Abstract
PURPOSE
Recombinant BMP-7 inhibits the pathogenesis of renal injury in response to a variety of stimuli. However, little is known about the molecular regulation of endogenous BMP-7 and its renal protective functions. This study examines the transcriptional regulation of Bmp-7 and its role in the pathogenesis of renal injuries resulting from urinary tract dysfunction.
MATERIALS AND METHODS
Obstruction-induced renal injury was modeled in vivo in mice by unilateral ureteral obstruction (UUO) and in vitro in primary kidney cells by treatment with TGF-β, a pro-fibrotic cytokine that is elevated in the obstructed kidney.
RESULTS
UUO results in the loss of BMP-7 expression in conjunction with histone deacetylation and transcriptional repression of the Bmp-7 promoter. The histone deacetylase (HDAC) inhibitor trichostatin A (TSA) stimulates Bmp-7 expression in primary kidney cells. Furthermore, TSA inhibits the expression of TGF-β-dependent pro-fibrotic genes in a manner that is dependent upon BMP receptor signaling. These findings extend to the obstructed kidney in vivo where treatment with TSA restores the expression of Bmp-7 along with the BMP-7-mediated suppression of TGF-β-dependent signaling pathways. Finally, the TSA-stimulated activation of the BMP-7 pathway ameliorates obstruction-induced renal injuries by preventing the disruption of renal architecture and the development of renal fibrosis.
CONCLUSIONS
Together, these findings demonstrate that the HDAC-dependent repression of Bmp-7 transcription is a critical event during the pathogenesis of renal injury in obstructive uropathies. Accordingly, treatment with HDAC inhibitors represents a potentially effective strategy to restore BMP-7 expression and its renal protective functions during the treatment of obstructive uropathies.
Keywords: Bone Morphogenic Protein 7, Fibrosis, Histone Deacetylases, Transforming Growth Factor Beta, Ureteral Obstruction
INTRODUCTION
Obstructive uropathies are a leading cause of pediatric kidney disease, accounting for 16.5% of kidney transplants in children.1 While the kidney possesses innate repair mechanisms that are capable of restoring renal structure and function following acute injury due to urinary obstruction and other stimuli,2–4 the clinical manifestation of chronic renal injury stems in part from a dysregulated wound healing response that results in renal fibrosis.3, 4 At the molecular level, the activation of the TGF-β pathway has classically been described as a critical pathologic step in the development of renal fibrosis that promotes apoptosis, cellular de-differentiation, myofibroblast activation, and matrix protein synthesis.5 Given that these same processes are involved in injury repair,4 it is likely that TGF-β also plays a physiologic role in the early stages of injury repair but, when TGF-β activity is dysregulated, it instead promotes disease progression. Nonetheless, the mechanisms that counterregulate TGF-β activity during the response to renal injury are poorly understood.
Interestingly, another member of the TGF-β superfamily, BMP-7, has been demonstrated to inhibit TGF-β-dependent signaling pathways.6 The inhibitory effects of BMP-7 are mediated by the activation of the SMAD1/5/8 proteins which, in turn, suppress the activity of TGF-β-dependent transcription factors and their ability to stimulate pro-fibrotic gene expression.7, 8 Importantly, treatment with recombinant BMP-7 inhibits the development of renal injury in response to urinary obstruction and a variety of other stimuli.6–17 While the renal protective effects of recombinant BMP-7 are well established, our laboratory recently demonstrated that endogenous BMP-7 activity is required for the cessation of the TGF-β response along with the restoration of renal architecture and the resolution of fibrotic changes in the kidney that occur during the repair of obstruction-induced renal injuries.8 The importance of BMP-7 in the repair of renal injuries is further supported by studies in our laboratory and by others which have demonstrated that treatment with recombinant BMP-7 is capable of reversing the progression of chronic renal injuries.8–10
Despite the biologic importance of BMP-7, little is known about the molecular mechanisms that regulate endogenous BMP-7 activity and its renal protective functions. However, our laboratory found that BMP-7 expression is upregulated during kidney repair following acute obstruction-induced renal injury.7, 8 Conversely, chronic renal injury in response to a variety of stimuli, including urinary obstruction, is associated with the loss of BMP-7 expression.8, 11–13, 18 Along with the previously discussed findings, this suggests that the loss of BMP-7 expression is a critical event during the pathogenesis of chronic renal injuries that may lead to both the suppression of BMP-7-dependent repair mechanisms and, in part, the persistent and inappropriate activation of TGF-β-dependent pro-fibrotic pathways. In this study, we delineate the molecular mechanisms that lead to the loss of BMP-7 expression in the obstructed kidney and examine their potential importance during the treatment of patients with obstructive uropathies and other conditions that lead to chronic renal injury.
MATERIALS AND METHODS
Unilateral Ureteral Obstruction
A unilateral ureteral obstruction was created in 8–10 week old C57BL/6J mice by placing a microvascular clamp on the proximal ureter.2 When indicated, mice were treated with 500 μg/kg of trichostatin A (Sigma) daily by intraperitoneal injection. All procedures were approved by institutional review.
Histology
Trichrome staining was performed using the Accustain Masson’s Trichrome Staining Kit (Sigma). Immunofluorescence was performed by using rabbit anti-BMP-7 and rabbit anti-Type IV Collagen (Abcam), as previously described.8 Tubular volume was quantified in samples stained for Type IV Collagen by digitally overlaying a grid on microscopy images and determining the percentage of grid points located in the interstitial/tubular regions.14
Collagen Quantification
Kidney samples were hydrolyzed and hydroxyproline was quantified by comparison to standards in a colorimetric reaction, as previously described.19 Collagen content was calculated using the approximation that collagen contains approximately 14% (w/w) hydroxyproline and expressed as a ratio to the dry tissue weight of the sample.
BMP-7 ELISA
Kidneys were pulverized in liquid nitrogen and homogenized in 100 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, and Complete Protease Inhibitor Cocktail (Roche). Samples were normalized according to total protein content using the BCA Protein Assay Kit (ThermoScientific). BMP-7 levels were quantified by using the BMP-7 ELISA Kit (R&D Systems) according to product specifications.
