, and we encourage other authors to incorporate such analyses into their work.
Keywords: Coloration, Luminance, Vocal signals, Multimodal, Primate, Rhesus macaques
Introduction
Over the past decade, there has been an increasing shift in the animal communication literature towards studies that incorporate multimodal aspects of signaling. This move has taken place in part in response to an increasing awareness that much animal communication is in fact multimodal in nature (Partan and Marler 1999, 2005) and has taken place mainly in well-studied animal taxa within behavioral ecology, such as frogs (Taylor et al. 2007, 2008, 2011), insects (Rowe and Guilford 1999; 2001), spiders (Hebets and Uetz 1999; Uetz and Roberts 2002; Uetz et al. 2009), lizards (Thompson et al. 2008), and birds (Partan et al. 2005; Smith and Evans 2008; Uy et al. 2009). In contrast, despite some studies of multimodal signaling in primates (Partan 2002), most research on free-ranging primates has lagged behind in its incorporation of multisensory signal elements. Recent studies have called specifically for more multimodal research on primates (Slocombe et al. 2011), but there remain few primate studies that have reported measures of different signal elements in different sensory modalities in the same manuscript and assessed signal interrelationships.
Despite this, primates are an excellent group for studying multimodal signaling. Unlike the majority of mammals, which exhibit few colorful sexually selected traits, primates exhibit an extremely vivid and colorful array of visual signals (Bradley and Mundy 2008; Higham 2009). Some of these signals have a classical position within sexual selection (see discussion by Darwin 1871, 1872, 1876), such as the pink sexual swellings of baboons (Gesquiere et al. 2007; Higham et al. 2008) and chimpanzees (Emery and Whitten 2003; Deschner et al. 2004), and the red and blue facial colors of mandrills (Setchell and Dixson 2001; Setchell and Wickings 2005). At the same time, primate signals in the auditory modality are also well described. Well-studied vocal signals include food calls (e.g., rhesus macaques Hauser 1992), the alarm calls of species such as vervet monkeys (Seyfarth et al. 1980) and Diana monkeys (Zuberbühler et al. 1997), but also signals that are almost certainly related to sexual selection, such as male loud calls (e.g., gelada, Gustison et al. 2012; crested macaques, Neumann et al. 2010) and copulation calls, which may occur in both females (e.g., Barbary macaques, Pfefferle et al. 2008a, b; Semple and McComb 2000; long-tailed macaques, Engelhardt et al. 2012; yellow baboons, Semple et al. 2002) and males (rhesus macaques, Hauser 1993; Manson 1996). Although olfactory signals are less well studied (Heymann 2006a), there is increasing evidence that they play a significant role not just in strepsirrhines such as lemurs (Boulet et al. 2009; Charpentier et al. 2010), but also in platyrrhines (Ziegler et al. 1993; Heymann 2006b) and even catarrhines (e.g., mandrills, Setchell et al. 2011; chacma baboons, Clarke et al. 2009; stump-tailed macaques, Cerda-Molina et al. 2006).
These diverse signals may stem from different selective pressures even when they appear homologous between species. For example, red coloration among primate males is often linked to social status, with higher ranked individuals expressing “redder” signals (e.g., mandrills, Setchell and Dixson 2001; drills, Marty et al. 2009; gelada, Bergman et al. 2009). This has led to an assumption that redder individuals should be more dominant almost ubiquitously across the trichromatic primates (Khan et al. 2011). However, such coloration may not always be driven by selective pressures on social status signaling, but (nonmutually exclusively) by selective pressures on attractiveness to the opposite sex. In species with strong levels of male–male competition over dominance rank and high levels of sexual dimorphism, we might expect clear signals of dominance, in order to reduce costly male–male conflict (Andersson 1994). However, in a number of other primate species in which there is lower rank-related male reproductive skew (van Noordwijk and van Schaik 2004), male characteristics other than high rank may be attractive to females. In these species, sexual dimorphism is typically less marked (Plavcan 2001), and male signals of social status aimed at reducing aggression may be less important. As male dominance rank in such species may primarily indicate group tenure length rather than physical competitiveness (e.g., in rhesus macaques Macaca mulatta, Manson 1995), females may be less able to use dominance rank as surrogate for male quality (Higham et al. 2012) and may need quality signals to assess mates. We might therefore expect signals in these species not to be a product of male–male competition and social status signaling, but more related to intersexual communication and mate choice (Dubuc et al., unpublished manuscript). As such, even seemingly similar signals such as red coloration in closely related species could actually be under different selection pressures.
Rhesus macaques are a particularly interesting primate species for studying multimodal signaling. Both males and females express red coloration in their faces and rumps (Rhodes et al. 1997; Waitt et al. 2003, 2006; Dubuc et al. 2009; Higham et al. 2010), while males additionally express red coloration on their genitals. This red coloration is related to the degree of blood flow in the area, with this increasing in response to estrogen binding at receptors in the hairless face, genitals, and rump (Rhodes et al. 1997). Experimental data on captive rhesus macaques have shown that males respond to testosterone injections with increased reddening of the face, as a result of this testosterone being aromatized to estrogen at these receptors by aromatase (Rhodes et al. 1997). These estrogen receptors are not present in other parts of the skin, which is highly consistent with a functional signaling role for sex skin coloration. Experimental studies have shown that both male and female color variation is salient to members of the opposite sex (Waitt et al. 2003, 2006; Higham et al. 2011a). Both sexes also give a wide repertoire of facial expressions and accompanying vocalizations during affiliative and aggressive interactions (Partan 2002), and males also sometimes vocalize during mating (Hauser 1993; Manson 1996). Studies of captive rhesus macaques have also shown that olfactory signals are important in this species, with female olfactory signals collected during the fertile phase seemingly sufficient to elicit male arousal independently of other cues (Michael and Keverne 1968). In the rhesus brain, cells in parts of the auditory cortex are more responsive to visual and vocal multimodal signals than to vocal signals alone, demonstrating cross-modal signal salience effects directly in the brain (Ghazanfar et al. 2005).
In the free-ranging rhesus macaque population on Cayo Santiago, Puerto Rico, newly immigrated adult males enter the dominance hierarchy at the bottom and queue for rank over time, rather than challenging the resident alpha male upon immigration (Manson 1995; van Noordwijk and van Schaik 2004). Furthermore, although high dominance rank is correlated with mating success in this population (Berard et al. 1994; Widdig et al. 2004; Dubuc et al. 2011), sexually receptive females also actively solicit and mate with middle and low ranking males, indicating that there are male characteristics other than high rank that females find attractive. We might therefore predict that male signals should be related primarily to some aspect of quality that is attractive to females, rather than to social status. For example, previous research has shown that females attend more to redder male faces, suggesting that they find them more attractive (Waitt et al. 2003).
Despite these intriguing results, there are as yet no tests of relationships between male dominance status, androgen levels, visual and vocal signals, and female mate choice in free-ranging rhesus macaques. Such questions are interesting because we still have little information about whether signals in different modalities have similar potential information content (Hebets and Papaj 2005) and are redundant (Partan and Marler 1999, 2005) or are potentially informative about different aspects of individual condition. Further, it is unclear how information extracted from multiple signals by receivers may be used to assess social status or attractiveness.
In the present study, we studied male rhesus macaques on Cayo Santiago, focusing on assessing the potential information that might be inferred from signals, rather than on signal efficacy. We collected fecal samples for the measurement of androgen levels, color-calibrated images of male faces, genitals, and rumps which we modeled to rhesus perception, recordings of male threat vocalizations (barks, sensu Rowell and Hinde 1962; Hauser et al. 1993), and behavioral data on male dominance and female mate choice. Barks are brief, noisy, broadband calls typically emitted in the context of aggressive interactions (Rowell and Hinde 1962; Hauser et al. 1993; Partan 2002). Like other vocalizations (e.g., copulation calls; Pfefferle et al. 2011), their structural modulation may depend on changes in the level of reproductive hormones. These elicit morphological changes in the organs responsible for sound production, such as the vocal cords (Gerritsma et al. 1994) and larynx, tissues known to contain receptors for sex steroids (Newman et al. 2000; Saez and Sakai 1976; Voelter et al. 2008; cf. Schneider et al. 2007). Barks are regularly uttered and we have observed them being used extensively during rare periods of direct competition over dominance rank, when they are expressed very frequently (Higham and Maestripieri 2010). As sex skin color is visible at these (and indeed all) times, these two signals can be considered as “free” (Smith 1977) parts of a multimodal display. Barks have been shown to contain information about caller anatomy (e.g., body size, Fitch 1997), and experiments have shown that rhesus macaque receivers are able to match pictures of different conspecifics of different body size to their matching calls (Ghazanfar et al. 2007). Barks are therefore salient to receivers and potentially informative for conspecifics about aspects of signaler condition.
