Abstract
Background
The annual mortality rate of human rabies in rural Africa is 3.6 deaths per 100,000 individuals. Rabies can be prevented by prompt post-exposure prophylaxis, but this is costly and often inaccessible in rural Africa. As 99% of human exposures occur through rabid dogs, canine vaccination also prevents transmission of rabies to humans.
Objective
Evaluate the cost-effectiveness of rabies control through annual canine vaccination campaigns in rural sub-Saharan Africa.
Design
We model transmission dynamics in dogs and wildlife and assess empirical uncertainty in the biological parameters to make probability-based evaluations of cost-effectiveness.
Data Sources
Epidemiological parameters from contact tracing study and literature; cost data from ongoing vaccination campaigns
Target Population
Two districts of rural Tanzania, Ngorongoro and Serengeti
Time Horizon
Ten years
Perspective
Health policymaker
Interventions
Vaccination coverage ranging from 0 to 95% in increments of 5%
Outcome Measures
Life-years for health outcomes and 2010 USD for economic outcomes
Results of Base-Case Analysis
Annual canine vaccination campaigns are very cost-effective in both districts compared with no canine vaccination. In Serengeti, annual campaigns up to 70% coverage are cost-saving.
Results of Sensitivity Analysis
Across a wide range of parameter assumptions and levels of societal willingness-to-pay for life-years, the optimal vaccination coverage for Serengeti is 70%. In Ngorongoro, though optimal coverage depends on willingness-to-pay, vaccination campaigns are always cost-effective and life-saving, and therefore preferred.
Limitations
Canine vaccination is very cost-effective in both districts, but there is greater uncertainty regarding the optimal coverage in Ngorongoro.
Conclusions
Annual canine rabies vaccination campaigns confer extraordinary value and dramatically reduce the health burden of rabies.
Primary Funding Source
US National Institutes of Health (U01 GM087719)
Introduction
Rabies is a viral encephalitic disease of mammals which is responsible for an estimated 61,000 human deaths each year (1), nearly one-third of which occur in rural Africa (2). Once symptoms appear, rabies is almost universally fatal (3). Control of the disease in canines is a potential approach to reducing human rabies as more than 99% of all human cases worldwide result from the bite of a domestic dog (4).
Post-exposure prophylaxis (PEP), including a series of vaccinations and administration of immunoglobulin, can prevent rabies following a dog bite. Worldwide, over 7.5 million rabies PEP regimens are delivered annually (5) at an estimated cost of more than US$1.5 billion (6). Given that a disproportionate rabies burden occurs in sub-Saharan Africa, these costs often fall to those countries least able to afford them. In addition, PEP is frequently unavailable in rural areas within the 24-hour period recommended for treatment initiation after exposure to rabies (7).
Concerns about program costs and the efficient use of health resources have been identified as major barriers to the implementation of canine vaccination programs (8). One-time canine rabies vaccination campaigns have been evaluated as cost-effective prevention against human rabies in urban Chad (9). However, over 75% of rabies mortality in Africa occurs in rural areas (2) and disease dynamics vary between these two settings due to different densities and contact patterns among humans, dogs, and other wildlife (10). Additionally, high birth and death rates in domestic dogs as well as re-introduction of rabies from dogs or wildlife in neighboring, unvaccinated regions make it unlikely that a one-time vaccination campaign will control canine rabies in rural Africa indefinitely (11). We therefore evaluate the cost-effectiveness of rabies control in rural Africa through a strategy of annual canine vaccination campaigns.
Methods
We developed a mathematical model of rabies transmission to estimate the epidemiological effects, clinical benefits, economic costs, and cost-effectiveness of canine vaccination coverage strategies ranging from 0 to 95% in rural Tanzania. No vaccination, the status quo in most parts of Tanzania, is considered the baseline for our analysis. Outcome measures included numbers of dogs vaccinated, incidence of human rabies, and economic costs (in 2010 USD). The analysis was conducted from the perspective of a health policymaker, and we therefore considered health burden in terms of life-years, which in this context are equivalent to Disability Adjusted Life Years (DALYs) given that rabies is inevitably fatal and thus the entire health burden accrues from mortality rather than morbidity. We assessed economic costs associated with both a canine vaccination campaign and post-exposure prophylaxis to prevent rabies in exposed people. In conformity with World Health Organization guidelines (12) and other recommendations for best practices (13), cost-effectiveness outcomes are reported across both one-year and ten-year time horizons on a present-value basis with a 3% annual discount rate. We evaluated the robustness of the results to model inputs, using both probabilistic uncertainty analysis and one-way sensitivity analyses. We applied World Health Organization recommendations (14,15) to denote strategies with incremental cost-effectiveness ratios less than the per capita GDP for a life-year saved (GDP, $1430 for Tanzania (16)) as “very cost-effective” and ratios less than three times the per capita GDP ($4290) as “cost-effective.”
We compared pastoral (Ngorongoro) and agro-pastoral (Serengeti) districts in rural Tanzania as representative of two major settlement patterns and canine densities in rural Africa. While both are sparsely populated compared to cities, agro-pastoral areas generally consist of larger, more closely located villages than found in pastoral areas. Canine density, measured as dogs per km2, is nearly seven times higher in Serengeti than in Ngorongoro. Rabies in Serengeti is endemic, with cases continuously observed, while rabies in Ngorongoro is epidemic, with no observed cases between outbreaks (17,18). Additionally, pilot rabies vaccination campaigns in the two districts have required different strategies to achieve high coverage (19). Both districts border Serengeti National Park and are home to abundant and diverse wildlife populations. Although rabies cannot persist solely in wildlife in either district (17), we address concerns that vaccination coverage which has been sufficient for control in some regions might be insufficient in these wildlife-rich areas (8) by explicitly including wildlife hosts and their contribution to transmission in our dynamic model. Additional details and a map of these districts are included in the Appendix.