RT-PCR
Kidneys were pulverized in liquid nitrogen, homogenized in Trizol (Invitrogen), and RNA was isolated. RT-PCR was conducted by using the Superscript RT-PCR system (Invitrogen) and primers specific for BMP-7 (5′-GAAAACAGCAGCAGTGACCA-3′; 5′-GCTCAGGAGAGGT TGGTCTG-3′), TGF-β1 (5′-CAAACGTCGGGGCGACCTGG-3′; 5′-TGCTCCACCTTGGGC TTGCG-3′), α-smooth muscle actin (5′-CCCTGAGACGCTGCTCCAGCTA-3′; 5′-GGCATAG AGGGACAGCACAGCCT-3′), the α1 chain of type I collagen (5′-TGGTCCTGCCGGTCCT CCTG-3′; 5′-ACACATTGGGGGTAGGAACA-3′), or GAPDH (5′-AACTTTGGCATTGTGG AAGG-3′; 5′-ACACATTGGGGGTAGGAACA-3′). Relative intensities of PCR bands were quantified using ImageJ (NIH) analysis software and normalized as a ratio to GAPDH levels.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed using the Imprint ChIP kit (Sigma). DNA was sheared to 2 kB fragments by sonication. Immunoprecipitation was performed using either rabbit IgG or rabbit anti-acetyl-histone H3 (Sigma). PCR was performed using primers specific to the −407 to −151 bp region of the proximal Bmp-7 promoter (5′-GTTTGTTGCTGGTGCCCGCG-3′; 5′-GCTCTACGCGCGATCCGGG-3′). Relative intensities of PCR bands were quantified using ImageJ (NIH) analysis software and normalized as a ratio to PCR products obtained from reactions performed on input DNA.
Culture of Primary Kidney Cells
Murine inner medullary collecting duct cells (IMCD-3) were obtained from ATCC. Cells were cultured in DMEM: F12 growth media supplemented with 10% FBS. When indicated, cells were treated with 100 nM trichostatin A (Sigma), 1 ng/mL TGF-β (R&D Systems), 10 ng/mL BMP-7 (R&D Systems), or 10 μM dorsomorphin (Sigma).
Statistical Analysis
Statistical significance was analyzed by t-test or ANOVA with Bonferroni correction for multiple comparisons as appropriate. Data are presented as mean values ± standard deviation.
RESULTS
Obstruction-Induced Renal Injury is Associated with the Transcriptional Repression of Bmp-7 Expression
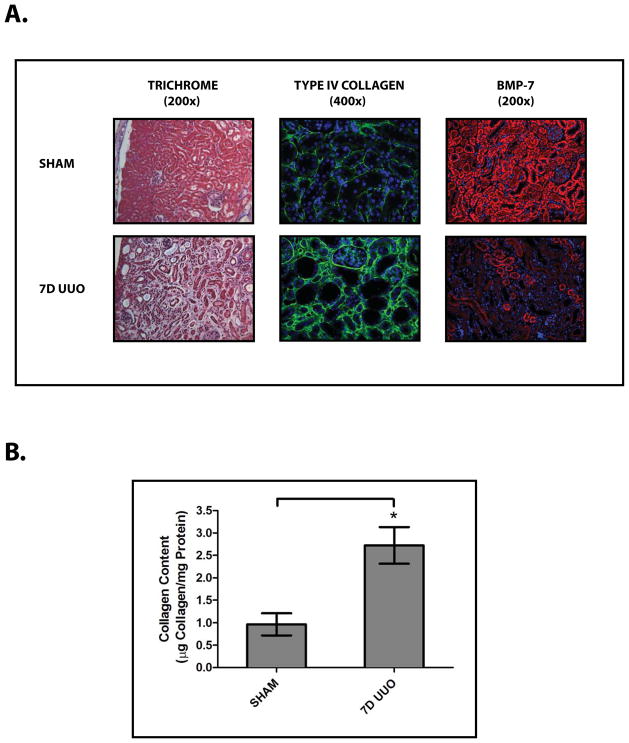
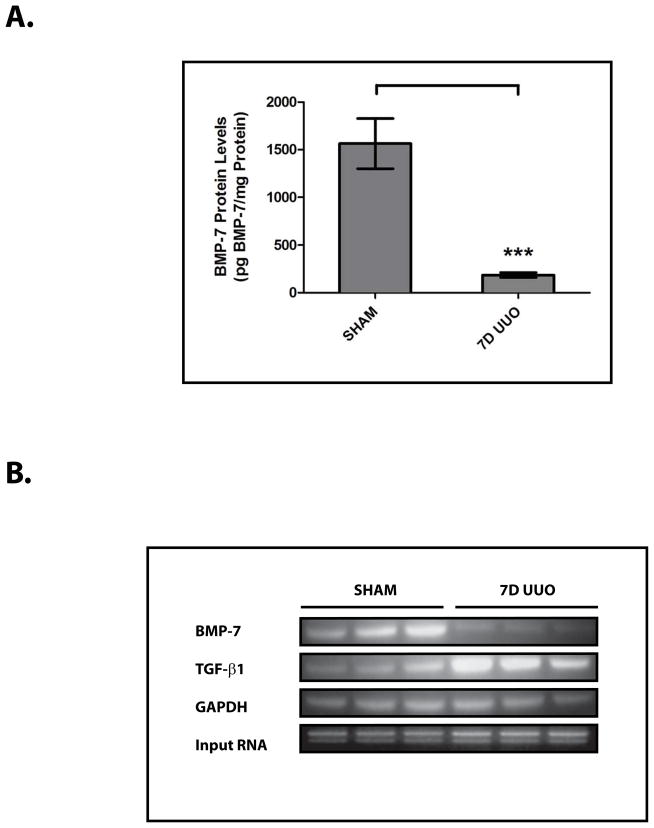
To examine the molecular regulation of BMP-7, we studied a murine model of obstruction-induced renal injury following unilateral ureteral obstruction (UUO) (Fig. 1A, 1B).2 UUO results in a dramatic decrease in BMP-7 expression in a variety of cell types throughout the kidney (Fig. 1A). In quantifying the loss of BMP-7 expression, we found that UUO-induced renal injury is associated with an 88.1±11.1% decrease in BMP-7 protein levels (p<0.005) (Fig. 2A) along with a corresponding 83.1±12.8% decrease in BMP-7 mRNA levels (p<0.01) (Fig. 2B, 2C). Together, these data suggested the possibility that the loss of BMP-7 expression is mediated at the transcriptional level.