We investigated these visual (sex skin coloration) and vocal (barks) signals in rhesus males. We predicted that, consistent with results from females (Higham et al. 2010), sex skin colors will vary primarily in achromatic (luminance) rather than chromatic (color) measures. We also predicted that color expression shown in the three different sex skin regions should all correlate, consistent with the results from the few other primate species where the color of multiple sex skin regions has been assessed (mandrills, Setchell and Dixson 2001; drills, Marty et al. 2009). We made no prediction about whether color and bark vocalizations would correlate in their expression patterns, but note that differences in expression would give the potential for different information to be signaled in different modalities. We predicted that color and vocal parameters should not be potentially informative about dominance, but should correlate with male androgen output, and that females should be more attracted to males with increased levels of signal expression, consistent with results from captive experiments (Waitt et al. 2006).
Methods
Study site and population
The study was undertaken on Cayo Santiago, a 15.2-ha island located 1 km off the East coast of Puerto Rico. A rhesus macaque colony was established on Cayo Santiago in 1938 using free-ranging individuals from India (Rawlins and Kessler 1986). The population is currently managed by the Caribbean Primate Research Center of the University of Puerto Rico. Animals range freely in naturally formed social groups, but are provisioned with commercial monkey chow. Reproduction is seasonal, with a 6-month mating season from March to August, and a 6-month birth season from September to February (Hoffman et al. 2008). Data presented in the present manuscript were collected during the 2009 mating season, from 1st June to 7th August 2009. During the study period, the population consisted of around 1,000 individuals living in six social groups. Data collection took place in group R, which consisted of an average of 268 individuals (range=243–307) (group size variation was due to births, deaths, and male emigration/immigration). We collected dominance rank data (see below for rank calculations) on all adult group males. A maximum of 44 males were found in the group at any point during the study period, but only 35 were observed to interact with other males with sufficient regularity to be included in the group's dominance hierarchy. Focal behavioral data, fecal samples, and color and vocal data were collected on a subset of 20 males (mean age 12.9 years, range 5.5–22.4). Ranks of focal males ranged from 1 to 35, representing the full range of group ranks. Males were chosen as focals on the basis of their central physical and apparent social positions in the group. Data on male ages were available for all males from long-term records.
Fecal sample collection and androgen analyses
Fecal samples were collected and analyzed from males as described elsewhere (Higham et al. 2013). Briefly, samples were collected between 7:00 and 14:30, as previous studies have shown little diurnal variation in endocrine metabolite values measured from primate feces (e.g., androgens, long-tailed macaques, Girard-Buttoz et al. 2009). During the present study period, we collected 137 fecal samples, with a mean of 7.6±0.7 (range 3–13) samples per male. Fecal samples (uncontaminated with urine) were collected from known individuals and homogenized, and a small bolus of approximately 0.5–2 g wet weight was placed in a 20-ml tube, which was in turn placed into a cooler containing ice packs (Hodges and Heistermann 2011) until frozen at −20 °C on return to the mainland. Tubes were shipped on ice to the German Primate Center; all samples arrived frozen.
Fecal samples were lyophilized and pulverized, and an aliquot (50–70 mg) of the fecal powder was extracted with 3 ml 80 % methanol by vortexing for 15 min (Heistermann et al. 1995). All fecal extracts were analyzed for androgen metabolites using an enzyme immunoassay for epiandosterone (Palme and Möstl 1994), a major metabolite of testosterone in primate feces (Möhle et al. 2002; Girard-Buttoz et al. 2009). The assay was carried out as described in detail elsewhere (Girard-Buttoz et al. 2009) and has been successfully used to monitor androgen status previously in rhesus males in the present population (Higham et al. 2013). Interassay variation, determined by repeated measurement of high and low value quality controls in each assay, was 9.8 % (high) and 16.6 % (low), while intra-assay variation was 7.1 % (high) and 8.8 % (low).
Collection and measurement of color signal data
Rhesus macaque males have three different red sex skin regions—the face, genitals, and hindquarters. We collected digital images of all three regions, captured from 1 to 3 m away from the subjects using a Canon EOS Digital Rebel XTi camera with a 10.1 megapixel CMOS censor and an EF28-135mm f/3. 5-5.6 IS USM lens. Photographs were taken in RAW format to avoid lossy compression (Stevens et al. 2007). In order to standardize images for ambient light and camera settings, we employed the “sequential method” (Higham 2006; Higham et al. 2008; Bergman and Beehner 2008; Stevens et al. 2009), in which a second photograph was taken of a GretagMacbeth ColorChecker immediately after the animal image, in the same location as the subject, using identical camera settings. Following our previous work on rhesus macaque female coloration (Higham et al. 2010; 2011a, b), we used the program ColourWorker™ (http://www.chrometrics.com) to determine the reflectance spectrum of the signal from the photographs. For the required reference spectra, we used samples of red–pink coloration: the human skin samples which are preloaded with the software, and long-tailed macaque (Macaca fascicularis), red uakari (Cacajao rubicundus), and mandrill (Mandrillus sphinx) red colors taken from http://vision.psychol.cam.ac.uk/spectra/. We obtained mean spectra from 10 reflectance spectra of each male sex skin region from each image. We calculated the quantal catch of the rhesus longwave (LW), mediumwave (MW), and shortwave (SW) cones in response to the colors, using the reflectance spectra and a standard daylight “D65” irradiance spectrum (see, e.g., Endler and Mielke 2005; Stevens et al. 2009). We used rhesus LW and MW cone data from Bowmaker et al. (1978), and substituted human SW cone (Dartnall et al. 1983) for rhesus SW cone data (the error associated with this should be very minor, see Higham et al. 2010). We then used a log form of the Vorobyev–Osorio receptor noise model (Vorobyev and Osorio 1998) to determine how different two colors are likely to appear to the rhesus visual system (Higham et al. 2010; 2011a). We used cone abundance data in the proportion of 1:16:16 (see Higham et al. 2010) and Weber fraction values of 0.08 for the SW cones and 0.02 for both the MW and LW cones (Osorio and Vorobyev 1996; Osorio et al. 2004). For luminance modeling, we used a Weber value of 0.08 and calculated luminance as (LW+ MW)/2, as luminance vision stems from a combination of the LW and MW cones in trichromatic primates (Osorio and Vorobyev 2005). The units of the model (just noticeable differences, JNDs) gives values where numbers <1 mean that two signals cannot be discriminated, values between 1 and 3 are discriminably different under good lighting, and increasing values above 3 indicate that two signals are increasingly easy to tell apart, even as light levels deteriorate (Siddiqi et al. 2004).
As the Vorobyev–Osorio receptor noise model works on comparison between two colors, for each image, we calculated a male intraindividual color and luminance score and a male interindividual color and luminance score for comparison with those of other individuals. For the intraindividual scores, we assessed for each male the lightest (maximum luminance) image and compared all others to this image, such that positive intraindividual JND scores represent increasing darkness. For scores used in interindividual analyses, we compared each male image to the lightest image displayed by any male in the whole dataset. These comparisons were made because the model produces values that represent a comparison between two colors, and so should be considered simply as a scaling process. Maximum lightness was used as prior results in females indicated that facial “reddening” during ovulation actually represents darkening of the face from a lighter phase (Higham et al. 2010).
Collection and measurement of vocal signal data
We recorded barks (Rowell and Hinde 1962) ad libitum from males using a Marantz PMD 661 Professional Portable Solid State Flash Card Recorder (D & M Professional, Longford, UK) and a Sennheiser directional microphone (Sennheiser, Wedemark, Germany; K6 power module with Rycote Modular Windshield System and a Rycote Windjammer, Rycote Ltd., Stroud, UK). Since the rhesus macaques on Cayo Santiago are well habituated, we could make recordings at very close distances (mean ± SD, 1.79±1.05 m). We recorded and transferred calls to a computer at 16 bit accuracy and a sampling frequency of 44.1 kHz. For analyses, we used data only from eight males from whom we had a minimum of 5 (range 5–8, mean 6.38±0.32) different high-quality recordings undisturbed by background noise.
