SUMMARY
The germline of the nematode Caenorhabditis elegans exhibits a remarkable ability to specifically silence transgenic DNA. We have shown that this silencing mechanism is disrupted in animals mutant for the maternal effect sterile genes mes-2, mes-3, mes-4 and mes-6. The proteins encoded by mes-2 and mes-6 have been shown to be related to the Polycomb Group of transcriptional repressors (Holdeman, R., Nehrt, S. and Strome, S. (1998). Development 125, 2457-2467; Korf, I., Fan, F. and Strome, S. (1998). Development 125, 2469-2478). These results suggest that a genetic silencing process is essential for sustained germline function, and that this silencing is mediated, at least in part, by Polycomb Group proteins.
Keywords: Caenorhabditis elegans, mes genes, Polycomb group, Repeat dependent silencing, Germline
INTRODUCTION
The versatile genetic, molecular and cytological approaches available in C. elegans provide an excellent opportunity to investigate the fundamental dichotomy between somatic and germline tissue (Schedl, 1997). In the early embryo, C. elegans produces two primordial germ cells, named Z2 and Z3 (Sulston et al., 1983). These cells are surrounded by rapidly dividing and differentiating somatic cells, yet they remain essentially quiescent throughout embryogenesis (Sulston et al., 1983). When the larva hatches and feeds, Z2 and Z3 begin a concerted proliferation that ultimately gives rise to the thousand or so germ cells in the adult gonad (Kimble and Hirsch, 1979). The suppressive mechanisms that maintain germ cell ‘character’ in the early C. elegans embryo involve a complete (or near complete) inhibition of RNA polymerase II transcription mediated by the maternally encoded factor pie-1 (Mello et al., 1996; Seydoux et al., 1996; Seydoux and Dunn, 1997). Later in embryogenesis, and during larval development, pie-1 is absent (Mello et al., 1996) and a variety of genes are activated in the germline (Schedl, 1997). Little is known about how the germline maintains its totipotency during postembryonic development.
Certain aspects of the germline/soma dichotomy are reflected by the distinct treatment of repetitive transgenes in the two tissues. Transgenes in C. elegans are generally transmitted as large repetitive arrays composed of many tandem copies of the injected DNA(s) (Stinchcomb et al., 1985; Mello et al., 1991). The arrays are physically present in both soma and germline. As has been seen in a variety of organisms (Sabl and Henikoff, 1996; Martin and Whitelaw, 1996), C. elegans is capable of efficiently silencing certain repetitive transgene arrays (Okkema et al., 1993; Mello and Fire, 1995; J. Hsieh, S. Xu, and A. Fire, unpublished results). The C. elegans germline exhibits a particularly striking ability to silence transgene arrays. For several ‘housekeeping’ genes tested, repetitive transgene arrays that show activity in all somatic lineages are continuously silenced in the germline (Kelly et al., 1997). The silencing of transgenes in the germline is dependent on chromatin context: transgenes in non-repetitive arrays consisting of mixed fragments of C. elegans DNA can resist these silencing effects (Kelly et al., 1997). These observations suggest that the germline contains a unique system for chromatin organization, and that transgene silencing is a specific result of this mechanism.
We have taken a genetic approach based on transgene activity to identify components responsible for germline identity in general, and germline-specific silencing processes in particular. This has included (1) transgene-based genetic screens for mutations affecting germline silencing and (2) analyses of silencing in existing mutants with germline defects. One group of mutants with characterized germline defects are the mes (maternal effect sterile) mutants first identified in the laboratory of Susan Strome (Capowski et al., 1991). These genes are of particular interest in this respect, given that two of them (mes-2 and mes-6) have been shown to encode members of the Polycomb Group of transcriptional repressors (Holdeman et al., 1998; Korf et al., 1998).