Figure 1. Obstruction-Induced Renal Injury Leads to the Loss of BMP-7 Expression.
Mice (n=5) underwent either a sham operation or unilateral ureteral obstruction (UUO) for 7 days. Kidneys were analyzed by (A) (left) Masson’s Trichrome staining (200x), (middle) Type IV Collagen immunofluorescence (green) with DAPI counterstaining (blue) (400x), (right) BMP-7 immunofluorescence (red) with DAPI counterstaining (blue) (200x), and (B) quantification of collagen content. * denotes p<0.05.
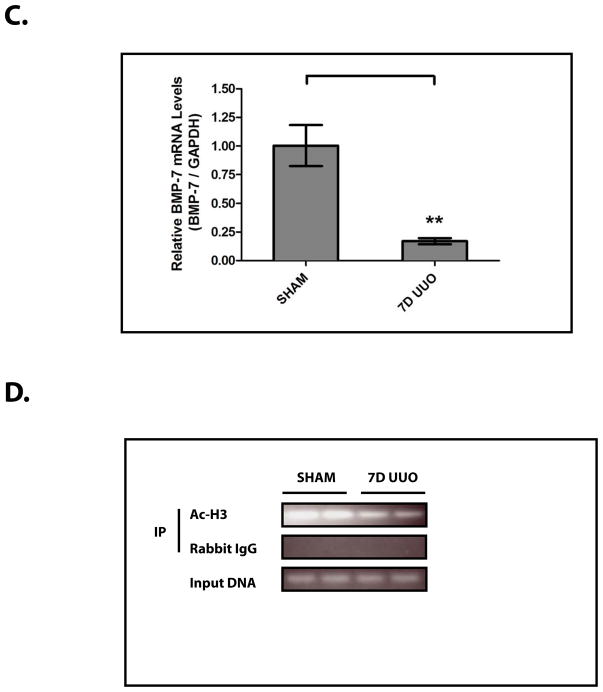
Figure 2. The Loss of BMP-7 Expression is Mediated at the mRNA Level in Conjunction with Histone Deacetylation in the Proximal Bmp-7 Promoter.
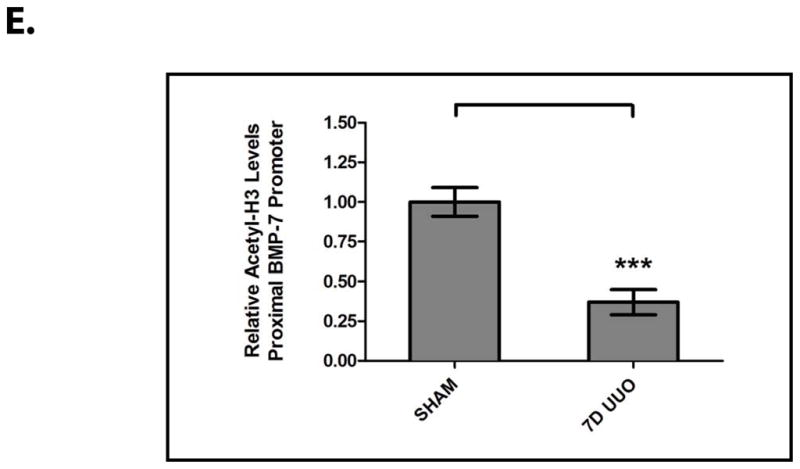
Mice (n=3) underwent either a sham operation or unilateral ureteral obstruction (UUO) for 7 days. Kidneys were analyzed by (A) BMP-7 ELISA, (B) RT-PCR for Bmp-7, TGF-β1, and GAPDH (control), (C) quantification of Bmp-7 expression from Fig. 2B, (D) chromatin immunoprecipitation with anti-acetylated-histone H3 or rabbit IgG (control) followed by PCR directed against the proximal region (−407 to −151 bp) of the Bmp-7 promoter, and (E) quantification of histone acetylation in the Bmp-7 promoter from Fig. 2D. ** denotes p<0.01; *** denotes p<0.005.
Accordingly, we sought to determine whether the loss of BMP-7 mRNA expression is associated with chromatin modifications in the regulatory regions of the Bmp-7 gene. Indeed, we found that UUO results in a 63.0±8.5% decrease in the acetylation of histone H3 proteins bound to the proximal Bmp-7 promoter (p<0.005) (Fig. 2D, 2E). This is a significant finding given that a number of transcriptional factors function by recruiting histone deacetylase (HDAC) complexes to target genes, a process that subsequently promotes chromatin condensation and transcriptional repression.20
HDAC Proteins Regulate Bmp-7 Expression and the BMP-7-Mediated Suppression of TGF-β-Dependent Pro-Fibrotic Pathways In Vitro
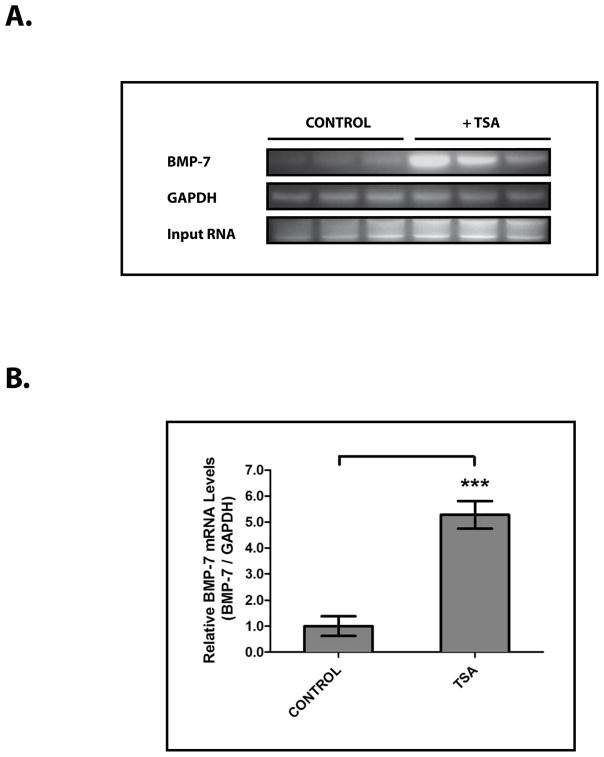
To begin to investigate the role that changes in histone acetylation play in the regulation of BMP-7 expression, we examined the transcriptional regulation of Bmp-7 in inner medullary collecting duct cells (IMCD-3), a significant source of BMP-7 in the kidney.6 Subsequently, we found that treatment with the pan-HDAC inhibitor trichostatin A (TSA) 20 stimulates a 5.3 ± 1.2 fold increase in BMP-7 mRNA levels in IMCD-3 cells (p<0.005) (Fig. 3A, 3B). This finding, along with those in Fig. 2D and Fig. 2E, demonstrates that Bmp-7 expression is repressed by HDAC proteins.