Model Structure
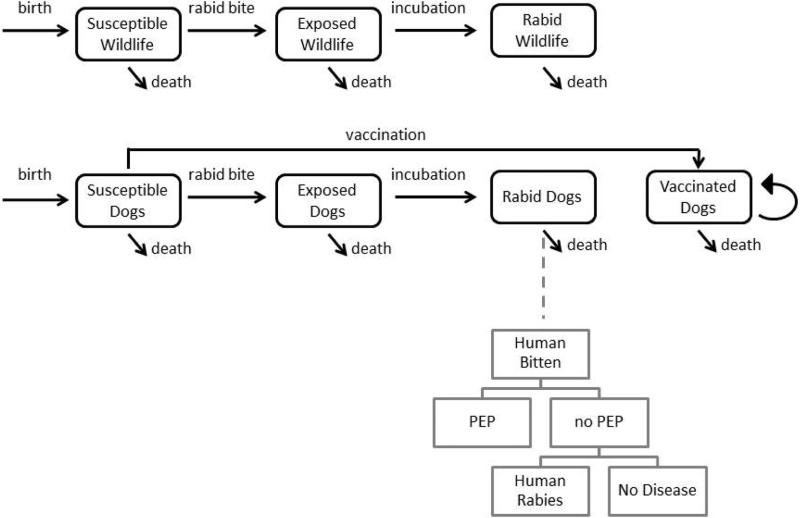
We developed a compartmental transmission model with two strata, one for domestic dogs and another for wildlife, which includes all other carnivores in the area (Figure 1). Each stratum contains the three disease classes of susceptible, exposed/latent, and infectious, measured in units of animals per km2. The canine stratum also includes a vaccinated class. As rabies is fatal, there is no recovered class (9,20–22). Canine demography is explicitly considered through birth into the susceptible class, all-cause death (excluding rabies) at constant rates from all classes, and death due to carrying capacity, or the resource constraints that exist as the population grows and exceeds the limits of the human and geographic environment. We assume that infected animals do not have fecundity because of the typically short incubation period of rabies and the low likelihood that their puppies would survive. To evaluate the impact of uncertainty on our cost-effectiveness assessments, we conducted probabilistic sensitivity analyses and threshold analyses. A full specification of the model equations, parameter values, distributions derived from field and published data, sensitivity analyses, and sources is provided (Appendix, Appendix Tables 1 – 3). We compared our model output to the incidence of canine and human rabies in these two districts before large-scale annual vaccination campaigns began. Due to prior sporadic vaccination efforts, 5 - 10% dogs in these districts had been previously vaccinated when the annual canine incidence was observed to be 1 - 2%. For human rabies, we have previously estimated an incidence of 1.48 - 4.28 deaths per 100,000 people in Ngorongoro prior to large scale implementation of canine vaccination, resulting in 2 - 6 rabies deaths per year for the district (23). From animal bite injury data and availability of PEP, we have estimated that the incidence of human rabies in unvaccinated areas near Serengeti was 4.9 (2.9 - 7.2) deaths annually per 100,000 persons in the late 1990s (24), leading to 5 - 13 human cases of rabies annually.
Figure 1. Rabies transmission model.
Our dynamic compartmental model is stratified by host type. Rabid dogs are linked to human deaths through a probability tree of human health outcomes. The equations governing the movement between classes are given in the Appendix.
Costs of Vaccination
We parameterized the costs in our analysis using field data we collected during annual vaccination campaigns in Serengeti and Ngorongoro and from published literature (19). We considered only the direct costs of vaccination, as dogs are often brought to vaccination stations by children and the average income loss from bringing a dog to the central point is therefore considered to be minimal. We generate functions of cost with increasing vaccination coverage. The costs vary between the two districts. In the agro-pastoralist district of Serengeti, central point vaccination campaigns are sufficient to achieve high coverage, whereas in the more sparsely populated pastoral district of Ngorongoro, central point vaccination campaigns must be supplemented with door-to-door vaccinators in order to achieve high coverage, increasing the costs per dog vaccinated for Ngorongoro compared with Serengeti. We estimated costs as a function of coverage level, taking into account both the fixed costs of program startup and the decreasing efficiency associated with searching for additional dogs to vaccinate as coverage levels increase (Appendix).
Costs of Disease
An untreated rabies bite to a human was estimated to result in the loss of 31.4 life-years on average (2), taking into account the typical age-distribution of rabies victims. Monetary losses accrue through the cost of post-exposure prophylaxis (PEP), estimated to be $111.29 per regimen (25), which includes both direct costs of treatment and indirect costs of transportation and lost income for the days on which treatment is administered. We assume a full course of PEP is 100% effective, both consistent with clinical data (7) and conservative given that this assumption would bias against canine vaccination. When victims who do not receive PEP progress to rabies, we consider only the health burden, as medical care is not effective and usually not provided in rural African settings. We constructed a probability tree to model the chain of events leading from a rabid dog to PEP, a case of rabies, or neither (Figure 1, Appendix Table 2). We do not consider transmission from wildlife to humans, as this represents less than 1% of human cases (4). However, our previous findings do show that mass vaccination of domestic dogs could concomitantly eliminate disease from wildlife (17), and this would potentially be an additional benefit for conservation (26,27) as well as human health.
Data collected through contact tracing of all rabies cases detected from January 2002 through December 2006 (17,18) were used to estimate that each rabid dog bites 0.51 humans (Appendix). We estimated that each rabid dog leads on average to $36.89 in costs from PEP administration and to a loss of 1.07 human life-years (Appendix Table 2). To estimate the cost from disease for each strategy that we considered, we multiplied each of these measures by the canine rabies incidence predicted through simulation. We calculate the cumulative economic cost of disease and vaccination on two time scales, annually and over a decade.
Cost-Effectiveness
Within each district, any strategy which has both greater monetary cost and more lives lost than some other strategy or combination of strategies is considered to be dominated by the latter strategy. For each non-dominated scenario, we found the incremental cost-effectiveness ratio (ICER) as compared to the next-lowest cost scenario. The ICER measures the additional cost per life-year saved of expanding canine vaccination to the next coverage level.
To compare the cost-effectiveness results from our entire range of 10,000 simulations, we used a net benefits framework (28). Net health benefits are defined as the difference between the average health benefit of an intervention (for us, in life-years saved), and the absolute intervention cost divided by the threshold cost-effectiveness ratio (29). This framework yields a single outcome measure that that simplifies the identification of the program that provides the largest health benefit for a given societal willingness-to-pay for life-years. We calculated the net health benefit of each incremental level of coverage from each simulation across a range of cost-effectiveness ratios. From this, we found the probability that a given coverage had the greatest net health benefit across a wide range of alternative cost-effectiveness ratios or levels of willingness-to-pay.
Role of the Funding Source
Vaccination campaigns were conducted and field data were generated with the support of the National Science Foundation and National Institutes of Health (DEB0225453, DEB0513994), the Wellcome Trust, and Lincoln Park Zoo (LPZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Rabies Burden
To determine the cost-effectiveness of using annual canine vaccination to prevent human cases of rabies, we modeled rabies in dogs and wildlife using a dynamic transmission model and assessed the costs of vaccination campaigns and expected outcomes across a range of vaccine coverage levels. Our results show that the expected number of rabies cases in both domestic dogs and wildlife hosts decreases monotonically with increasing canine vaccination coverage (Figure 2, Appendix Figure 2). In a scenario with no vaccination, the cumulative burden of rabies after ten years is estimated to be 0.13 rabid dogs/km2 undiscounted in Ngorongoro and 5.43 rabid dogs/km2 undiscounted in Serengeti (0.11 rabid dogs/km2 and 4.7 rabid dogs/km2, respectively, discounted to present value terms). These values are consistent with our observation that the per annum rabies loss is 1-2% of the dog population under conditions of very low vaccination coverage. The herd immunity threshold, or the coverage at which rabies will no longer persist in the dog population, is reached at roughly 10% coverage in Ngorongoro and 30% coverage in Serengeti (Figure 2).