MATERIALS AND METHODS
Alleles and strains
Animals were maintained as described (Brenner, 1974). Strains carrying mes mutations were as follows (Capowski et al., 1991):
mes-3(bn21ts) I, or mes-3(bn35) dpy-5(e61); sDp2(I;f)I
mes-2(bn11)/mnC1[dpy-10(e128)unc-52(e444)]II
-
mes-6(bn66)IV/DnT1[unc(n754)let](IV,V)
mes-6(bn66)IV/dpy-20(e1282ts)
mes-4(bn67)V/DnT1[unc(n754)let](IV,V)
Assay for germline desilencing
A stable transgenic line expressing a LET-858∷GFP reporter was generated by co-injecting a gfp-tagged let-858 reporter plasmid (pBK48.1; Kelly et al., 1997) with a selectable marker plasmid carrying the native pha-1 gene (pC1, Schnabel et al., 1990; Granato et al., 1994) into a host carrying the temperature-sensitive mutation pha-1(e2123)III. The plasmids were injected as circular DNAs. The resulting repetitive transgenic array was heritably transmitted. When maintained at 22-25°C, all viable animals carry the transgene, since viability requires the pha-1 rescuing activity of the array. All of these animals are capable of passing the array to a fraction of progeny; thus these animals carry the transgene array physically in the germline. Expression of the GFP-tagged LET-858 reporter from the transgene array is detectable in the nuclei of all somatic tissues in this line, but has not been observed in the germline (Kelly et al., 1997). The requirement for mes contributions by maternal (M) and zygotic (Z) genomes was tested by introducing the array into the appropriate genetic background and examining GFP activity in germline and somatic tissue. Requirements for zygotic MES contribution were tested by crossing array-bearing males with mes heterozygote hermaphrodites, cloning green (array bearing) cross progeny and selfing these animals to identify those with a mes/+ genotype. Adult progeny of mes/+ parents were cloned, allowed to lay eggs overnight and then scored for germline desilencing by fluorescence microscopy (Fig. 1A). The genotypic mes status for each cloned F2 animal was determined by the fertility of offspring [i.e., sterile progeny indicated the parent was a fertile mes/mes (M+Z−) animal]. This procedure also allowed us to assess the germline transgene silencing status of the sterile M−Z− F3 animals.
Fig. 1.
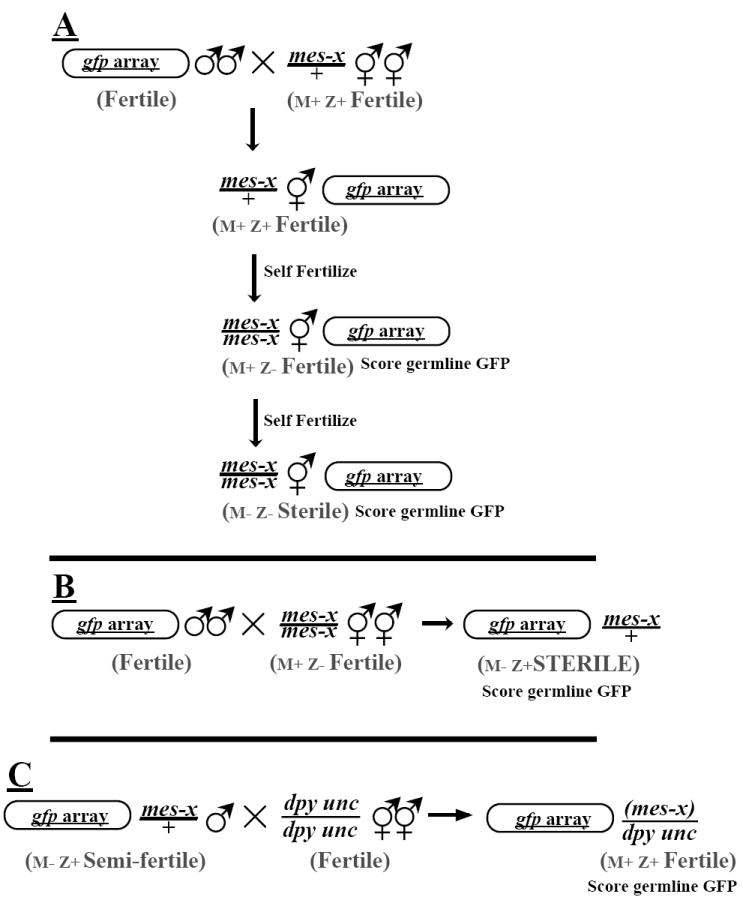