Figure 3. HDAC Proteins Repress BMP-7 mRNA Expression in Primary Kidney Cells.
IMCD-3 cells (n=3 plates/sample) were treated with PBS (control) or 100 nM trichostatin A (TSA) for 24 hours. Cells were analyzed by (A) RT-PCR for Bmp-7 and GAPDH (control) and (B) quantification of Bmp-7 expression from Fig. 3A. *** denotes p<0.005.
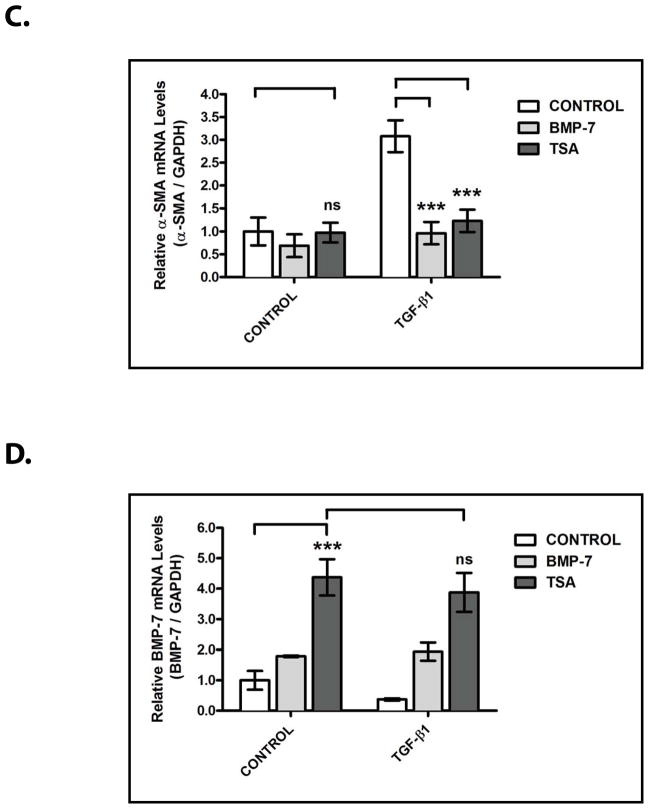
We next sought to determine the importance of the HDAC-dependent regulation of Bmp-7 expression in the renal protective functions of the BMP-7 pathway. To accomplish this, we studied an in vitro model of TGF-β-mediated renal fibrosis and used the suppression of TGF-β-dependent pro-fibrotic gene expression as a functional readout for BMP-7 activity. As previously demonstrated, treatment with TGF-β induces the expression of several pro-fibrotic genes that are central to the pathogenesis of renal injury including the α1 chain of type I collagen (COLIα1; a gene that encodes a protein that is a significant contributor to fibrosis)21 (p<0.005) (Fig. 4A, 4B) and α-smooth muscle actin (α-SMA; a gene that encodes a protein that contributes to the differentiation of pro-fibrotic myofibroblasts)22 (p<0.005) (Fig. 4A, 4C). Importantly, co-treatment with recombinant BMP-7 results in a 67.3 ± 25.8% decrease in COLIα1 induction (p<0.005) (Fig. 4A, 4B) and a 68.7 ± 19.1% decrease in α-SMA induction (p<0.005) (Fig. 4A, 4C), demonstrating that the activation of the BMP-7 pathway is sufficient to suppress TGF-β-dependent pro-fibrotic gene expression in this model system.
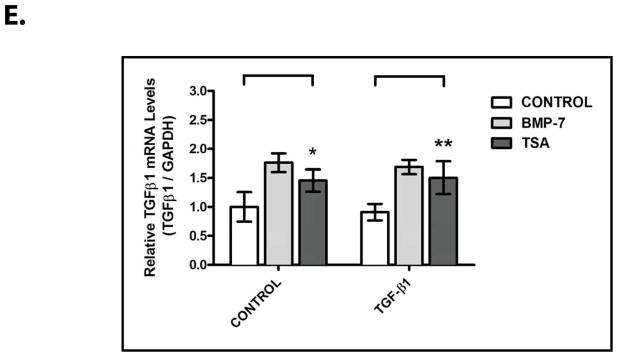
Figure 4. HDAC Inhibition Suppresses the Activity of TGF-β-Dependent Pro-Fibrotic Pathways in Primary Kidney Cells.
IMCD-3 cells (n=3 plates/sample) were serum starved for 24 hours, reintroduced to serum-containing medium, and treated with either PBS (control) or 1 ng/mL TGF-β and either PBS (control), 10 ng/mL BMP-7, or 100 nM TSA for 24 hours, as indicated. Cells were analyzed by (A) RT-PCR for Bmp-7, TGF-β1, COLIα1, α-SMA and GAPDH (control), (B) quantification of COLIα1 expression from Fig. 4A, (C) quantification of α-SMA expression from Fig. 4A, (D) quantification of BMP-7 expression from Fig. 4A, and (E) quantification of TGF-β1 expression from Fig. 4A. ns denotes p>0.05; * denotes p<0.05; ** denotes p<0.01; *** denotes p<0.005.