Figure 2. Cumulative rabies cases after ten years of annual canine vaccination campaigns at increasing vaccination coverage.
Cases are undiscounted.
Higher densities of rabid dogs lead to humans being exposed to rabies through dog bites, causing mortality in the absence of prompt PEP. Each death corresponds to a loss of 31.4 life-years (2). Our model projects the loss of 0.14 life-years per km2 undiscounted (0.12 life-years discounted) after ten years in pastoral Ngorongoro when dogs are not vaccinated. In agro-pastoral Serengeti, this estimate is 5.8 life-years per km2 undiscounted (5.0 life-years discounted), reflecting the higher population density of both dogs and humans in this district. The expected loss of life decreases monotonically to approach zero with increasing canine vaccination coverage. We compared model predictions of annual human rabies burden for the entirety of each district against data collected prior to large-scale vaccination campaigns. Our model predicts 2.0 rabies deaths annually at 5% vaccination coverage and 0.6 deaths annually at 15% coverage in Ngorongoro, as well as 39.3 and 8.3 rabies deaths annually in Serengeti, at 5% and 15% coverage respectively. These model results are consistent with observations of 2 – 6 deaths in Ngorongoro and 5 – 13 deaths in Serengeti during years of low vaccination coverage (23).
Economic Costs
The expected cumulative cost of providing PEP to victims of rabies bites at current levels for ten years is estimated to be $57,280 ($4.08/km2) for Ngorongoro and $584,484 ($173.28/km2) for Serengeti (Table 1) in present-value terms. As the incidence of canine rabies declines, these costs decline simultaneously (Figure 3). Conversely, the cost of canine vaccination increases with increasing coverage. The strategy with lowest total cost in Ngorongoro is no canine vaccination. In Serengeti, the lowest cost strategy is 25% coverage, as the high cost of PEP outweighs the cost of campaigns below that coverage. Regardless of the time horizon, no canine vaccination always remained the lowest cost strategy in Ngorongoro. However, in Serengeti, the costs of vaccination strategies up to 80% coverage break even with the costs of not vaccinating by the end of the first year (Appendix Figures 3 – 4).
Table 1.
Costs, benefits, and incremental cost-effectiveness ratios for control strategies. Costs are in 2010 USD, and both costs and life-years saved are cumulative over ten years and discounted to present-value terms with a 3% discount rate. Dominated strategies, which are italicized, are those which are more expensive and which provide less benefit than another strategy orcombination of strategies.
| Vaccination Coverage (%) | Ngorongoro District | Serengeti District | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost ($/km2) | Life-years saved (/km2) | District Cost ($) | District life- years saved | ICER1 m($/life-year) | Cost ($/km2) | Life-years saved (/km2) | District Cost ($) | District life- years saved | ICER1 ($/life-year) | |
| 0 | 4.08 | 0.000 | 57 280 | 0 | minimum cost | 173.28 | 0.000 | 584 484 | 0 | dominated |
| 5 | 14.66 | 0.078 | 205 816 | 1 098 | dominated | 180.71 | 1.842 | 609 533 | 6 213 | dominated |
| 10 | 14.41 | 0.098 | 202 248 | 1 382 | dominated | 129.16 | 3.449 | 435 646 | 11 635 | dominated |
| 15 | 14.59 | 0.106 | 204 718 | 1 484 | dominated | 103.32 | 4.299 | 348 494 | 14 499 | dominated |
| 20 | 14.90 | 0.109 | 209 105 | 1 535 | 98.90 | 96.00 | 4.598 | 323 807 | 15 510 | dominated |
| 25 | 17.19 | 0.112 | 241 305 | 1 565 | 1 070.10 | 94.44 | 4.727 | 318 535 | 15 945 | minimum cost |
| 30 | 19.51 | 0.113 | 273 856 | 1 585 | 1 644.92 | 94.79 | 4.799 | 319 737 | 16 188 | 4.95 |
| 35 | 21.87 | 0.114 | 306 976 | 1 599 | 2 382.42 | 96.19 | 4.846 | 324 446 | 16 344 | 30.11 |
| 40 | 24.21 | 0.115 | 339 853 | 1 609 | 3 215.87 | 97.88 | 4.878 | 330 165 | 16 453 | 52.50 |
| 45 | 26.55 | 0.115 | 372 719 | 1 617 | 4 227.39 | 100.09 | 4.902 | 337 595 | 16 533 | 92.98 |
| 50 | 28.95 | 0.116 | 406 383 | 1 623 | 5 547.53 | 102.31 | 4.920 | 345 084 | 16 594 | 123.25 |
| 55 | 31.32 | 0.116 | 439 627 | 1 628 | 6 874.51 | 104.53 | 4.934 | 352 580 | 16 641 | 157.93 |
| 60 | 33.68 | 0.116 | 472 716 | 1 632 | 8 439.08 | 107.11 | 4.945 | 361 279 | 16 679 | 229.58 |
| 65 | 36.04 | 0.116 | 505 922 | 1 635 | 10 290.46 | 109.28 | 4.954 | 368 603 | 16 710 | 237.95 |
| 70 | 38.44 | 0.117 | 539 515 | 1 638 | 12 486.69 | 111.77 | 4.962 | 376 989 | 16 735 | 330.27 |
| 75 | 40.78 | 0.117 | 572 383 | 1 640 | 14 485.27 | 129.16 | 4.968 | 435 662 | 16 757 | 2 764.60 |
| 80 | 43.17 | 0.117 | 606 001 | 1 642 | 17 382.35 | 146.50 | 4.973 | 494 157 | 16 775 | 3 258.65 |
| 85 | 45.53 | 0.117 | 639 075 | 1 644 | 19 872.75 | 162.79 | 4.978 | 549 076 | 16 790 | 3 578.78 |
| 90 | 52.11 | 0.117 | 731 397 | 1 645 | 63 903.77 | 178.39 | 4.982 | 601 723 | 16 803 | 3 974.04 |
| 95 | 65.24 | 0.117 | 915 727 | 1 646 | 145 801.16 | 217.85 | 4.985 | 734 792 | 16 815 | 11 532.80 |
Incremental Cost-Effectiveness Ratio
Figure 3. Component and total costs of rabies control with increasing canine vaccination coverage.