We first calculated the temporal call parameter duration by determining the start and end of each call based on its spectrogram view. In order to achieve better frequency resolution for spectral analyses, we subsequently reduced the sampling frequency of the calls to 22.05 kHz (corresponding frequency range 11.025 kHz) using the program Avisoft SASLab Pro 3. 92. (R. Specht, Berlin, Germany). Since barks are noisy, they are not suitable for measuring fundamental frequencies, but their broadband nature makes them well suited for formant frequency measurements (Fitch 1997). Formants are resonance frequencies of the vocal tract. Their number and locations depend on the callers’ vocal tract length, with individuals having shorter vocal tracts (smaller animals) producing fewer formants than individuals with longer ones (bigger individuals). We determined format frequencies by linear predictive coding (LPC) using the program Speechstation2 (Sensimetrics, Somerville, MA, USA). We used a 512-point Hamming analysis window and pre-emphasis filter. All LPC measurements were checked by superimposing them on a corresponding 512-point FFT of the same time slice, allowing the empirical determination of the optimum number of formants for each call. We subsequently used the locations of the formants to calculate the minimum formant spacing (ΔF, see Reby and McComb 2003). We fitted a linear regression line with an intercept equal to 0 to the set of observed formant locations plotted against (2i–1)/2, with i indicating the number of formant measured. The slope of the regression line indicates the minimum formant spacing (Reby and McComb 2003). Frequencies below 50 Hz resample background noise and so this was used as a threshold cutoff, with frequencies below this not included in calculations. The start and end points of the call were determined by assessing all parts of each call segment for their amplitude relative to the mean maximum amplitude of all time segments in that call. A start/end threshold of 10 % was then used, such that call elements registering less than 10 % of the maximum amplitude were excluded from calculations.
To describe calls further, we ran a fast Fourier transformation (FFT length 1,024 pt, time step 5 ms, frequency range 11. 025 kHz, frequency resolution 21.6 Hz) and submitted the resulting frequency time spectra to the custom software program LMA 2010, extracting different sets of call parameters from acoustic signals (Hammerschmidt 1990). We focused on variables that describe the distribution of the amplitudes in the frequency spectrum (DFA) and the location of the dominant frequency bands (DFB). We first determined the overall amplitude (energy) for each time segment and assessed the mean frequency at which the distribution of the amplitude in the frequency spectrum reached the first (DFA1) and second quartiles (DFA2) of the total value. We then calculated parameters that describe the first two DFBs. The DFBs are characterized by amplitudes that exceed a given threshold in a consecutive number of frequency bins. In addition to DFAs and DFBs, we calculated the frequency range, which is the distance between the minimum and maximum frequency in a call. For a description of the algorithms used, see Hammerschmidt (1990) and Schrader and Hammerschmidt (1997). A schematic illustration describing DFA and DFB can be found in Pfefferle et al. (2007).
We took the mean scores for each male from these barks for the following variables: the frequency range (min, mean, max), the frequency of the first two dominant frequency bands (DFB1: min, median, mean, max; DFB2: min, median, mean, max), and the frequency at the first and second quartiles of the total amplitude in the frequency spectrum (DFA1: min, median, mean, max; DFA2: min, median, mean, max). We checked that these measures were suitable for use in parametric principal component analysis (PCA) and found that all did not significantly differ from a normal distribution (Shapiro–Wilks tests, all p>0.05). We then explored the relationships between these multiple measures using principal component analysis. PCA using all variables produced multiple component axes that explained significant (eigenvalues >1) portions of the overall variance. However, these axes were difficult to interpret, with each one loading with multiple measures across (rather than merely within) the different DFA and DFB types. Five separate PCAs were therefore undertaken on the different measures (min, median, mean, max) of frequency range, DFB1, DFB2, DFA1, and DFA2. Four of the PCAs produced one axis which loaded strongly for all constituents. The DFA1 PCA produced two axes, with the second axis primarily representing DFA1 min. This second axis was ignored and the actual DFA1 min value used instead. The component scores of these five PCA axes were utilized in all analyses and from hereon are referred to as frequency range, DFB1, DFB2, DFA1 (overall), and DFA2. These PCA axes were not significantly correlated with each other (Spearman's correlations, all p>0.05). In addition to these five axes, we assessed the DF1 min value, ΔF, and call duration.
Collection of morphometric data
As reported elsewhere (Higham et al. 2011b), between 22nd January and 24th February 2009, 12 of the males that were focal individuals during the present study period were captured during the annual colony trapping procedures. Trained staff members captured males between 8:30 and 11:00 in a 100-m2 feeding corral provisioned with monkey chow, netting or capturing the monkeys by hand, transferring them to a holding cage (0.62×0.42×0.62 m), and then on to a field laboratory where they remained overnight. The following morning, veterinary technicians anesthetized the males with ketamine (approximately 10 mg/kg via IM injection), and we weighed the anesthetized males in a standard hanging scale. Using a 1-m ruler with 1 mm gradations, we measured crown-rump length (hereafter body length) of each male in a standardized position with his back fully straight. We calculated body mass index (BMI) for each individual by dividing mass (kilogram) by body length squared (square meter) (Campbell and Gerald 2004). This gave us three morphometric variables for analysis in the present manuscript: mass, BMI, and body length.
Collection of behavioral data
We undertook 30-min continuous focal observations of adult male behavior collected using Behaviour software loaded on to Psion hand-held Workabout hardware. From a wider ethogram, we here present data on mating behaviors, recorded as copulation, and ejaculatory copulation (considered separately, since rhesus macaques are serial mount ejaculators). During observations, we also documented the presence of consortships between males and females, which we defined as occurring when males and females consistently stayed within close (<2 m) association, with exclusive mating (see a similar definition in Berard et al. 1994). We also recorded the presence of all individuals within a 2-m and 5-m radius around the focal male at 5-min intervals using instantaneous sampling. Observations for each male were balanced between two time blocks, from 7:00 to 10:00 and from 10:00 to 14:30, such that each male was followed at least twice within each time block each week. The total number of 30-min focal observations included here is 631 (mean 31.5±0.3 observations per male, range 28–33). Data cannot usually be collected before 7:00 or after 14:30 on Cayo Santiago because of restrictions on access to the island.
Data were parsed into Access (Microsoft Corp., Redmond, WA, USA), and Access queries were then used to generate behavioral rates and durations. Behavioral data were analyzed to assess male attractiveness to females. Variables analyzed were as follows: rate of copulation and rate of ejaculatory copulation (separately, both as N per hour of observation), total time spent in consortship (as percentage of observation time), and mean number of adult and subadult females found within 2 m and within 5 m.
Calculation of dominance rank
During the 2009 mating season, a period of rank instability began at the start of June, which resulted in several high ranking males being attacked repeatedly over the course of the following weeks by coalitions of subordinates (Higham and Maestripieri 2010). As such, the data presented in this study (data collection; June–August) represent a period of relative rank instability. For the present study, we used a dominance hierarchy that included data only from July and early August, so ignoring data from June, during which most instability occurred (a hierarchy previously described and used in Higham et al. 2011b and referred to there as the “end of mating season” hierarchy). Dominance ranks were assigned on the basis of the following “winner–loser” agonistic interactions involving all adult (≥5.5 years) males: fear grins (winner is the individual grinned at); avoidance (where one individual moves out of the way of an approaching individual; winner is the individual avoided); displacements (winner is the displacer); and threats, chases, and lunges (winner is the aggressor in all cases). We compiled interactions into a winner–loser matrix, and used Matman 1.1 (Noldus) to create our hierarchy. Following 10,000 iterations, a significant linear hierarchy was produced (linearity test using Landau's linearity index corrected for unknown relationships, p=0.049), which was highly directionally consistent (DCI=0.93).