In examining the effects of HDAC inhibition on the activity of the BMP-7 pathway, we found that treatment with TSA results in a 4.4 ± 1.5 fold increase in Bmp-7 expression (p<0.005) and that the stimulation of Bmp-7 expression is not affected by co-treatment with TGF-β (Fig. 4A, 4D). In a manner similar to treatment with BMP-7, co-treatment with TSA results in a 73.7 ± 21.9% decrease in TGF-β-induced COLIα1 expression (p<0.005) (Fig. 4A, 4B) and a 60.0 ± 13.6% decrease in TGF-β-induced α-SMA expression (p<0.005) (Fig. 4A, 4C). Given that TGF-β-dependent Smad-binding sites have previously been shown to be required for promoter activity in the COLIα1 and α-SMA genes,21, 22 it is likely that the inhibitory effects of TSA treatment are mediated by the suppression of downstream steps in the TGF-β pathway. In support of this possibility, treatment with TSA has only minimal effects on the baseline expression of COLIα1 (Fig. 4A, 4B), α-SMA (Fig. 4A, 4C), and endogenous TGF-β1 (Fig. 4A, 4E) in the absence of recombinant TGF-β.
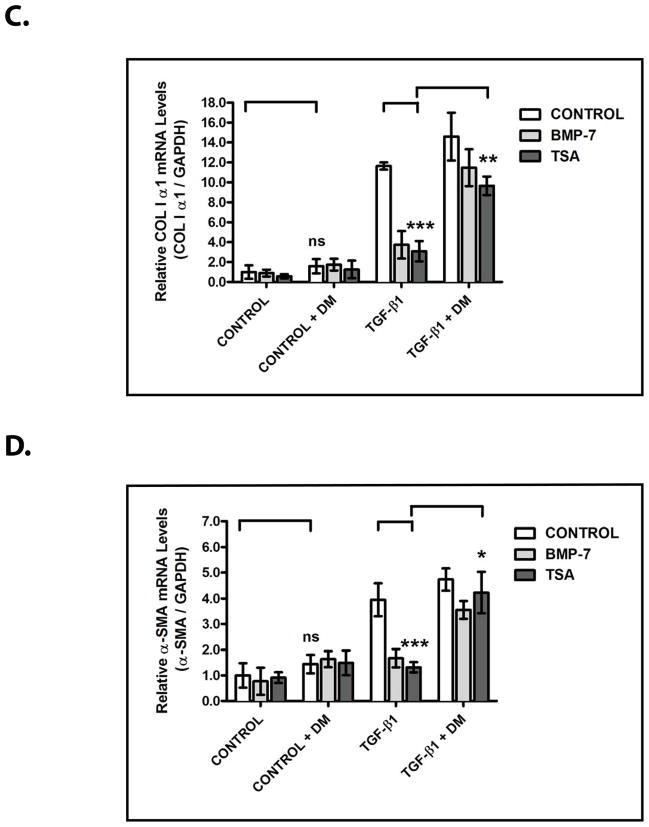
We next sought to determine the requirement for BMP-7 activity in the ability of TSA to inhibit TGF-β-dependent pro-fibrotic gene expression. To accomplish this, we examined the effects of dorsomorphin, a pharmacologic inhibitor of BMP receptor activity,23 on the inhibitory effects of TSA. As in Fig. 4, treatment with TSA stimulates Bmp-7 expression (p<0.005) and suppresses TGF-β-induced COLIα1 (p<0.005) and α-SMA (p<0.005) expression (Fig. 5A–5D). However, when combined with dorsomorphin treatment, the inhibitory effects of TSA on TGF-β-induced COLIα1 expression are reduced by 66.8 ± 35.5% (p<0.01) (Fig. 5A, 5C) and on TGF-β-induced α-SMA expression are reduced by 77.9 ± 17.1% (p<0.05) (Fig. 5A, 5D). Importantly, treatment with dorsomorphin does not affect TSA-stimulated Bmp-7 expression (Fig. 5A, 5B) and has only minimal effects on the baseline expression of COLIα1 (Fig. 5A, 5C), α-SMA (Fig. 5A, 5D), and endogenous TGF-β1 (Fig. 5A, 5E) in the absence of recombinant TGF-β. Together, these findings demonstrate that the TGF-β-suppressing effects of HDAC inhibition are largely dependent upon stimulating the activity of the BMP-7 pathway.
Figure 5. The TGF-β-Suppressing Effects of HDAC Inhibition Are Dependent Upon the Stimulation of BMP Activity.

IMCD-3 cells (n=3 plates/sample) were serum starved for 24 hours, reintroduced to serum-containing medium, and treated with either PBS (control) or 1 ng/mL TGF-β, either PBS (control), 10 ng/mL BMP-7, or 100 nM TSA, and either PBS (control) or 10 μM dorsomorphin (DM) for 24 hours, as indicated. Cells were analyzed by (A) RT-PCR for Bmp-7, TGF-β1, COLIα1, α-SMA and GAPDH (control), (B) quantification of Bmp-7 expression from Fig. 5A, (C) quantification of COLIα1 expression from Fig. 5A, (D) quantification of α-SMA expression from Fig. 5A, and (E) quantification of TGF-β1 expression from Fig. 5A. ns denotes p>0.05; * denotes p<0.05; ** denotes p<0.01; *** denotes p<0.005.