Dotted lines, costs due to canine vaccination programs achieving a given coverage level; dashed lines, costs due to human post-exposure prophylaxis in the presence of canine vaccination at a given coverage level; and solid lines, sum of these two costs. All costs are cumulative over ten years.
Cost-Effectiveness of Vaccination
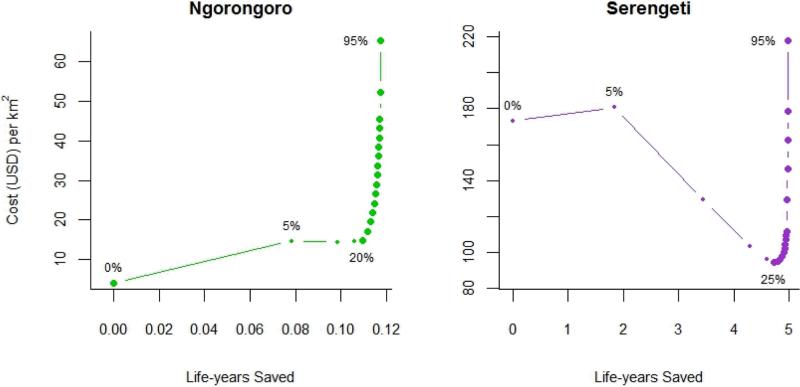
In Serengeti, vaccination coverage at 25% has lower monetary costs and higher health benefits than coverage below 25% (Table 1, Figure 4). Therefore, strategies with coverage below 25% are considered to be dominated. As coverage increases above 25%, both life-years saved and costs increase. In Ngorongoro, 5 – 15% coverage is dominated. With the thresholds set by per-capita GDP at $1430 and $4290 per life-years saved (16) , canine vaccination in Ngorongoro is very cost effective for annual campaigns that reach 20% to 30% coverage, and cost effective for campaigns reaching 35% to 50%. In Serengeti, vaccination would be very cost-effective at coverage from 25% to 70%, and cost-effective for coverage ranging from 75% to 85%.
Figure 4. Cost of vaccination coverage and life-years saved.
Points indicate increasing canine vaccination coverage. Smaller points indicate dominated strategies, which achieve fewer health benefits than other strategies of equal or lesser cost. Costs and life-years saved are cumulative over ten years. Please note that the two districts are represented on different scales.
Uncertainty and Sensitivity Analyses
When considering all of the uncertainty in the epidemiological parameters, a cost-effectiveness acceptability curve identifies the optimal strategy as the one having the highest probability of being cost-effective for each willingness-to-pay threshold. At the very cost-effective threshold of $1430 per life-year gained, 80% vaccine coverage is optimal for Ngorongoro with a probability of 0.28 and 70% coverage is optimal for Serengeti with a probability of 0.85 (Figure 5). At the “cost-effective” threshold of $4290, campaigns achieving 90% coverage are most likely to be optimal for both districts. Regardless of willingness-to-pay, canine vaccination is the optimal choice at a probability of at least 0.68 in Ngorongoro and 0.86 in Serengeti. At any level of PEP availability below 98%, canine vaccination remains cost-effective (Appendix Figures 5 – 6). This is true irrespective of the price of PEP (Appendix Figure 6).
Figure 5. Cost-effectiveness acceptability curves.
Curves show the probability that a given canine vaccination coverage is optimal, i.e. providing the largest net health benefit at a given willingness-to-pay threshold. At willingness-to-pay thresholds of $1430/life-year and $4290/life-year, the World Health Organization thresholds for “very cost-effective” and “cost-effective” interventions in Tanzania, optimal annual coverage ranges between 70 - 90% in both districts. These thresholds are indicated by solid vertical lines.
It is common in northwest Tanzania to kill, tie, or otherwise restrain rabid dogs. Our baseline analysis includes consideration of these practices. Given the possibility that these practices might change in the future or across different settings, we consider the impact that a change in these practices would have on the cost-effectiveness of the system. Without rabid dog removal, rabies transmission increases dramatically. Consequently, canine vaccination would be cost-saving in Ngorongoro as well as Serengeti, and would be cost-effective at up to 90% coverage in Ngorongoro and 95% coverage in Serengeti (Appendix Table 5).
Vaccination campaign costs are lower in both districts if dogs are not repeatedly vaccinated every year, by approximately 15% regardless of district or coverage acheived (Appendix Table 6). The cost differences represent the largest additional expenditure that a program should be willing to spend on education, tagging, or other methods to avoid revaccinating the same dogs.
Discussion
We found that canine vaccination against rabies is a very cost-effective approach to prevent human rabies in two distinct settings of rural Africa. In an agro-pastoral region such as Serengeti, canine vaccination is even cost-saving relative to PEP alone. Yet throughout most of sub-Saharan Africa, canine vaccination is exceedingly limited and rarely implemented with sufficient coverage to achieve these benefits. In Serengeti, the health burden and economic cost of maintaining the status quo of no canine vaccination is higher than the cost of establishing canine vaccination programs, even at high levels of coverage (85%). Coverage below 25% in Serengeti generates both higher spending and more deaths than coverage at 25%. In pastoral Ngorongoro, lives could be saved both efficiently and inexpensively by achieving up to 50% coverage. Even the highest-cost strategy, 95% coverage in Ngorongoro, would consume less than 0.2% of the overall health budget for Tanzania (30). These results are robust to empirical uncertainty, and we find that the optimal strategy to prevent human rabies under any assumption about societal willingness-to-pay is one that includes annual canine vaccination campaigns.
While Serengeti and Ngorongoro are representative of agro-pastoral and pastoral communities in sub-Saharan Africa, they differ from each other with regard to dog and human density, to wildlife transmission dynamics, and, consequently, to the most suitable approach for program implementation. Nonetheless, annual canine rabies vaccination is broadly cost-effective in both regions, suggesting that this finding is applicable across different rural settings. Specifically, Ngorongoro is more sparsely populated by both humans and dogs, requiring a more expensive house-to-house approach to achieve the same coverage that central-point campaigns would yield in Serengeti. Additionally, the rabies burden is lower in Ngorongoro than in Serengeti, so the potential health impact is less dramatic. These differences suggest that the optimal level of coverage for an annual campaign might differ across rural Africa, with higher coverage potentially both more necessary and more efficient in rural areas of higher human and dog density.