Introduction of a germline-silenced transgene into mes mutants. (A) Array-carrying males were mated with animals heterozygous for a mes mutation. GFP+ (F1) offspring heterozygous for the mutation were then self-fertilized and GFP+ (F2) animals were cloned. These animals were then assayed for both germline GFP expression and mes genotype (F3 sterility). (B) Array-carrying males were mated to fertile, homozygous mes hermaphrodites. Since mes mutations are strict maternal-effect (revealing a requirement for maternal Mes+ activity), the heterozygous F1 offspring of these matings were phenotypically Mes. Adult F1 offspring carrying the reporter transgene (as determined by somatic GFP) were assayed for the presence of fluorescent germ cell nuclei, indicating expression of the transgene in the germline. (C) Individual F1 mes/+ (M−Z+) male progeny from the cross in B were mated to dpy10(e128) unc-4(e120) animals before assaying their germline for GFP fluorescence. The presence of phenotypically wild-type (non-DpyUnc) offspring from these single-male matings indicated the male was fertile. In such experiments with mes-4, two fertile germline-GFP+ mes-4/+ males were identified. The cross-progeny sired by these males were analyzed: a fraction showed somatic GFP expression, indicating transmission of the transgene array, but none showed germline GFP.
M−Z+ animals for each of the mes mutants were obtained by mating males carrying the array to fertile mes/mes (M+Z−) animals. The sterile mes/+ (M−Z+) cross-progeny were then scored for silencing (Fig. 1B).
Desilencing of this array is not a general consequence of altered genetic backgrounds or passage through male sperm. In the course of genetic studies using this transgene, expression has been examined in genetic backgrounds with a variety of characterized morphological and neurological defects [dpy-5(e61)I, unc-54(e190)I, dpy-10(e128)II, unc-4(e120)II, dpy-18(e364)III, unc-32(e189)III, dpy-4(e1166), unc-17(e245)IV, dpy-11(e224)V, unc-60(e723)V, dpy-28(y1)III; Brenner, 1974; Plenefisch et al., 1989]. In no case was activation of gene expression in the germline observed. Crosses in which the array transgene was propagated through sperm to a non-mes host (e.g. animals with an unrelated genetic marker) also showed no reactivation of the transgene in the germline.
Further evidence for specificity comes from experiments making use of the temperature-sensitivity of the mes-3 allele bn21 (11,12). In this mutant, germline activation of the transgene only occurred under conditions (25°C) in which the germline phenotype is manifest (Table 1).
Table 1.