Statistical analyses
Following initial analyses, we focused on luminance and not color measures (see “Results”). To assess intraindividual relationships between the luminance of each sex skin area (i.e., whether intraindividual lightening or darkening in one area was indicative of lightening or darkening in the others), we first undertook three Spearman's correlations on each male separately for facial–genital luminance, facial–rump luminance, and genital–rump luminance. Three one-sample t tests were then used to compare the coefficients of these correlations for all males to an expected value of zero (predicted under the null hypothesis that there is no relationship between the intraindividual luminance scores of each sex skin). To assess relationships between signal expression in different sex skin regions, we undertook Pearson's correlations on male mean values. For relationships between luminance measures, as we had repeated measures of the same individuals over time, we also undertook two linear mixed models (LMMs), with genital and then facial luminance as dependent variables in each model and the other two variables included as fixed effects. Male ID was included as a random effect in all models to control for multiple observations of the same individuals. As sample sizes were small for vocal parameters, we then undertook Spearman's correlations to compare male mean values for vocal parameters with each sex skin region.
To assess relationships between mean androgen levels, morphometric variables, age and luminance measures, three general linear models (GLMs) were undertaken, one for each sex skin region. As we had multiple values from each male for sex skin luminance and androgen levels, we also undertook three LMMs (one for each sex skin region) utilizing all luminance and androgen values, with male ID as a random effect in all models to control for multiple observations of the same individual. Spearman's correlations were used to assess relationships between vocal measures and potentially predictive mean androgen levels and morphometric measures separately. To assess relationships between luminance and vocal measures and male dominance rank, Spearman's correlations were used (as rank is an ordinal variable and rank-based statistics are most appropriate). Finally, to assess relationships between our behavioral measures and luminance measures, we undertook partial correlations controlling for dominance rank. This enabled us to test whether, e.g., males who had darker faces than expected for a given rank spent more time in consortship with females than expected for a given rank. Spearman's correlations that did not control for dominance rank were used for vocal measures due to small sample sizes. All statistics were undertaken in SPSS version 18.0, with p<0.05 considered significant.
Results
Intersignal relationships
Visual modeling revealed some perceptual variation in male coloration, but most perceptual variation was in male luminance, with males being lighter vs darker rather than varying in color per se (Table 1). Across each sex skin region, some males showed as little variation as 0.5 JNDs in color from their own individual maximum, while all males showed at least ≈10 JNDs darkening from their individual maximum lightness value in all three regions. Of all regions, there was the greatest amount of intra- and interindividual variation in genital coloration. In this region, males showed up to 7.2 color JNDs and 50.9 luminance JNDs variation from their own individual maximum and up to 6.6 color JNDs and 67. 8 luminance JNDs variation from the “all male” maximum (Table 1). Given the marked variation in luminance scores and relatively small to imperceptible differences in color scores, we used the luminance measures only in the remaining analyses.
Table 1.
For each sex skin region, measures of the maximum JND variation that males showed from their own (intraindividual) and the all male (interindividual) maximum lightness images. Values presented represent the mean (with SEM) maximum variation (i.e., the average maximum JND score showed across all males), as well as the min and max JND scores showed by any male. Note that each image in the dataset was compared to the image of maximum lightness (of that male for intraindividual analyses and of all males for interindividual analyses). However, this scaling image may not necessarily be the least colorful image. As such, though luminance measures must necessarily always be higher for interindividual measures when compared to intraindividual measures, this is not the case for color measures
| Face | Rump | Genitals | ||
|---|---|---|---|---|
| Intraindividual color variation (JNDs) | x̄ | 1.5 | 1.7 | 2.4 |
| SEM | 0.1 | 0.2 | 0.4 | |
| Min | 0.5 | 0.8 | 0.7 | |
| Max | 2.3 | 3.2 | 7.2 | |
| Intraindividual luminance variation (JNDs) | x̄ | 17.5 | 19.3 | 27.1 |
| SEM | 1.4 | 1.6 | 2.4 | |
| Min | 10.1 | 9.9 | 14.3 | |
| Max | 34.2 | 36.5 | 50.9 | |
| Interindividual color variation (JNDs) | x̄ | 1.9 | 2.4 | 2.1 |
| SEM | 0.1 | 0.1 | 0.4 | |
| Min | 1.4 | 1.4 | 0.6 | |
| Max | 2.5 | 3.1 | 6.6 | |
| Interindividual luminance variation (JNDs) | x̄ | 28.2 | 28.2 | 39.2 |
| SEM | 1.5 | 1.4 | 2.1 | |
| Min | 18.8 | 18.9 | 25.4 | |
| Max | 40.4 | 46.4 | 67.8 |
Intraindividual changes in luminance in each sex skin area were significant positively correlated overall, such that when a male darkened or lightened in one area, he darkened and lightened in another too (one sample t tests on male correlation coefficients, male facial and genital luminance, t19=2.778, p=0.012; facial and rump luminance, t19=2.117, p = 0.048; genital and rump luminance, t19 = 2.282, p=0.034). Pearson's correlations on male mean values found that male facial and rump luminance were not correlated (r=0.221, n=20, p=0.349) and that there were nonsignificant trends for mean male facial and genital luminance (r=0.384, n=20, p=0.095) and mean male genital and rump luminance (r=0.385, n=20, p=0.094). However, results of an LMM found the relationship between genital luminance (dependent variable) and facial (F1, 165.6 =20.0, p<0.001) and rump luminance (F1, 143.6 =8.6, p=0.004) significant. In a second LMM with facial luminance as the dependent variables, again no relationship between facial and rump luminance was found (p>0.1), but the significant relationship between both facial and genital luminance was confirmed.
Spearman's correlations showed that male mean genital luminance was significantly negatively correlated with DFA1 min, such that males with darker genitals had lower minimum frequencies (r=−0.810, n=8, p=0.015). There was also a nonsignificant trend between male facial luminance and frequency range (r=0.667, n=8, p=0.071), such that there was a trend towards males with darker faces having larger frequency ranges. There were no other significant relationships between vocal and luminance measures (all p>0.1).
Hormonal and morphometric correlates of signals
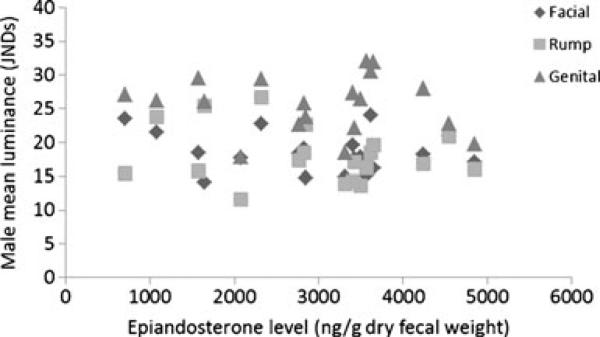
Three GLMs (one for each sex skin region) found no significant relationships between mean male facial, genital, and rump luminance and any morphometric measure (mass, BMI, body length, all p>0.1), age (all p>0.1) or mean androgen levels (all p>0.1, Fig. 1). Three LMMs (one for each sex skin region) utilizing all image values and all corresponding androgen values also found no significant relationships between androgen levels and luminance measures (all p>0.1).
Fig. 1.
Male mean luminance JNDs against androgen (epiandosterone) levels. No significant correlation between the two variables was found
For vocal measures, Spearman's correlations for androgen levels showed positive correlation coefficients for all scores, with a significant positive relationship between androgens levels and DFB1 (r=0.762, n=8, p=0.028). Body mass showed a strong significant negative correlation with ΔF, with males of increasing mass showing lower values (r=;0.943, n=6, p=0.005). BMI showed a significant positive correlation with DFB2 (r=0.886, n=6, p=0.019) and a nonsignificant positive trend with DFB1 (r=0.771, n=6, p=0.072). Body length showed a significant negative correlation with frequency range, with shorter males showing longer frequency ranges (r=−0.829, n=6, p=0.042), and a nonsignificant negative trend with DFA2 (r=−0.771, n=6, p=0.072). There were no significant relationships between male age and vocal measures (all p>0.1).