Our base case analysis recommends lower coverage for Ngorongoro than for Serengeti across all levels of societal willingness-to-pay. However, our uncertainty analysis suggests that commensurately high coverage might be optimal for Ngorongoro. The difference between the central-point estimate and the uncertainty analysis may be attributed to the threshold behavior of the transmission dynamics, i.e., that the R0 of rabies is close to 1 in Ngorongoro. Consequently, near this threshold even small shifts in the transmission parameters that we draw from the parameter distributions in our probabilistic analysis can significantly impact the coverage necessary to curtail transmission. Alternatively, the discrepancy between the probabilistic and deterministic results could be related to the exclusion of combinations of parameters that lead to R0 values below one. Their exclusion may lead to an average R0 in the uncertainty analysis that would be higher than that used in the base case and hence make high coverage vaccination campaigns more likely to be optimal in the uncertainty analysis. Additionally, the empirical distributions of the transmission parameters from which we draw are wider for Ngorongoro than for Serengeti, a result of the smaller sample size of rabies in Ngorongoro (10). Therefore, there is less certainty in choosing a particular coverage as optimal for Ngorongoro, although it is clear that the status quo of no vaccination is unlikely to be the best choice from neither economic nor public health perspectives.
Our model estimates the costs of vaccination campaigns along a wide range of coverage, but the original data was collected in association with a few specific coverage levels for each district. We assumed the most likely scenario that costs accumulate linearly between start-up costs and the observed coverage of central-point campaigns, but this may not be the case. Should the cost structure be different for vaccination campaigns achieving coverage below what we have observed, the optimal coverage levels might be different than what is reported here for low willingness-to-pay values. Similarly, the cost structure at very high coverage might change, as these costs were also estimated but not actually observed. However, neither limitation changes the general conclusion of our results, which is that canine vaccination campaigns achieving 70% coverage or higher are very cost-effective for both districts.
The World Health Organization Commission on Macroeconomics and Health recommends that interventions which confer DALYs (life-years, in the case of rabies) at an incremental cost less than the national per capita GDP ($1430 for Tanzania) or three times that GDP ($4290) be considered “very cost-effective” or “cost-effective,” respectively (14). Although these guidelines are considered simplistic (31), they are among the more stringent criteria and most typically used. The World Health Organization's Choosing Interventions that are Cost-Effective program (WHO-CHOICE) recommends threshold criteria at $2154 and $6461 per DALY, respectively, for Africa Region E, of which Tanzania is a part (15). Our analysis provides the incremental cost-effectiveness ratios across a large range of feasible coverage scenarios, including all these thresholds, equipping policymakers with the information necessary to select among these criteria based on their priorities and on the incremental benefits of competing health programs.
Our study is the first to reveal that repeated annual canine vaccination against rabies may be cost-saving. We find that even high-coverage annual vaccination campaigns in Serengeti are cost-saving relative to PEP alone within the first year. This is a more rapid recouping of expenditure than predicted in an urban setting (9) or from static models that do not incorporate transmission (32). In N'Djaména, Chad, the canine density is three times that in Serengeti and more than ten times that in Ngorongoro (9), elevating the total costs of canine vaccination programs in cities relative to those in agro-pastoral areas, and extending the time required to recoup costs.
Compared with a one-time campaign, annual vaccination protects better against the threat of rabies reintroduction from bordering unvaccinated populations. True elimination of rabies in any country would rely on a coordinated effort across political boundaries (33,34). Without such international cooperation in East Africa, permanent elimination of rabies in northwest Tanzania is not a feasible goal and sustained vaccination efforts will be required for control.
When access to the relatively expensive PEP is limited, as is usually the case in rural Africa, canine vaccination is imperative to prevent human death. A study in northwest Tanzania traced 699 victims of confirmed rabid dogs and found that only 456 (65%) of these victims received PEP (23). Without PEP, 19% of bite victims die of rabies (25). Lack of education about rabies, distance from the nearest clinic, or an inability to afford the fees contribute to imperfect rates of PEP administration (23). In addition, clinics do not always have PEP in stock, immunoglobulin is almost inevitably absent (23,35), and dog owners may be mistaken about the vaccination status of their dog (23). Although improved access to PEP is itself cost-effective (25,36), we found that canine vaccination would remain cost-effective even if PEP were more accessible (Appendix Figures 5 – 6).
Vaccination up to the coverage of herd immunity ensures the eventual control of the disease, but vaccinating beyond herd immunity continues to be cost-effective and even cost-saving. While herd immunity indicates the coverage at which vaccination will ultimately control rabies, higher coverage controls rabies even faster and likewise averts further human cases earlier. Additional coverage is inexpensive relative to the cost of PEP and the willingness-to-pay thresholds. Therefore, optimal coverage even at the lowest willingness-to-pay threshold is higher than herd immunity alone might suggest.
In our current vaccination trials in Tanzania, every dog brought to the vaccination site is vaccinated, regardless of whether the dog has been previously vaccinated. This current practice is implemented to reduce confusion about which dogs should be brought in a given year, because vaccination certificates are often lost and veterinary registers incomplete. However, our household surveys suggest that, once vaccinated, dogs will usually be brought each subsequent year for revaccination. We found that the cost of annual campaigns could be reduced by about 15% over a decade in both districts by eliminating these repeat vaccinations. This suggests that a vaccination campaign might achieve significant savings by investing in record-keeping practices, marking dogs, and/or discouraging serial canine vaccination. In addition, the implications of relatively less straight-forward promotion and the practicalities of such changes to campaigns would also require further consideration.
A challenge for any vaccination program is the integration of canine vaccination into existing infrastructure and ongoing health programs. Health authorities must balance the investment of scarce resources, and veterinary programs are often perceived as low priority. However, our results demonstrate the tremendous human health benefits of canine rabies vaccination and that annual canine vaccination may actually release resources currently being used for rabies post-exposure prevention, so that other health goals may be pursued. Ongoing campaigns in Tanzania may serve as a model for implementation of such programs in other parts of sub-Saharan Africa. Efforts in Tanzania demonstrate that necessary levels of coverage are achievable, but they do require considerably more effort in terms of organization than is typically allocated to canine vaccination in sub-Saharan Africa. During the initial phase of vaccination program scale-up in areas without prior experience in canine vaccination, coverage lower than the targets are likely to be achieved, but even these lower levels are likely to be beneficial and cost-effective.
In summary, canine vaccination is a highly cost-effective approach to reducing human rabies fatalities in rural Tanzania. In some settings, canine vaccination is even cost-saving relative to the current status quo of providing PEP without vaccination of the canine reservoir. These results, modeled both in pastoral and agro-pastoral areas, are likely to be applicable across a wide range of rural African settings. We recommend the continuation of annual canine vaccination in rural Tanzania, and the immediate implementation of campaigns in other areas of rural Africa. This is particularly imperative in regions where PEP is expensive or unavailable, and it is important for policymakers and the medical community to recognize that this basic veterinary measure can prevent human death from a devastating disease. Although the precise quantitative recommendations of optimal coverage may be specific to a region, it is clear from our results that high coverage campaigns confer extraordinary value. An investment in canine vaccination throughout Tanzania specifically and sub-Saharan Africa generally will be repaid both in dollars and in lives.