HDAC Inhibition Restores BMP-7 Expression, Suppresses TGF-β Activity, and Ameliorates Renal Injury in Obstructive Uropathies
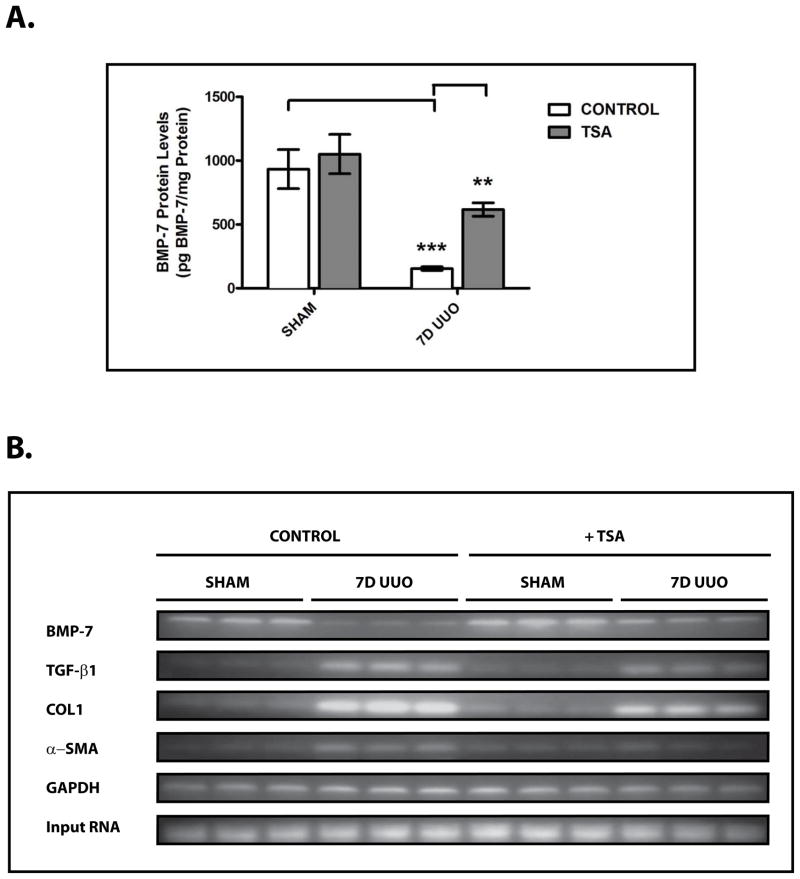
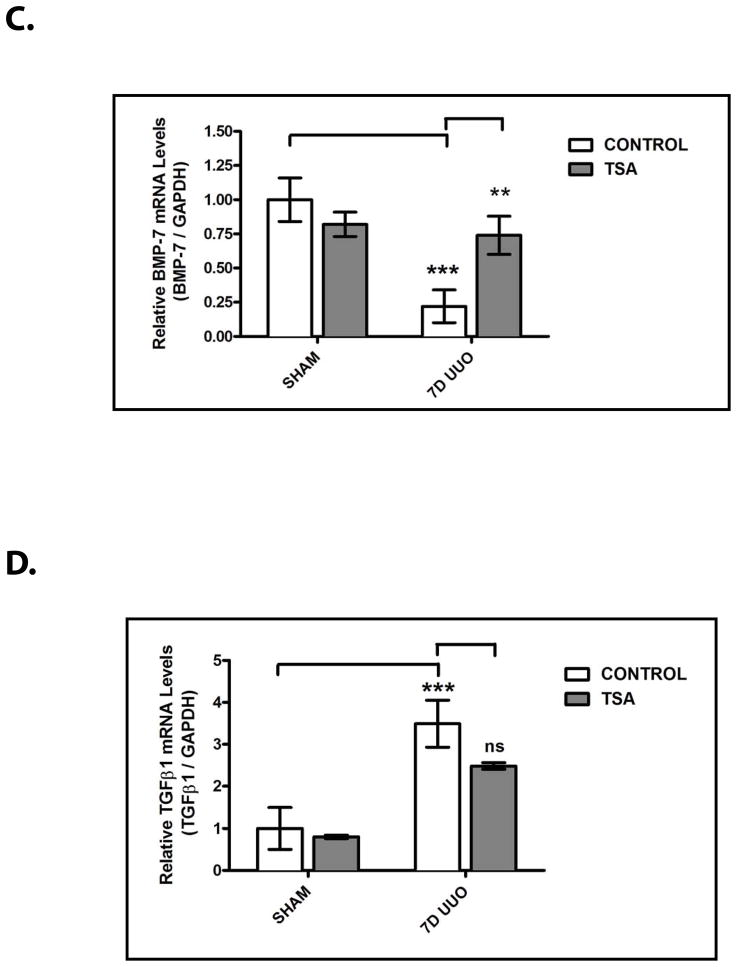
To extend these findings to the pathogenesis of chronic renal injury in vivo, we next sought to examine the effects of HDAC inhibition on BMP-7 expression in our murine model of obstructive uropathy. While UUO results in a significant decrease in BMP-7 protein levels, treatment with TSA restores BMP-7 expression in the obstructed kidney by stimulating a 4.0 ± 0.5 fold increase in BMP-7 protein levels (p<0.01) (Fig. 6A). Importantly, these changes in BMP-7 expression at the protein level are paralleled by a 3.4 ± 1.9 fold increase in BMP-7 mRNA expression (p<0.01) (Fig. 6B, 6C). Along with our findings in Fig. 2D and Fig. 2E, these results demonstrate that Bmp-7 transcription is regulated by HDAC-dependent mechanisms in vivo.
Figure 6. HDAC Inhibition Restores BMP-7 Expression and Suppresses TGF-β-Dependent Pro-Fibrotic Pathways Following Obstruction-Induced Renal Injury.
Mice (n=3) underwent either sham operation or unilateral ureteral obstruction (UUO) for 7 days and were treated with either PBS (control) or 500 μg/kg of trichostatin A (TSA). Kidneys were analyzed by (A) BMP-7 ELISA, (B) RT-PCR for Bmp-7, TGF-β1, COLIα1, α-SMA, or GAPDH (control), (C) Quantification of Bmp-7 expression from Fig. 6B, (D) Quantification of TGF-β1 expression from Fig. 6B, (E) Quantification of COLIα1 expression from Fig. 6B, and (F) Quantification of α-SMA expression from Fig. 6B. ns denotes p > 0.05; * denotes p < 0.05; ** denotes p < 0.01; *** denotes p < 0.005.
Subsequently, we sought to determine whether the HDAC-dependent repression of Bmp-7 expression plays a role in the molecular mechanisms that respond to renal injury. As shown in previous studies, UUO results in a significant upregulation in the expression of TGF-β1 (p<0.005) (Fig. 6B, 6D) along with its downstream pro-fibrotic target genes COLIα1 (p<0.005) (Fig. 6B, 6E) and α-SMA (p<0.05) (Fig. 6B, 6F) during the development of obstruction-induced renal fibrosis.5 Importantly, treatment with TSA not only restores BMP-7 expression in the obstructed kidney (p<0.01) (Fig. 6A–6C), but also reduces COLIα1 expression by 41.6 ± 7.0% (p<0.005) (Fig. 6B, 6E) and α-SMA expression by 61.6 ± 37.0% (p<0.05) (Fig. 6B, 6F). Furthermore, TSA treatment has only minimal effects on TGF-β1 expression (Fig. 6B, 6D), demonstrating that the inhibitory effects of HDAC inhibition are mediated by the suppression of downstream steps in the TGF-β pathway. Along with our prior findings (Fig. 4, 5), these results strongly suggest that the HDAC-dependent repression of Bmp-7 expression contributes to the dysregulation of the TGF-β pathway following chronic renal injury.