Maternal and zygotic effects on germline silencing in mes mutants
| mes mutant | Genotype | Phenotype | Maternal Complement | Zygotic Complement | Germline desilencing (%)
|
|
|---|---|---|---|---|---|---|
| Hermaphrodites | Males | |||||
| wt | +/+ | Fertile | M+ | Z+ | 0/85 (0) | 0/64 (0) |
| mes-2(bn11) | m/m | Fertile | M+ | Z− | 0/22 (0) | nd |
| m/m | Sterile | M− | Z− | 35/41 (85) | nd | |
| m/+ | Sterile | M− | Z+ | 35/40 (87) | 24/39 (61) | |
| mes-3 | ||||||
| (bn35) | m/m | Fertile | M+ | Z− | 14*/25 (56) | nd |
| (bn35) | m/m | Sterile | M− | Z− | 14/16 (88) | nd |
| (bn21ts) 25° | m/+ | Sterile | M− | Z+ | 15/24 (62) | 10/31 (32) |
| (bn21ts) 16° | m/+ | Sterile | M− | Z+ | 0/6 (0) | 1/9 (11) |
| mes-4 (bn67) | m/m | Fertile | M+ | Z− | 28/28 (100) | nd |
| m/m | Sterile | M− | Z− | 40/42 (95) | nd | |
| m/+ | Sterile | M− | Z+ | 29/30 (96) | 54/102(52) | |
| mes-6 (bn66) | m/m | Fertile | M+ | Z− | 5**/33 (15) | nd |
| m/m | Sterile | M− | Z− | 42/42 (100) | nd | |
| m/+ | Sterile | M− | Z+ | 32/36 (82) | 20/40 (50) | |
Animals carrying the let-858∷gfp array were identified by the presence of somatic GFP, then scored for germ cells (differential interference contrast microscopy) and for GFP (fluorescence microscopy) in these cells. nd= not determined.
(The GFP fluorescence observed in these animals was of weaker intensity, on average, than that observed in mes-4 M+Z− animals).
(Of these five mes-6 animals, four had very low levels of germline fluorescence limited to one of the two gonad arms. The fifth animal exhibited fluorescence comparable to the M+Z− mes-4 group).
RESULTS
As a reporter for germline silencing, we have used a transgene array carrying multiple tandem copies of a plasmid encoding a green fluorescent protein (GFP)-tagged version of a ubiquitously expressed C. elegans gene (let-858). For the purposes of this study, we have employed the let-858 transgene solely as a reporter: all experiments were carried out in a let-858+ genetic background, in which the expression of the reporter has no evident phenotypic consequence. Transgene arrays carrying this reporter construct were subject to germline silencing, i.e., the reporter construct is efficiently expressed in somatic lineages, but is silenced in the germ lineage (Kelly et al., 1997).
The ability of germline cells to silence the GFP-reporter transgene array was monitored in a set of mes mutant genetic backgrounds (Fig. 1). mes-2, mes-3, mes-4 and mes-6 animals all show a distinct germline phenotype. Germ cells are present in the newly hatched larva but proliferation is limited (Capowski et al., 1991). In later stages, a progressive degeneration of some germline cells is observed (Paulsen et al., 1995; Garvin et al., 1997). The magnitude of these phenotypic effects depends on the mes gene and allele (Capowski et al., 1991; Paulsen et al., 1995; Garvin et al., 1997). Because of the distinct maternal requirement for the MES products, it is important to follow silencing as a function of both maternal MES contribution (M+ or M−) and zygotic MES contribution (Z+ or Z−). For the purposes of this work, we will use the term zygotic to refer to all (non-maternal) products that are synthesized from transcripts produced after fertilization.
We first assessed the effects of maternal genotype on silencing of the transgene array. This involved assays for silencing in mes/+ progeny from a mes/mes mother. These ‘M−Z+’ animals are healthy but sterile (due to a lack of maternally supplied MES product). We found in this assay that mutations in four of the mes genes, mes-2, mes-3, mes-4 and mes-6, resulted in desilencing of the transgene array, as evidenced by the expression of the transgene array in the residual germ cells (Fig. 2). The observed desilencing was temporally limited; activation was most dramatically seen in adult animals, and was only rarely observed in late (L4) larvae (not shown). Germline transgene activity was not observed in earlier larval stages (L1,L2,L3). In adults, we observed a regional preference for accumulation of the transgene product, with activity frequently restricted to the medial region of each gonad arm (Fig. 2B). The desilencing was not limited to the let-858 transgene, as other reporter transgenes (based on ama-1 and unc-37) showed similar desilencing in the mes mutants (not shown).
Fig. 2.