Supplementary Material
Appendix Table 1 Transmission model equations
| dSd/dt = bd*(Sd + Vd) – μd*Sd – γd*Sd*Nd – β11*Id*Sd – β12*Iw*Sd – (v*Nd – Vd) |
| dSd/dt = β11*Id* Sd + β12*Iw* Sd – μd*Ed – γ*Ed*Nd – σ*Ed |
| dId/dt = σ*Ed – μd *Id – γ*Id*Nd – α*Id |
| dVd/dt = (v*Nd – Vd) – μd *Vd – γ*Vd*Nd |
| dSw/dt = (bw-μw)* Sw – γw*Sw*Nw – β22*Iw*Sw – β21*Id*Sw |
| dEw/dt = β22*Iw*Sw + β21*Id*Sw – μw*Ew – γ*Ew*Nw – σ*Ew |
| dIw/dt = σ*Ew – μw*Iw – γ*Iw*Nw – α*Iw |
| Nd = Sd + Ed + Id + Vd |
| Nw = Sw+ Ew + Id |
Blue portions are active only during vaccination campaigns.
Appendix Table 2 Parameters for transmission model, cost, and decision model. Estimates were used for the base-case analysis, and the full distribution was sampled for the uncertainty analysis.
| Parameter | Description | Estimate | Distribution | Reference |
|---|---|---|---|---|
| Transmission Model Parameters | ||||
| b d | Birth rate (domestic dogs) | 1.72/year | normal (1.72, 0.11) | (18) |
| μ a | Death rate (adult dogs) | .45/year | normal (0.45, 0.02) | (18) |
| bw,μw | Birth/death rate (wildlife) | same as for dogs | same as for dogs | |
| Kd,N | Carrying capacity (Ngorongoro, dogs) | 1.5 | (18) | |
| K d,S | Carrying capacity (Serengeti, dogs) | 10 | (18) | |
| Kw,N | Carrying capacity (Ngorongoro, wildlife) | 4.5 | Field data | |
| K w,S | Carrying capacity (Serengeti, wildlife) | 3.0 | Field data | |
| γ | Death from carrying capacity limits | (b-μ)/K | ||
| 1lσ | Incubation period of rabies | 22.3 days | normal (22.3, 1.28) | (18) |
| 1/α | Infectious period of rabies | 3.1 days | normal (3.10, 0.13) | (18) |
| V | Vaccination coverage | varies | 0 – 1 | |
| β l1,N | Dog to dog transmission (Ngorongoro) | 0.20 | tnorm1 (0.20, 0.020) | (10) |
| β 12,N | Wildlife to dog transmission (Ngorongoro) | 0.11 | tnorm1 (0.11, 0.035) | (10) |
| β 21,N | Dog to wildlife transmission (Ngorongoro) | 0.01 | tnorm1 (0.01, 0.002) | (10) |
| β 22,N | Wildlife to wildlife transmission (Ngorongoro) | 0.03 | tnorm1 (0.03, 0.009) | (10) |
| β 11,S | Dog to dog transmission (Serengeti) | 0.03 | tnorm1 (0.03, 0.001) | (10) |
| β 12,S | Wildlife to dog transmission (Serengeti) | 0.03 | tnorm1 (0.03, 0.004) | (10) |
| β 21,S | Dog to wildlife transmission (Serengeti) | 0.01 | tnorm1 (0.01, 0.001) | (10) |
| β 22,S | Wildlife to wildlife transmission (Serengeti) | 0.02 | tnorm1 (0.02, 0.006) | (10) |
| Cost Parameters | ||||
| Dog vaccination, Ngorongoro, per dog | $4.07 | uniform (3.41 –4.77) | (19) | |
| Dog vaccination, Serengeti, per dog | $2.05 | uniform (1.00 –3.19) | (19) | |
| PEP2 administration, per regimen | $111.29 | (25) | ||
| Life-year cost per human case | 31.426 | (2) | ||
| Decision Tree Parameters | ||||
| P1 | Number of humans bitten by a single rabid dog | .51 | Field data | |
| P2 | That a bite victim of a rabid dog goes to the hospital (.76) and receives PEP2 (.86) | .65 | (25) | |
| P3 | That a bite victim of a rabid dog who does not seek PEP2 will contract rabies | .19 | (25) | |
| PEP2 cost per bite (P1*P2*$111.29) | $36.89 | |||
| Human rabies health burden per bite (P1*(1-P2)*P3*31.426 life-years) | 1.07 life-years | |||
Truncated normal distribution
Post-exposure prophylaxis
Table 3.
Appendix Model sensitivity to variation in wildlife density. Health and economic outcomes associated with varying wildlife density are listed for Ngorongoro. Kw indicates the carrying capacity of wild carnivores (per km2). For central-point implementation, Kw = 4.5 carnivores per km2. Health and economic outcomes are cumulative over ten years and discounted at 3% annually.
| Vaccination Coverage (%) | Kw = 3 | Kw = 4.5 | Kw = 6 | |
|---|---|---|---|---|
| 30 | Life-years saved per km2 | 0.113 | 0.113 | 0.113 |
| 60 | 0.116 | 0.116 | 0.116 | |
| 90 | 0.117 | 0.117 | 0.117 | |
| 30 | Cost per km2 | $19.51 | $19.51 | $19.51 |
| 60 | $33.68 | $33.68 | $33.68 | |
| 90 | $52.11 | $52.11 | $52.11 |
Table 4.
Appendix Estimates of rabies epidemiological parameters. The parameter kij indicates the number of animals of type i that a single rabid animal of type j is likely to infect. Values are reported both for the scenario in which rabid dogs are killed or restrained ("w/ removal") and for that in which they are not ("w/o removal"). The 95% confidence interval is given in parentheses. We previously reported estimates of transmission parameters for the scenario without rabid canine removal (10).
| Description | Ngorongoro District | Serengeti District | ||||
|---|---|---|---|---|---|---|
| 1° Infection | 2° Infection | w/o removal | w/ removal | w/o removal | w/removal | |
| k11 | dog | dog | 1.16 (0.85 – 1.54) | 0.92 (0.75 – 1.11) | 1.09 (0.98 – 1.21) | 1.01 (0.94 – 1.07) |
| k21 | dog | other | 0.13 (0.05 – 0.27) | 0.12 (0.07 – 0.19) | 0.09 (0.06 – 0.13) | 0.09 (0.07 – 0.11) |
| k12 | other | dog | 0.49 (0.23 – 0.84) | 0.48 (0.23 – 0.86) | 0.95 (0.71 – 1.21) | 0.95 (0.71 – 1.20) |
| k22 | other | other | 0.39 (0.20 - 0.67) | 0.39 (0.19 – 0.67) | 0.23 (0.13 – 0.35) | 0.23 (0.12 – 0.35) |
| R0 | basic reproduction number | 1.24 | 1.02 | 1.18 | 1.11 | |
Table 5.