Male dominance, mating success, and signal expression
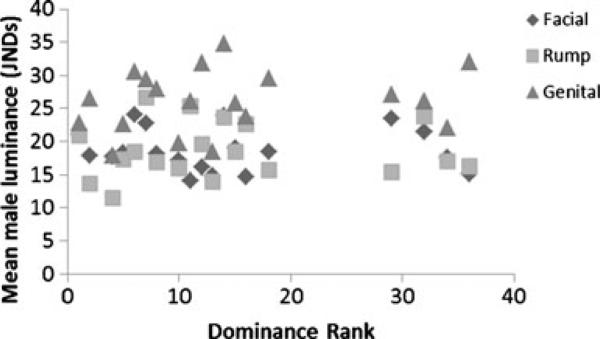
Male dominance rank was uncorrelated with sex skin luminance measures (all p>0.1; Fig. 2). Similarly, no vocal measure was correlated with male dominance rank, although there was a close to significant trend between male dominance rank and DFB1, such that males of higher rank tended towards higher frequencies (r=−0.690, n=8, p=0.058).
Fig. 2.
Male mean luminance JNDs against dominance rank. No significant correlation between the two variables was found
Partial correlations controlling for male dominance rank found no significant differences in male mean consort time, copulation rates, or rates of ejaculatory copulations and facial, genital, or rump luminance measures (all p>0.1). Similarly, partial correlations found that the total number of mating partners and the average number of females within 2 m and within 5 m were not related to male luminance measures (all p>0.1). No behavioral measures were correlated with vocal measures in Spearman's correlations (all p>0.1).
Discussion
To our knowledge, our study is the first of a primate species to report the expression of multiple signals in differing modalities with respect to their potential underlying hormonal correlates and to behavior and morphometric variables. Our focus was on signal function and the potential information that might be inferred from signals, rather than on signal form (efficacy). We found a number of significant relationships between signal expression in different sex skin regions, but limited evidence for intersignal covariance in different modalities. We also found no evidence for relationships between androgen levels and luminance levels, although vocal parameters were associated with both androgen levels and a number of morphometric variables.
Very few studies to date have measured primate color using a visual discrimination model, and our study provides further evidence that in rhesus macaques, both intra- and interindividual changes in red coloration occur primarily in luminance (achromatic variation) rather than color (chromatic variation). This is consistent with our previous findings in female rhesus macaques (Higham et al. 2010) and with the notion that, in this species, color primarily varies on an axis from light pink to dark red. This is also consistent with our understanding of how such color expression mechanisms work, with “color” change being associated with an increase or decrease in the degree of saturation with oxygenated blood. This oxygenated blood itself does not change in color to a large degree, but this change in saturation appears to affect the lightness–darkness of the area. Reflectance spectra were similar to those obtained from females and revealed this change in lightening and darkening visually (see examples in Higham et al. 2010, Supplementary Fig. 1).
Rhesus macaques, like a number of other primate species, exhibit color signals in different regions of the body. In most cases, it remains unclear whether colors in these multiple areas correlate and so potentially convey information about similar aspects of condition (have similar content, Hebets and Papaj 2005), or whether they do not. In the only previous studies to assess this, red coloration in different regions of the body was shown to correlate between the face and genitals (mandrills, Setchell and Dixson 2001; drills, Marty et al. 2009). Our study confirmed these findings, with facial and genital and genital and rump luminance values correlated both within and between individuals, and facial and rump luminance values correlated within but not between individuals. This result was also found in females, with no correlation between facial and rump luminance, and facial but not rump luminance indicating ovulation (Higham et al. 2010). In males, this result leaves some scope for face and rump luminance to contain different information too, though we found generally strong correlations between luminance scores of sex skin regions. One potential proximate reason for weak relationships between facial and rump luminance may be that the highly terrestrial Cayo Santiago rhesus macaques spend much of their time sitting on their rump, which may affect blood flow and saturation and hence luminance levels. Nonetheless, if indicating rump colors similar in appearance to those shown in the face was important, we would expect some compensation mechanism from signalers. It is also worth noting that variation in genital color and luminance is much more marked than that of the other areas. This could indicate increased selection for signal expression in this particular area, and it is worth noting that a range of catarrhines exhibit coloration specifically in this area (e.g., mandrills, Setchell and Dixson 2001; drills, Marty et al. 2009; vervet monkeys, Gerald 2001).
Although there were some weak relationships between luminance and vocal measures, with a significant correlation between genital luminance and minimum frequencies in DFA1, we found no compelling evidence for consistent relationships between signal expression in the two different modalities. This is interesting because it gives the potential for different signals to indicate different aspects of condition. However, the small sample sizes for vocal parameters should certainly be borne in mind. Although sex skin visual signals were analyzed using a visual model, whereas vocal parameters were measured using objective assessments of call parameters, this should not matter for analyses such as those presented here—either expression changes correlate or they do not, however, they are measured. However, more generally, there are many questions related to signal detectability where some measure of the likely biological relevance of acoustic signal variation would clearly be preferable to objective parameters, and methods for the analysis and presentation of animal vocalizations in perceptually meaningful units would be welcome.
Our study found no relationships between androgen levels and male luminance measures, but found some significant and close to significant relationships between androgen levels and vocal measures. There were also some relationships between vocal measures and morphometrics, with shorter males exhibiting larger frequency ranges, and males with higher BMIs displaying higher frequencies in the first (nonsignificant trend) and second (significant) dominant frequency bands. Additionally, we found a relationship between body mass and formant spacing, with males of bigger mass having smaller spacing. Formant spacing depends on tissue structure and the shape and length of the vocal tract (Fant 1960), which is fixed to the skull and therefore closely tied to overall body size and mass (e.g., Pfefferle and Fischer 2006; Fitch 1997). These findings give the potential for barks to be informative to other individuals about important physical aspects of condition. Experimental evidence shows that rhesus macaque receivers can match pictures of conspecifics of different body size to their calls, suggesting that such information genuinely is salient and useful (Ghazanfar et al. 2007). Collectively, these results further highlight the potential of vocal and color signals to indicate different aspects of condition to conspecifics. However, sample sizes for the vocal analyses are very small and multiple parameters were tested. Given these two problems, if corrections for multiple testing are undertaken, the vocal results would not remain significant. However, the consistency of the results with prior evidence (e.g., Fitch 1997) gives some confidence that they are not artifacts created by such issues.
The absence of significant relationships between androgen and sex skin luminance levels is curious at first glance, given that previous studies have demonstrated that artificial administration of testosterone causes increased reddening in rhesus macaques (Rhodes et al. 1997). One possible mechanism that could explain our results is tissue-specific expression in receptor sensitivity (in this case, sex skin region-specific expression). Tissue-specific gene expression seems likely to play a key role in many primate signals and may help to explain in particular how color signals function in species where males compete strongly over dominance. In the most sexually dimorphic of all primate species, where male–male competition for social status is strongest, red coloration appears to have evolved as a badge of status at least twice independently, with higher ranked males showing stronger color expression (genus Mandrillus, Setchell and Dixson 2001; Setchell and Wickings 2005; Marty et al. 2009; genus Theropithecus, Bergman et al. 2009). (Note, however, that this is not the case in rhesus macaques, as demonstrated in the present study.) Interestingly, studies of both mandrills and gelada have shown that subordinate males who defeat and usurp higher ranked individuals do not have stronger coloration before the challenge, but subsequently become more colorful, while deposed males lose their color (mandrills, Setchell and Dixson 2001; Setchell and Wickings 2005; gelada, Bergman et al. 2009). Challenger males show increased aggressive behavior leading up to their challenge (Bergman et al. 2009), which presumably is associated with an increase in testosterone (Dixson 2012) without an increase in red color expression. This dissociation between testosterone levels and status signal color in free-ranging primates could theoretically be related to changes in receptor expression in the sex skin, with sensitivity downgraded as androgen levels are increased in the absence of an increase in social status. This may be key in allowing males to undergo the endocrine changes necessary for increasing behavioral aggression (increased testosterone) without this necessarily altering signal expression and would allow coloration to be tied strictly to social status independently of circulating hormone levels. A recent study of female rhesus macaques has shown that gene expression is highly sensitive to changes in social status, such that expression patterns of functionally relevant genes such as androgen and glucocorticoid receptors respond quickly to experimentally assigned dominance ranks (Tung et al. 2012). Administration of testosterone to individuals exhibiting certain levels of receptor expression typically results in sex skin flushing, as experimentally demonstrated in rhesus macaques (Rhodes et al. 1997) and drills (Zuckerman and Parkes 1939). Without measuring both interindividual differences in androgen levels and receptor sensitivities in the relevant tissue simultaneously, it may be that studies of free-ranging primates often fail to find relationships between androgen levels and color signal expression.