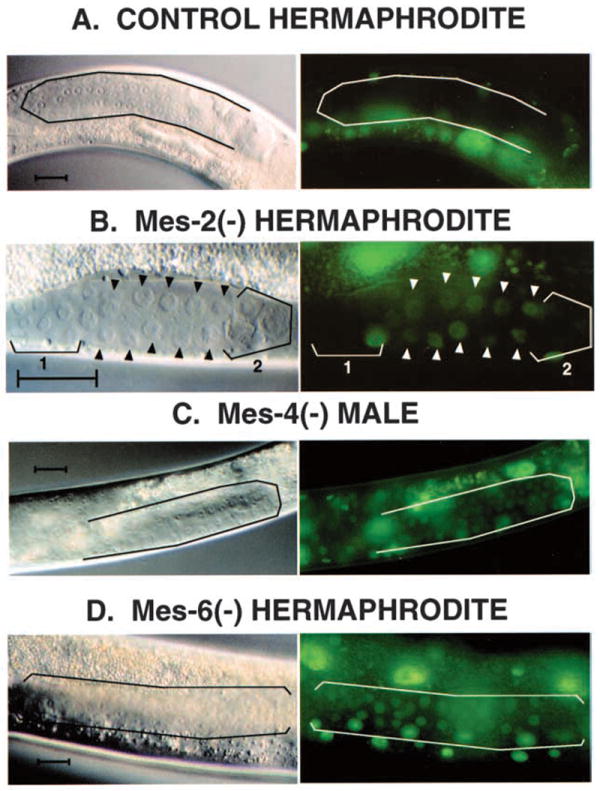
Desilencing of a repetitive transgene in Mes (M−Z+) mutants. A repetitive let-858∷gfp reporter extrachromosomal array was transferred into the different mes genetic backgrounds shown and assayed for desilencing in M−Z+ cross-progeny, as diagrammed in Fig. 1B. Adult gonad arms housing germline nuclei are outlined in A, C and D; arrowheads in B indicate representative germ cell nuclei. (A,B,D) Hermaphrodite gonads; (C) male. To illustrate the complete silencing of the array in a mes+ background, a control animal from the original line carrying the array is shown in A. (B,C,D) Expression of the reporter in the germlines of Mes worms. The bracket 1 in B outlines germ cells that are more distal, and presumably immature, and bracket 2 outlines others that are beginning to degenerate; GFP activity is not evident in these bracketed cells. Desilencing in the mes mutants was also not observed in earlier larval stages (L2,L3), where the germline is more generally proliferative (not shown). Scale bar, approximately 30 μm.
We likewise addressed potential roles in the silencing process for zygotically encoded MES products. For these experiments, mes/+ mothers were self fertilized to yield fertile mes/mes offspring as a fraction of their progeny. These M+Z− animals were then assayed for germline silencing of the transgene. In the mes-2 and mes-6 mutants tested in this manner, there was infrequent (mes-6) or no (mes-2) desilencing observed (Table 1), supporting a strict maternal requirement for these proteins in germline silencing. In contrast, a degree of desilencing was observed in mes-3 M+Z− adults: 56% of these animals showed germline GFP fluorescence (Table 1). The strongest desilencing in M+Z− adults was seen with mes-4: 100% of these animals showed desilencing (Table 1). The requirement for zygotic MES-4 in germline desilencing appeared to be adult-specific: M+Z− larvae showed no evidence of desilencing. As the animals reached adulthood, desilencing was observed throughout the germline, with the reporter easily detected in all proximal regions of the gonad including oocytes (Fig. 3). Desilencing of the array in M+Z− adults appeared to extend to mid-stage embryos of the next generation, as reporter expression level in progeny embryos was notably higher than in embryos from mes-4/+ controls (data not shown).
Fig. 3.

Desilencing of a repetitive transgene in mes-4 (M+Z−) animals. Differential interference contrast (A) and fluorescent (B) microscopy images of part of one gonad arm of an array-carrying, fertile mes-4/mes-4 (M+Z−) hermaphrodite. Arrowheads point to oocyte nuclei; bracket indicates germ cell nuclei in distal syncytium of gonad arm.