Appendix Costs, benefits, and incremental cost-effectiveness ratios for control strategies when canine removal is not practiced. Costs are in 2010 USD, and both costs and life-years saved are cumulative over ten years and discounted to present-value terms with a 3% discount rate. Dominated strategies, which are italicized, are those which are more expensive and which provide less benefit than another strategy or combination of strategies.
| Ngorongoro District | Serengeti District | |||||
|---|---|---|---|---|---|---|
| Vaccination Coverage (%) | Total Cost ($/km2) | Life-years saved | ICER1 ($/life-year) | Total Cost ($/km2) | Life-years saved | ICER1 ($/life-year) |
| 0 | 61.87 | 0.000 | dominated | 240.02 | 0.000 | dominated |
| 5 | 64.65 | 0.182 | dominated | 246.09 | 1.440 | dominated |
| 10 | 58.32 | 0.381 | dominated | 198.05 | 2.929 | dominated |
| 15 | 51.44 | 0.596 | dominated | 151.45 | 4.376 | dominated |
| 20 | 44.12 | 0.824 | dominated | 115.72 | 5.499 | dominated |
| 25 | 37.87 | 1.063 | dominated | 97.34 | 6.111 | dominated |
| 30 | 31.25 | 1.316 | dominated | 90.09 | 6.393 | dominated |
| 35 | 25.09 | 1.556 | dominated | 87.59 | 6.539 | dominated |
| 40 | 22.69 | 1.683 | minimum cost | 86.87 | 6.628 | minimum cost |
| 45 | 23.27 | 1.722 | 14.84 | 87.28 | 6.688 | 6.76 |
| 50 | 24.65 | 1.738 | 86.63 | 88.10 | 6.732 | 18.67 |
| 55 | 26.21 | 1.748 | 163.26 | 89.17 | 6.766 | 31.99 |
| 60 | 27.86 | 1.754 | 243.12 | 90.69 | 6.792 | 58.15 |
| 65 | 29.56 | 1.760 | 332.10 | 92.01 | 6.813 | 62.33 |
| 70 | 31.33 | 1.764 | 435.01 | 93.66 | 6.830 | 96.20 |
| 75 | 33.07 | 1.767 | 532.34 | 107.51 | 6.844 | 972.03 |
| 80 | 34.88 | 1.770 | 666.88 | 121.37 | 6.856 | 1158.79 |
| 85 | 36.67 | 1.772 | 787.46 | 134.40 | 6.867 | 1284.46 |
| 90 | 41.80 | 1.774 | 2654.69 | 146.93 | 6.875 | 1439.37 |
| 95 | 52.11 | 1.776 | 6201.99 | 178.88 | 6.883 | 4247.69 |
1 Incremental Cost-Effectiveness Ratio
Table 6.
Appendix Comparative cost of revaccination. The estimated cost per km2 of a vaccination campaign is listed for scenarios where dogs are vaccinated every year regardless of vaccine history compared with scenarios where dogs are never revaccinated. Costs are in 2010 USD, discounted, and cumulative over ten years.
| Ngorongoro | Serengeti | |||
|---|---|---|---|---|
| standard | no repeat vaccination | standard | no repeat vaccination | |
| 30% | $19.32 | $16.24 | $86.97 | $72.97 |
| 60% | $33.61 | $28.24 | $104.31 | $87.57 |
| 90% | $52.07 | $43.75 | $176.86 | $148.48 |
Acknowledgements
We thank Dr. Jamie Childs for insightful conversation about the rabies system, and Mr. Israel Silaa and Mr. Kaneja Ibrahim Mangaru of the Serengeti Health Initiative for informative discussions regarding campaign logistics. We are grateful to Angelika Hofmann for editorial assistance. We thank the Ministries of Health and Social Welfare, and Ministry of Livestock and Fisheries Development in Tanzania, TANAPA, TAWIRI, NCA Authority, the Tanzanian Commission for Science and Technology and National Institute for Medical Research for permissions and collaboration, as well as colleagues from the Serengeti Viral Transmission Dynamics team, medical officers, field officers, paravets and village officers in Serengeti and Ngorongoro District.
Financial Support: We are grateful for financial support from the National Institute of General Medical Sciences (U01 GM087719, Models of Infectious Disease Agent Study (MIDAS)) and the Miriam Burnett Trust for Ms. Fitzpatrick and Dr. Galvani. Ms. Fitzpatrick also received support from the NIAID Multidisciplinary Parasitology Training Program (T32AI007404) and the Lindsay Fellowship for Research in Africa. Support for Dr. Hampson was provided by the Wellcome Trust. Dr. Paltiel receives support from the National Institute on Drug Abuse (R01 DA015612).
References
- 1.World Health Organization WHO Expert Consultation on rabies. World Health Organization Technical Report Series. 2013:982. [PubMed] [Google Scholar]
- 2.Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda MEG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bulletin of the World Health Organization. 2005;83(5):360–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurology. 2002;1(2):101–9. doi: 10.1016/s1474-4422(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 4.WHO WHO Expert Consultation on Rabies. World Health Organization technical report series. 2005;931:1–88. [PubMed] [Google Scholar]
- 5.WHO Rabies vaccines. WHO Position Paper. 2007:425–36. [PubMed] [Google Scholar]
- 6.World Health Organization WHO Expert Consultation on rabies. World Health Organization Technical Report Series. 2013:982. [PubMed] [Google Scholar]
- 7.Quiambao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerczyk C. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine. 2005 Feb;23(14):1709–14. doi: 10.1016/j.vaccine.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Lembo T, Hampson K, Kaare M, Ernest E, Knobel DL, Kazwala RR, et al. The Feasibility of Canine Rabies Elimination in Africa: Dispelling Doubts with Data. Plos Neglected Tropical Diseases. 2010 doi: 10.1371/journal.pntd.0000626. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinsstag J, Dürr S, Penny MA, Mindekem R, Roth F, Menendez Gonzalez S, et al. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proceedings of the National Academy of Sciences of the United States of America. 2009 Sep 1;106(35):14996–5001. doi: 10.1073/pnas.0904740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick MC, Hampson K, Cleaveland S, Meyers LA, Townsend JP, Galvani AP. Reithinger R, editor. Potential for rabies control through dog vaccination in wildlife-abundant communities of Tanzania. PLoS neglected tropical diseases. 2012 Aug 21;6(8):e1796. doi: 10.1371/journal.pntd.0001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer HL, Hampson K, Lembo T, Cleaveland S, Kaare M, Haydon DT. Vaccine. 6. Vol. 30. Elsevier Ltd; 2011. The implications of metapopulation dynamics on the design of vaccination campaigns. pp. 1014–22. [DOI] [PubMed] [Google Scholar]
- 12.Mathers CD, Vos T, Lopez AD, Salomon J, Ezzati M. Health Policy. World Health Organization; Geneva: 2001. National Burden of Disease Studies: A practical guide. pp. 1–137. [Google Scholar]
- 13.Meltzer MI, Rupprecht CE. A review of the economics of the prevention and control of rabies. Part 2: Rabies in dogs, livestock and wildlife. PharmacoEconomics. 1998 Nov;14(5):481–98. doi: 10.2165/00019053-199814050-00003. [DOI] [PubMed] [Google Scholar]
- 14.WHO Commission on Macroeconomics and Health . Macroeconomics and Health: Investing in Health for Economic Development. Geneva: Jun, 2001. [Google Scholar]
- 15.WHO . Choosing interventions that are cost effective (WHO-CHOICE) World Health Organization; Geneva: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The World Bank The World Bank website [Internet] [cited 2013 Aug 1]. Available from: data.worldbank.org/country/tanzania.