We did not predict significant relationships between male coloration and dominance. It is worth bearing in mind that data were analyzed with respect to the hierarchy formed at the end of the mating season and that dominance data from June (part of the period of data collection) were not included in the present rank assessment due to a period of dominance instability. However, we have worked on this population for a number of years and have often observed that the high ranking males are not those with the darkest faces. We have also worked on other species exhibiting strong direct contest competition over dominance, and where a correlation between male coloration and dominance seems clear (e.g., drills, Marty et al. 2009; crested macaques, unpublished data). However, the absence of such a relationship in rhesus macaques was unsurprising to us not only from our prior observations, but also because male rhesus macaques experience less direct male–male competition over social status and access to mates compared to many other promiscuous primates. This is indicated by low sexual dimorphism in body size and in weaponry such as canine size (Plavcan 2001), in addition to behavioral evidence that newly immigrated males typically queue rather than fight over dominance (e.g., Manson 1995). If males do not compete directly over dominance, then it seems unlikely that signals of dominance would be likely to evolve. Instead of a role in dominance signaling, we hypothesized that coloration may play a role in mate choice by potentially conveying information about other (nondominance related) aspects of male condition. Male coloration differences may allow females, who are able to exert a relatively high degree of mate choice in this species, to select preferred males in the absence of a strong correlation between male dominance and competiveness (Higham et al. 2012; Dubuc et al., unpublished manuscript). Although we found no evidence that females preferred darker males, more detailed data on female proceptive behavior towards males, and controlled experiments in which color-calibrated stimuli of males are shown to females (Waitt et al. 2003), are needed to assess the potential role of coloration in mediating male attractiveness to females. Until such further assessments take place, the function of sex skin coloration in this species remains unclear.
Previous research on the evolutionary and behavioral ecology of primate signals has focused on specific signals in specific modalities, such as sexual swellings (visual) or copulation calls (aural). A shift in the broader communication literature towards studies of multiple signals in multiple modalities in other taxa has led to increased calls for multi-modal research on primates (Slocombe et al. 2011). For most primate species, there are multiple potential signals of social status and other aspects of individual condition that could be used in mate choice, but very few studies have attempted to assess the potential information content of such signals, and how this information may be used by receivers. Unfortunately, our small sample sizes for vocal parameters did not enable us to investigate the role that the interaction between the two signals may play in decision-making. Ideally, studies would include truly multimodal analyses where the effects of each signal separately, as well as their interaction during multimodal displays, could be assessed. These interaction effects could be determined experimentally (by presenting each signal separately and then together) or through observational study by the incorporation of both signal terms separately as well as their interaction term into the same model. Hopefully, our study will encourage further exploration of the relationships between signals in multiple modalities, their underlying endocrine determinants, and the way in which they are used by conspecifics in decision-making.
Acknowledgments
We thank Doreen Hess, Jenna Goldfein, Maria Rakhovskaya, and the staff of the Caribbean Primate Research Center for logistical support in the field and assistance with animal capturing and handling. We are extremely grateful to Andrea Heistermann and Petra Kiesel for analyzing the fecal samples and to Tara Mandalaywala and the Caribbean Primate Research Center for assisting with their transportation to Germany. We are also most grateful to John Addicott for first helping us to develop our behavioral data parser and Access database. We additionally thank Kurt Hammerschmidt for making his LMA sound analysis program available. Eileen Hebets and two anonymous reviewers provided constructive and helpful comments on the manuscript. This research was supported by NIH grant R21-AG029862 to D.M. M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1). This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Present Address: M. Stevens Centre for Ecology & Conservation, University of Exeter, Cornwall Campus, Penryn TR10 9EZ, UK
This manuscript is part of the special issue Multimodal Communication—Guest Editors: James P. Higham and Eileen A. Hebets.
Ethical standards This study was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and conformed to all laws of Puerto Rico, the USA, and Germany. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
James P. Higham, Institute for Mind and Biology, University of Chicago, 940 East 57th Street, Chicago, IL 60637, USA Department of Anthropology, New York University, 25 Waverly Place, New York, NY 10003, USA.
Dana Pfefferle, Behavioural Ecology Research Group, Institute of Biology, University of Leipzig, Leipzig, Germany; Junior Research Group of Primate Kin Selection, Department of Primatology, Max-Planck Institute for Evolutionary Anthropology, Leipzig, Germany.
Michael Heistermann, Endocrinology Laboratory, German Primate Centre, Kellnerweg 4, 37077 Göttingen, Germany.
Dario Maestripieri, Institute for Mind and Biology, University of Chicago, 940 East 57th Street, Chicago, IL 60637, USA.
Martin Stevens, Department of Zoology, University of Cambridge, Downing Street, Cambridge CB2 3EJ, UK.
References
- Andersson M. Sexual selection. Princeton University Press; Princeton: 1994. [Google Scholar]
- Berard JD, Nürnberg P, Epplen JT, Schmidtke J. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 1994;129:177–201. [Google Scholar]
- Bergman TJ, Beehner JC. A simple method for measuring colour in wild animals: validation and use on chest patch colour in geladas (Theropithecus gelada). Biol J Linn Soc. 2008;94:231–240. [Google Scholar]
- Bergman TJ, Ho L, Beehner JC. Chest color and social status in male geladas (Theropithecus gelada). Int J Primatol. 2009;30:791–806. [Google Scholar]
- Boulet M, Charpentier MJE, Drea CM. Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in primates. BMC Evol Biol. 2009;9:281. doi: 10.1186/1471-2148-9-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK, Dartnall HJA, Lythgoe JN, Mollon JD. The visual pigments of rods and cones in the rhesus monkey Macaca mulatta. J Physiol. 1978;274:329–348. doi: 10.1113/jphysiol.1978.sp012151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BJ, Mundy NI. The primate palette: the evolution of primate coloration. Evol Anthropol. 2008;17:97–111. [Google Scholar]
- Campbell BC, Gerald MS. Body composition, age and fertility among free-ranging female rhesus macaques (Macaca mulatta). J Med Primatol. 2004;33:70–77. doi: 10.1111/j.1600-0684.2004.00055.x. [DOI] [PubMed] [Google Scholar]
- Cerda-Molina AL, Hernández-López L, Rojas-Maya S, Murcia-mejía C, Mondragón-Ceballos R. Male-induced sociosexual behavior by vaginal secretions in Macaca arctoides. Int J Primatol. 2006;27:791–807. [Google Scholar]
- Charpentier MJE, Crawford J, Boulet M, Drea CM. Message‘scent’: lemurs detect the genetic relatedness and quality of con-specifics via olfactory cues. Anim Behav. 2010;80:101–108. [Google Scholar]
- Clarke PM, Barrett L, Henzi SP. What role do olfactory cues play in baboon mating? Am J Primatol. 2009;71:493–502. doi: 10.1002/ajp.20678. [DOI] [PubMed] [Google Scholar]
- Dartnall HJA, Bowmaker JK, Mollon JD. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B. 1983;220:115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. John Murray; London: 1871. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. John Murray; London: 1872. [Google Scholar]
- Darwin C. Sexual selection in relation to monkeys. Nature. 1876;15:18–19. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation and male behavior in wild West African chimpanzees. Horm Behav. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Primate sexuality: comparative studies of prosimians, monkeys, apes, and human beings. 2nd edn Oxford University Press; Oxford: 2012. [Google Scholar]
- Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. Sexual skin color contains information about the timing of the fertile phase in free-ranging rhesus macaques. Int J Primatol. 2009;30:777–789. [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. Testing the priority-of-access model in a seasonally breeding primate species Macaca mulatta). Behav Ecol Sociobiol. 2011;65:1615–1627. doi: 10.1007/s00265-011-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery MA, Whitten PL. Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes). Behav Ecol Sociobiol. 2003;54:340–351. [Google Scholar]
- Endler JA, Mielke PW. Comparing entire colour patterns as birds see them. Biol J Linn Soc. 2005;86:405–431. [Google Scholar]
- Engelhardt A, Fischer J, Neumann C, Pfeifer J-B, Heistermann M. Information content of female copulation calls in wild long-tailed macaques (Macaca fascicularis). Behav Ecol Sociobiol. 2012;66:121–134. doi: 10.1007/s00265-011-1260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant G. Acoustic theory of speech production. Mouton & Co; The Hague: 1960. [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Gerald MS. Primate colour predicts social status and aggressive outcome. Anim Behav. 2001;61:559–566. [Google Scholar]
- Gerritsma EJ, Brocaar MP, Hakkesteegt MM, Birkenhager JC. Virilization of the voice in postmenopausal women due to the anabolic-steroid Nandrolone Decanoate (Decadurabolin)—the effects of medication for one-year. Clin Otolaryngol. 1994;19:79–84. doi: 10.1111/j.1365-2273.1994.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in primate auditory cortex. Journal of Neuroscience. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Turesson HK, Maier JX, van Dinther R, Patterson RD, Logothetis NK. Vocal tract resonances as indexical cues in rhesus monkeys. Curr Biol. 2007;17:425–430. doi: 10.1016/j.cub.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis). Physiol Behav. 2009;98:168–175. doi: 10.1016/j.physbeh.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Gustison ML, le Roux A, Bergman TJ. Derived vocalisations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Philos Trans R Soc B. 2012;367:1847–1859. doi: 10.1098/rstb.2011.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K. Individuelle Lautmuster bei Berberaffen (Macaca sylvanus): Ein Ansatz zum Verständnis ihrer vokalen Kommunikation. Diss Freie Universtät; Berlin: 1990. [Google Scholar]
- Hauser MD. Costs of deception: cheaters are punished in rhesus monkeys (Macaca mulatta). P Natl Acad Sci U S A. 1992;89:12137–12139. doi: 10.1073/pnas.89.24.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD. Rhesus monkey copulation calls: honest signals for female choice? Proc R Soc Lond B. 1993;254:93–96. doi: 10.1098/rspb.1993.0132. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Evans CS, Marler P. The role of articulation in the production of rhesus monkey, Macaca mulatta, vocalizations. Anim Behav. 1993;45:423–433. [Google Scholar]
- Hebets EA, Papaj DR. Complex signal function: developing a testable framework for testable hypotheses. Behav Ecol Sociobiol. 2005;57:197–214. [Google Scholar]
- Hebets EA, Uetz GW. Female responses to isolated signals from multimodal male courtship displays in the wolf spider genus Schizocosa (Araneae: Lycosidae). Anim Behav. 1999;57:865–872. doi: 10.1006/anbe.1998.1048. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Finke M, Hodges JK. Assessment of female reproductive status in captive-housed Hanuman langurs (Presbytis entellus) by measurement of urinary and fecal steroid excretion patterns. Am J Primatol. 1995;37:275–284. doi: 10.1002/ajp.1350370402. [DOI] [PubMed] [Google Scholar]
- Heymann EW. Introduction: the neglected sense—olfaction in primate behaviour, ecology, and evolution. Am J Primatol. 2006a;68:519–524. doi: 10.1002/ajp.20249. [DOI] [PubMed] [Google Scholar]
- Heymann EW. Scent marking strategies of New World primates. Am J Primatol. 2006b;68:650–661. doi: 10.1002/ajp.20258. [DOI] [PubMed] [Google Scholar]
- Higham JP. PhD thesis. Roehampton University; London: 2006. The reproductive ecology of female olive baboons (Papio hamdryas anubis) at Gashaka-Gumti National Park, Nigeria. [Google Scholar]
- Higham JP. Primate coloration: an introduction to the special issue. Int J Primatol. 2009;30:749–751. [Google Scholar]
- Higham JP, Maestripieri D. Revolutionary coalitions in male rhesus macaques. Behaviour. 2010;147:1889–1908. [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: Information content of size and color. Horm Behav. 2008;53:452–462. doi: 10.1016/j.yhbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Higham JP, Brent LJN, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, Heistermann M, Stevens M. Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behav Ecol. 2010;21:739–746. doi: 10.1093/beheco/arq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJN, Dubuc C, Engelhardt A, Heistermann M, Maestripieri D, Santos LR, Stevens M. Familiarity affects assessment of facial signals of female fertility by free-ranging male rhesus macaques. Proc R Soc Lond B. 2011a;278:3452–3458. doi: 10.1098/rspb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. The energetics of male–male endurance rivalry in rhesus macaques. Anim Behav. 2011b;81:1001–1007. [Google Scholar]
- Higham JP, Heistermann M, Saggau C, Agil M, Perwitasari-Farajallah D, Engelhardt A. Sexual signaling in the crested macaque and the evolution of primate fertility signals. BMC Evol Biol. 2012;12:89. doi: 10.1186/1471-2148-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. The endocrinology of male rhesus macaque social and reproductive status: a test of the challenge and social stress hypotheses. Behav Ecol Sociobiol. 2013;67:19–30. doi: 10.1007/s00265-012-1420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JK, Heistermann M. Field endocrinology: monitoring hormonal changes in free-ranging primates. In: Setchell JM, Curtis DJ, editors. Field and laboratory methods in primatology. Cambridge. 2nd edn. Cambridge University Press; 2011. pp. 282–294. [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behav Ecol Sociobiol. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Levine WJ, Dobson SD, Kralik JD. Red signals dominance in male rhesus macaques. Psychol Sci. 2011;22:1001–1003. doi: 10.1177/0956797611415543. [DOI] [PubMed] [Google Scholar]
- Manson JH. Do female rhesus macaques choose novel males? Am J Primatol. 1995;37:285–296. doi: 10.1002/ajp.1350370403. [DOI] [PubMed] [Google Scholar]
- Manson JH. Rhesus macaque copulation calls: re-evaluating the “honest signal” hypothesis. Primates. 1996;37:145–154. [Google Scholar]
- Marty JS, Higham JP, Gadsby EL, Ross C. Dominance, coloration and social and sexual behavior in male drills (Mandrillus leucophaeus). Int J Primatol. 2009;30:807–823. [Google Scholar]
- Michael RP, Keverne EB. Pheromones in the communication of sexual status in primates. Nature. 1968;218:746–749. doi: 10.1038/218746a0. [DOI] [PubMed] [Google Scholar]
- Möhle U, Heistermann M, Palme R, Hodges JK. Characterization of urinary and fecal metabolites of testosterone and their measurement for assessing gonadal endocrine function in male nonhuman primates. Gen Comp Endocrinol. 2002;129:135–145. doi: 10.1016/s0016-6480(02)00525-7. [DOI] [PubMed] [Google Scholar]
- Neumann C, Assahad G, Hammerschmidt K, Farajallah DP, Engelhardt A. Loud calls in male Macaca nigra—a signal of dominance in a tolerant primate species. Anim Behav. 2010;79:187–193. [Google Scholar]
- Newman SR, Butler J, Hammond EH, Gray SD. Preliminary report on hormone receptors in the human vocal fold. J Voice. 2000;14:72–81. doi: 10.1016/s0892-1997(00)80096-x. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proc R Soc Lond B. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc Lond B. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Smith AC, Vorobyev M, Buchanan-Smith HM. Detection of fruit and the selection of primate visual pigments for color vision. Am Nat. 2004;164:696–708. doi: 10.1086/425332. [DOI] [PubMed] [Google Scholar]
- Palme R, Möstl E. Biotin–streptavidin enzyme immunoassay for the determination of oestrogens and androgens in boar faeces. In: Görög S, editor. Advances in steroid analysis. Akadémiai Kiadó; Budapest: 1994. pp. 11–117. [Google Scholar]