Since the findings in Fig. 6 demonstrated that HDAC inhibition restores BMP-7 expression and the balance in TGF-β/BMP-7 signaling in the obstructed kidney, we next examined the therapeutic effects of treatment with HDAC inhibitors during the progression of chronic renal injury. While UUO results in the loss of BMP-7 expression in a variety of cell types in the obstructed kidney, treatment with TSA effectively targets each of these cell populations to restore BMP-7 expression (Fig. 7A). More importantly, treatment with TSA also markedly inhibits the development of UUO-induced renal injury. TSA treatment is associated with a 31.1 ± 7.6% decrease in total renal collagen content (p<0.05) (Fig. 7A, 7B), a striking finding given that this corresponds to a 65.3% reduction in the increase in collagen expression above baseline levels following UUO. Furthermore, TSA treatment results in a 43.4 ± 10.1% decrease in the loss of tubular volume following UUO (p<0.01) (Fig. 7A, 7C). Together, these findings demonstrate that the administration of pharmacologic HDAC inhibitors is an effective therapeutic approach not only to prevent the loss of BMP-7 expression in the injured kidney, but also to inhibit the development of renal fibrosis and the disruption of renal architecture during the progression of chronic renal injury.
Figure 7. HDAC Inhibition Ameliorates Obstruction-Induced Renal Injuries.
Mice (n=5) underwent either sham operation or unilateral ureteral obstruction (UUO) for 7 days and were treated with either PBS (control) or 500 μg/kg of trichostatin A (TSA). Kidneys were analyzed by (A) (left) Masson’s Trichrome staining (200x), (middle) Type IV Collagen immunofluorescence (green) with DAPI counterstaining (blue) (400x), (right) BMP-7 immunofluorescence (red) with DAPI counterstaining (blue) (200x), (B) quantification of collagen content, and (C) quantification of tubular volume. * denotes p < 0.05; ** denotes p < 0.01.
DISCUSSION
Over the last decade, BMP-7 has emerged as a critical renal protective protein that safeguards the kidney against a variety of stimuli that cause renal injury.6–17 However, the loss of BMP-7 expression occurs in obstructive uropathies and other conditions that lead to chronic renal injury.8, 11–13, 18 While the mechanisms that lead to the loss of BMP-7 expression have remained unclear, here we demonstrate in a mouse model of obstructive uropathy that the loss of BMP-7 expression occurs at the mRNA level in conjuction with histone deacetylation and transcriptional repression of the Bmp-7 promoter (Fig. 2). Importantly, treatment with the pan-specific HDAC inhibitor TSA restores BMP-7 expression in the obstructed kidney (Fig. 6).
In examining the role that the HDAC-dependent repression of Bmp-7 expression plays in the molecular mechanisms that respond to renal injury, we found that HDAC inhibition limits the pro-fibrotic potential of renal epithelial cells in vitro by suppressing the downstream steps in the TGF-β signaling pathway in a BMP-7-dependent manner (Fig. 4, 5). These signaling mechanisms (summarized in Fig. 8) also have considerable implications during the in vivo response to renal injury as demonstrated by the finding that treatment with TSA inhibits the expression of TGF-β-induced pro-fibrotic genes in the obstructed kidney (Fig. 6). We have previously published that BMP-7 is required for both the cessation of the TGF-β response and the repair of obstruction-induced renal injuries.8 The findings in this study strongly suggest that the HDAC-dependent repression of Bmp-7 expression contributes to the persistent activation of the TGF-β pathway and the dysregulation of kidney repair following chronic renal injury.
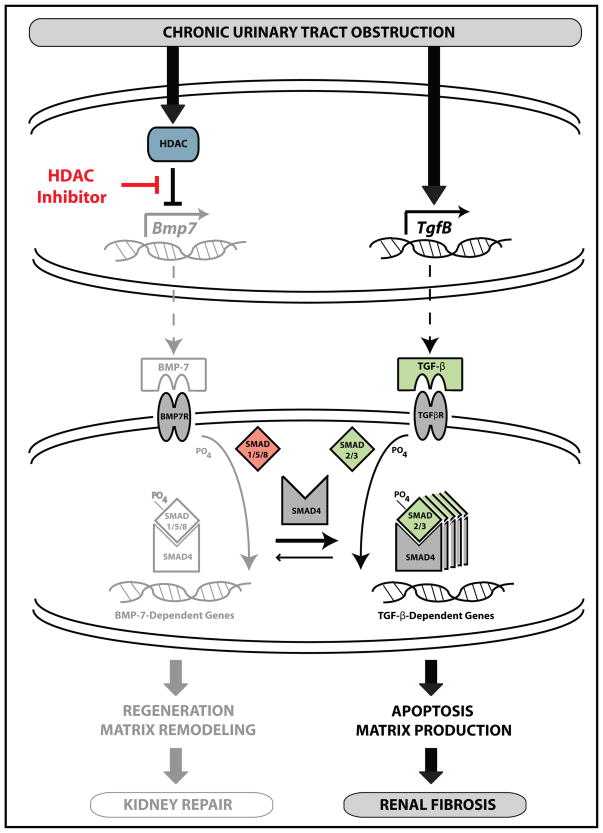
Figure 8. The HDAC-Dependent Transcriptional Repression of Bmp-7 Potentiates TGF-β-Mediated Renal Fibrosis in Obstructive Uropathies.
The BMP-7 protein is a secreted cytokine that normally functions to stimulate gene transcription by binding to its receptor and activating the Smad1/5/8 transcription factors. The activation of the BMP-7 pathway also suppresses the pro-fibrotic TGF-β pathway by sequestering Smad4, a protein necessary for the transcription of many TGF-β-dependent genes.6 These counter-regulatory mechanisms are important for the suppression of TGF-β-dependent pro-fibrotic pathways during kidney repair.8 However, chronic urinary tract obstruction results in the HDAC-dependent suppression of Bmp-7 transcription. The loss of BMP-7 results in the dysregulation of kidney repair along with the persistent and inappropriate activation of the TGF-β pathway. Importantly, treatment with pharmacologic HDAC inhibitors restores BMP-7 expression, suppresses TGF-β-dependent signaling pathways, and prevents renal fibrosis following chronic urinary tract obstruction.