- 17.Lembo T, Hampson K, Haydon DT, Craft M, Dobson A, Dushoff J, et al. Exploring reservoir dynamics: a case study of rabies in the Serengeti ecosystem. Journal of Applied Ecology. 2008:1246–57. doi: 10.1111/j.1365-2664.2008.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission Dynamics and Prospects for the Elimination of Canine Rabies. Plos Biology. 2009:462–71. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaare M, Lembo T, Hampson K, Ernest E, Estes A, Mentzel C, et al. Rabies control in rural Africa: evaluating strategies for effective domestic dog vaccination. Vaccine. 2009 Jan 1;27(1):152–60. doi: 10.1016/j.vaccine.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman PG, Dye C. Immunization coverage required to prevent outbreaks of dog rabies. Vaccine. 1996 Feb;14(3):185–6. doi: 10.1016/0264-410x(95)00197-9. [DOI] [PubMed] [Google Scholar]
- 21.Kitala PM, McDermott JJ, Coleman PG, Dye C. Comparison of vaccination strategies for the control of dog rabies in Machakos District, Kenya. Epidemiology and Infection. 2002 Sep 2;129(01):215–22. doi: 10.1017/s0950268802006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampson K, Dushoff J, Bingham J, Bruckner GK, Ali YH, Dobson A. Synchronous cycles of domestic dog rabies in sub-Saharan Africa and the impact of control efforts. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7717–22. doi: 10.1073/pnas.0609122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampson K, Dobson A, Kaare M, Dushoff J, Magoto M, Sindoya E, et al. Rabies Exposures, Post-Exposure Prophylaxis and Deaths in a Region of Endemic Canine Rabies. Plos Neglected Tropical Diseases. 2008 doi: 10.1371/journal.pntd.0000339. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleaveland S, Fevre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bulletin of the World Health Organization. 2002;80(4):304–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Shim E, Hampson K, Cleaveland S, Galvani AP. Evaluating the cost-effectiveness of rabies post-exposure prophylaxis: a case study in Tanzania. Vaccine. 2009 Nov 27;27(51):7167–72. doi: 10.1016/j.vaccine.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurenson M, Mlengeya T, Shiferaw F, Cleaveland S. Approaches to disease control in domestic canids for the conservation of endangered wild carnivores. Occasional Papers of the IUCN Species Survival Commission. 2003:30. [Google Scholar]
- 27.Randall DA, Marino J, Haydon DT, Sillero-Zubiri C, Knobel DL, Tallents LA, et al. An integrated disease management strategy for the control of rabies in Ethiopian wolves. Biological Conservation. 2006 Aug;131(2):151–62. [Google Scholar]
- 28.Briggs AH, Goeree R, Blackhouse G, O'Brien BJ. Probabilistic analysis of cost-effectiveness models: Choosing between treatment strategies for gastroesophageal reflux disease. Medical Decision Making. 2002;22(4):290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- 29.Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making. 1998;18(2):S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 30.Tanzania Global Health Initiative Strategy 2010 - 2015 [Internet] Washington, DC: 2011. [cited 2013 Aug 23]. Available from: http://www.ghi.gov/documents/organization/175135.pdf. [Google Scholar]
- 31.Braithwaite RS, Meltzer DO, King JT, Leslie D, Roberts MS. What does the value of modern medicine say about the 50,000 per quality-adjusted life-year decision rule? Medical Care. 2008;46(4):349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 32.Bogel K, Meslin FX. Economics of human and canine rabies elimination: guidelines for programme orientation. Bulletin of the World Health Organization. 1990;68(3):281–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider MC, Belotto A, Ade MP, Hendrickx S, Leanes LF, Rodrigues MJD, et al. Current status of human rabies transmitted by dogs in Latin America. Cadernos De Saude Publica. 2007;23(9):2049–63. doi: 10.1590/s0102-311x2007000900013. [DOI] [PubMed] [Google Scholar]
- 34.Cliquet F, Robardet E, Must K, Laine M, Peik K, Picard-Meyer E, et al. Eliminating rabies in Estonia. PLoS neglected tropical diseases. 2012 Jan;6(2):e1535. doi: 10.1371/journal.pntd.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeil D., Jr. Rabies: Rabies Outbreak in Angola, Caused By Roaming Dogs, Kills 93 Children. The New York Times. 2009 Mar 17; Section D; Column 0; Science Desk; Global Update. [Google Scholar]
- 36.Hampson K, Cleaveland S, Briggs D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS neglected tropical diseases. 2011 Jan;5(3):e982. doi: 10.1371/journal.pntd.0000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davlin SL, Vonville HM. Canine rabies vaccination and domestic dog population characteristics in the developing world: a systematic review. Vaccine. 2012 May 21;30(24):3492–502. doi: 10.1016/j.vaccine.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 38.Brooks R. The Veterinary record. 24. Vol. 127. BMJ Publishing Group Limited; Dec 15, 1990. Survey of the dog population of Zimbabwe and its level of rabies vaccination. pp. 592–6. [PubMed] [Google Scholar]
- 39.Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, et al. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Veterinary Microbiology. 2009;133(3):283–6. doi: 10.1016/j.vetmic.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Kayali U, Mindekem R, Yemadji N, Vounatsou P, Kaninga Y, Ndoutamia AG, et al. Coverage of pilot parenteral vaccination campaign against canine rabies in N'Djamena, Chad. Bulletin of the World Health Organization. 2003;81(10):739–44. [PMC free article] [PubMed] [Google Scholar]
- 41.R Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria; 2010. (01/19) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.