- Partan SR. Signal and multichannel signal composition: facial expressions and vocalizations of rhesus macaques (Macaca mulatta). Behaviour. 2002;139:993–1028. [Google Scholar]
- Partan SR, Marler P. Communication goes multimodal. Science. 1999;283:1272–1273. doi: 10.1126/science.283.5406.1272. [DOI] [PubMed] [Google Scholar]
- Partan SR, Marler P. Issues in the classification of multisensory communication signals. Am Nat. 2005;166:231–245. doi: 10.1086/431246. [DOI] [PubMed] [Google Scholar]
- Partan SR, Yelda S, Price V, Shimizu T. Female pigeons, Columba livia, respond to multisensory audio-video playbacks of male courtship behaviour. Anim Behav. 2005;70:957–966. [Google Scholar]
- Pfefferle D, Fischer J. Sounds and size: identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas. Anim Behav. 2006;72:43–51. [Google Scholar]
- Pfefferle D, West PM, Grinnell J, Packer C, Fischer J. Do acoustic features of lion, Panthera leo, roars reflect sex and male condition? J. Acoust Soc Am. 2007;121:3947–3953. doi: 10.1121/1.2722507. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Brauch K, Heistermann M, Hodges JK, Fischer J. Female Barbary macaque (Macaca sylvanus) copulation calls do not reveal the fertile phase but influence mating outcome. Proc R Soc B. 2008a;275:571–578. doi: 10.1098/rspb.2007.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle D, Heistermann M, Hodges JK, Fischer J. Male Barbary macaques eavesdrop on mating outcome: a playback study. Anim Behav. 2008b;75:1885–1891. [Google Scholar]
- Pfefferle D, Heistermann M, Pirow R, Hodges JK, Fischer J. Estrogen and progestogen correlates of the structure of female copulation calls in semi-free-ranging Barbary macaques (Macaca sylvanus). Int J Primatol. 2011;32:992–1006. doi: 10.1007/s10764-011-9517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavcan JM. Sexual dimorphism in primate evolution. Am J Phys Anthropol. 2001;116:25–53. doi: 10.1002/ajpa.10011.abs. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. The Cayo Santiago macaques: history, behavior, and biology. SUNY Press; Albany: 1986. [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003;65:519–530. [Google Scholar]
- Rhodes L, Argersinger ME, Gantert LT, Friscino BH, Hom G, Pikounis B, Hess DL, Rhodes WL. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, and aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J Reprod Fertil. 1997;111:51–57. doi: 10.1530/jrf.0.1110051. [DOI] [PubMed] [Google Scholar]
- Rowe C, Guilford T. Novelty in a multimodal warning signal. Anim Behav. 1999;57:341–346. doi: 10.1006/anbe.1998.0974. [DOI] [PubMed] [Google Scholar]
- Rowe C, Guilford T. The evolution of multimodal warning signals. Evol Ecol. 2001;13:655–671. [Google Scholar]
- Rowell TE, Hinde RA. Vocal communication by the rhesus monkey (Macaca mulatta). Proc Zool Soc Lond. 1962;138:279–294. [Google Scholar]
- Saez S, Sakai F. Androgen receptors in human pharyngolaryngeal mucosa and pharyngo-laryngeal epithelioma. J Steroid Biochem Mol Biol. 1976;7:919–921. doi: 10.1016/0022-4731(76)90011-x. [DOI] [PubMed] [Google Scholar]
- Schneider B, Cohen E, Stani J, Kolbus A, Rudas M, Horvat R, van Trotsenburg M. Towards the expression of sex hormone receptors in the human vocal fold. J Voice. 2007;21:502–507. doi: 10.1016/j.jvoice.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Schrader L, Hammerschmidt K. Computer-aided analysis of acoustic parameters in animal vocalisations: a multi-parametric approach. Bioacoustics. 1997;7:247–265. [Google Scholar]
- Semple S, McComb K. Perception of female reproductive state from vocal cues in a mammal species. Proc R Soc Lond B. 2000;267:707–712. doi: 10.1098/rspb.2000.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S, McComb K, Alberts SC, Altmann J. Information content of female copulation calls in yellow baboons. Am J Primatol. 2002;56:43–56. doi: 10.1002/ajp.1062. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Dixson AF. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm Behav. 2001;39:177–184. doi: 10.1006/hbeh.2000.1628. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology. 2005;111:25–50. [Google Scholar]
- Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, Knapp LA. Odour signals major histocompatibility complex genotype in an Old World monkey. Proc R Soc Lond B. 2011;278:274–280. doi: 10.1098/rspb.2010.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL, Marler P. Vervey monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav. 1980;28:1070–1094. [Google Scholar]
- Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- Slocombe K, Waller B, Liebal K. The language void: the need for multimodality in primate communication research. Anim Behav. 2011;81:919–924. [Google Scholar]
- Smith WJ. The behavior of communicating: an ethological approach. Harvard University Press; Cambridge: 1977. [Google Scholar]
- Smith CL, Evans CS. Multimodal signaling in fowl Gallus gallus. J Exp Biol. 2008;211:2052–2057. doi: 10.1242/jeb.017194. [DOI] [PubMed] [Google Scholar]
- Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko T. Using digital photography to study animal coloration. Biol J Linn Soc. 2007;90:211–237. [Google Scholar]
- Stevens M, Stoddard MC, Higham JP. Studying primate color: towards visual system-dependent methods. Int J Primatol. 2009;30:893–917. [Google Scholar]
- Taylor RC, Buchanan BW, Doherty JL. Sexual selection in the squirrel treefrog Hyla squirella: the role of multimodal cue assessment in female choice. Anim Behav. 2007;74:1753–1763. [Google Scholar]
- Taylor RC, Klein BA, Stein J, Ryan MJ. Faux frogs: multi-modal signalling and the value of robotics in animal behavior. Anim Behav. 2008;76:1089–1097. [Google Scholar]
- Taylor RC, Klein BA, Stein J, Ryan MJ. Multimodal signal variation in space and time: how important is matching a signal with its signaler? J Exp Biol. 2011;214:815–820. doi: 10.1242/jeb.043638. [DOI] [PubMed] [Google Scholar]
- Thompson JT, Bissell AN, Martins EP. Inhibitory interactions between multimodal behavioral responses may influence the evolution of complex signals. Anim Behav. 2008;76:113–121. [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulous V, Toufexis D, Michelini KM, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. P Natl Acad Sci U S A. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz GW, Roberts JA. Multisensory cues and multimodal communication in spiders: insights from video/audio playback studies. Brain Behav Evol. 2002;59:222–230. doi: 10.1159/000064909. [DOI] [PubMed] [Google Scholar]
- Uetz GW, Roberts JA, Taylor PW. Multimodal communication and mate choice in wolf spiders: female response to multimodal versus unimodal signals. Anim Behav. 2009;78:299–305. [Google Scholar]
- Uy JAC, Moyle R, Filardi CR. Plumage color and song differences mediate species recognition between incipient flycatcher species of the Solomon Islands. Evolution. 2009;63:153–164. doi: 10.1111/j.1558-5646.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- van Noordwijk MA, van Schaik CP. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In: Kappeler P, van Schaik CP, editors. Sexual selection in primates. Cambridge University Press; Cambridge: 2004. pp. 208–229. [Google Scholar]
- Voelter C, Kleinsasser N, Joa P, Nowak I, Martinez R, Hagen R, Voelker HU. Detection of hormone receptors in the human vocal fold. Eur Arch Otorhinolaryngol. 2008;265:1239–1244. doi: 10.1007/s00405-008-0632-x. [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt C, Little AC, Wolfensohn S, Honess P, Brown AP, Buchanan-Smith HM, Perrett DI. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc R Soc Lond B. 2003;B270:S144–S146. doi: 10.1098/rsbl.2003.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt C, Gerald MS, Little AC, Kraiselburd E. Selective attention toward female secondary sexual color in male rhesus macaques. Am J Primatol. 2006;68:738–744. doi: 10.1002/ajp.20264. [DOI] [PubMed] [Google Scholar]
- Widdig A, Bercovitch FB, Streich WJ, Nürnberg P, Krawczak M. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc R Soc Lond B. 2004;271:819–826. doi: 10.1098/rspb.2003.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Epple G, Snowdon CT, Porter TA, Belcher AM, Kuderling I. Detection of the chemical signals of ovulation in the cotton-top tamarin, Saguinus oedipus. Anim Behav. 1993;45:313–322. [Google Scholar]
- Zuberbühler K, Noë R, Seyfarth RM. Diana monkey long-distance calls: messages for conspecifics and predators. Anim Behav. 1997;53:589–604. [Google Scholar]
- Zuckerman S, Parkes AS. Observations on the secondary sexual characteristics in monkeys. J Endocrinol. 1939;1:430–439. [Google Scholar]