Based on these findings, we have begun to examine the therapeutic potential of administering pharmacologic HDAC inhibitors as a means of limiting the progression of chronic renal injuries. Importantly, treatment with TSA significantly inhibits the development of renal fibrosis and the loss of renal architecture in response to urinary tract dysfunction (Fig. 7). While the suppression of TGF-β signaling is likely to account for many of the anti-fibrotic effects of TSA-stimulated BMP-7 expression, TGF-β-independent mechanisms likely also play an important role in inhibiting the pathogenesis of renal injury. We and others have previously shown that recombinant BMP-7 promotes the regeneration of renal architecture following injury 8–10, 17 in a manner that recapitulates the requirement for BMP-7 during kidney development.24 A possible role for these functions in the renal protective effects of HDAC inhibition is suggested by a recent study that identified an HDAC inhibitor as the leading candidate in a high throughput screen designed to identify compounds that stimulate regenerative cell populations within the kidney.25
To fully realize the therapeutic potential of HDAC inhibitors, it will be necessary to translate these findings to in vivo models which more closely parallel the development of kidney disease following congenital urinary obstruction. While our study examined obstruction-induced renal injury in the mature kidney, surgical models of fetal/neonatal obstruction as well as genetic models of congenital obstruction may provide useful tools for examining the importance of these biological pathways in the developing kidney.26 Additionally, while our treatment regimen for the administration of TSA is supported by prior pharmacokinetic studies,27 these protocols may be improved through further optimization. Finally, the use of isoform-specific HDAC inhibitors may provide a means of developing more specific and effective therapies for kidney disease.
CONCLUSIONS
In working towards the development of novel therapies for chronic kidney disease, it has become increasingly evident that the loss of BMP-7 is a critical molecular event that contributes to disease progression across a broad spectrum of etiologies.6–18 This study demonstrates that the loss of BMP-7 occurs by the HDAC-dependent repression of Bmp-7 transcription in a murine model of obstructive uropathy. Furthermore, treatment with HDAC inhibitors is an effective therapeutic approach to restore BMP-7 expression and limit the progression of chronic renal injury in the obstructed kidney. The importance of these findings is accentuated by studies which have demonstrated the renal protective effects of HDAC inhibition in other settings.28–30 Although the mechanisms underlying these effects have remained unclear, this study demonstrates that the remarkable therapeutic potential of HDAC inhibitors is, in part, due to their ability to target the BMP-7 pathway. Given that HDAC inhibitors are already in clinical use, it is imperative to further examine the utility of these compounds in the treatment of obstructive uropathies and other conditions that lead to chronic kidney disease.
Footnotes
DISCLOSURE OF FUNDING SOURCES
This research was supported by funding from the NIH/NIDDK (1R01DK096177).
References
- 1.Roth KS, Koo HP, Spottswood SE, et al. Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr (Phila) 2002;41:309. doi: 10.1177/000992280204100503. [DOI] [PubMed] [Google Scholar]
- 2.Cochrane AL, Kett MM, Samuel CS, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16:3623. doi: 10.1681/ASN.2004090771. [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- 5.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124:243. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 7.Manson SR, Niederhoff RA, Hruska KA, et al. The BMP-7-Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol. 2011;185:2523. doi: 10.1016/j.juro.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson SR, Niederhoff RA, Hruska KA, et al. Endogenous BMP-7 is a critical molecular determinant of the reversibility of obstruction-induced renal injuries. Am J Physiol Renal Physiol. 2011;301:F1293. doi: 10.1152/ajprenal.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 11.Zeisberg M, Bottiglio C, Kumar N, et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003;285:F1060. doi: 10.1152/ajprenal.00191.2002. [DOI] [PubMed] [Google Scholar]
- 12.Hruska KA, Guo G, Wozniak M, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279:F130. doi: 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, de Caestecker M, Kopp J, et al. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006;17:2504. doi: 10.1681/ASN.2006030278. [DOI] [PubMed] [Google Scholar]
- 14.Morrissey J, Hruska K, Guo G, et al. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002;13 (Suppl 1):S14. [PubMed] [Google Scholar]
- 15.Wang S, Chen Q, Simon TC, et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003;63:2037. doi: 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 16.Vukicevic S, Basic V, Rogic D, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102:202. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen-Qiang F, Bing-Wei Y, Yong-Liang L, et al. Localized expression of human BMP-7 by BM-MSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells. 2012;17:53. doi: 10.1111/j.1365-2443.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang SN, Lapage J, Hirschberg R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol. 2001;12:2392. doi: 10.1681/ASN.V12112392. [DOI] [PubMed] [Google Scholar]
- 19.Samuel CS. Determination of collagen content, concentration, and sub-types in kidney tissue. Methods Mol Biol. 2009;466:223. doi: 10.1007/978-1-59745-352-3_16. [DOI] [PubMed] [Google Scholar]
- 20.de Ruijter AJ, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritzenthaler JD, Goldstein RH, Fine A, et al. Regulation of the alpha 1(I) collagen promoter via a transforming growth factor-beta activation element. J Biol Chem. 1993;268:13625. [PubMed] [Google Scholar]
- 22.Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 23.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 25.de Groh ED, Swanhart LM, Cosentino CC, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21:794. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson L, Taylor GW, Aboagye EO, et al. Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice. Drug Metab Dispos. 2004;32:1132. doi: 10.1124/dmd.104.000638. [DOI] [PubMed] [Google Scholar]
- 28.Mishra N, Reilly CM, Brown DR, et al. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010;335:266. doi: 10.1124/jpet.110.168385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HB, Noh H, Seo JY, et al. Histone deacetylase inhibitors: a novel class of therapeutic agents in diabetic nephropathy. Kidney Int Suppl. 2007:S61. doi: 10.1038/sj.ki.5002388. [DOI] [PubMed] [Google Scholar]