We looked for additional correlation between the Mes phenotypic effect and germline desilencing by examining effects of gender on desilencing. For each of the mes genes, we observed an enhanced frequency of germline transgene activation in hermaphrodites as compared to males (Table 1). The intensity of fluorescence observed in desilenced male germ cells was also consistently lower than that observed in hermaphrodites (not shown). This decreased effect in males parallels the differential severity of the mes germline defects seen in the morphological characterization of the Mes phenotypes by Garvin et al. (1997).
The decreased severity of the Mes defect in males allowed us to also test the possibility that partial desilencing might be compatible with male germ cell fate. We found that a fraction of male progeny of mes (M− Z−) mothers exhibited both fertility and germline GFP-reporter expression (Fig. 1B). Hence, the modest desilencing effects observed in these males was compatible with the production of functional germ cells. The desilencing of the array in the male germline is not a heritable state, since fertilization of a wild-type oocyte with sperm from a desilenced male germline yield animals that are fully silenced in their germline (Fig. 1C, legend).
It was conceivable that passage through a mes− mutant background might result in epigenetic and heritable changes resulting in activation of the transgene in future generations. This appears not to be the case, as evidenced by the resilencing of the transgene array in Mes+ progeny sired by GFP-positive male germlines (Fig. 1C).
DISCUSSION
The transgene silencing effects described above provide a molecular correlate to the earlier descriptions of the mes mutant phenotypes. Maternally provided mes-2, mes-3, mes-4 and mes-6 products had previously been shown to be required for proper germline proliferation, cell viability and germ cell differentiation (Capowski et al., 1991; Paulsen et al., 1995; Garvin et al., 1997). The transgene expression effects that we have described indicate a molecular requirement for these mes genes in a generally active germline silencing process. Such a correlation could reflect one of two situations: (1) a direct molecular function for the mes genes in the silencing process, or (2) an indirect effect in which cell degeneration, or other cytological defects resulting from the mes mutations, might lead to defects in germline silencing.
We favor a direct role for the mes genes in germline silencing, based on several of our experimental observations. (1) Germline desilencing can precede the appearance of cytological defects. The separation between these two effects is most striking with mes-4. (2) Not all mutations with degenerative germlines have silencing defects. A screen for sterile mutations with degenerative germ cells yielded 17 such recessive mutants, with only 1 exhibiting detectable desilencing in the residual germ cells (W. G. K., unpublished observations). (3) Mes males exhibit partial desilencing under conditions where few distinct cytological defects are seen in the germline. These observations indicate that cellular degeneration is not a prerequisite for desilencing. Instead, it seems likely that the desilencing is directly related to events that lead to the degenerative germ cell death.
An additional argument for direct involvement of the mes genes in the silencing process comes from the structure of the mes-2 and mes-6 proteins. mes-2 and mes-6 encode proteins that are members of the Polycomb Group (Pc-G) of transcriptional repressors: MES-2 is related to the Drosophila Enhancer of Zeste, and MES-6 to Extra Sex Combs (Holdeman et al., 1998; Korf et al., 1998). Members of the Polycomb group have been shown to function in context- and repeat-dependent silencing in Drosophila (Kennison, 1995; Pal-Bhadra et al., 1997). The phenotypic analysis of Mes mutations in C. elegans and Pc-G mutations in Drosophila provide a means to compare roles for this conserved family in the two diverged species. In both cases, a function in genetic silencing has been observed. The apparent restriction of C. elegans mes gene activity to germline tissue is not observed for the Drosophila Pc-G genes, and may define an acquired or primordial role for the Pc-G group in maintenance of the germline.
It is interesting to note that the degree of M+Z− desilencing observed for the different mes mutants forms a series, with mes-4 >mes-3 >mes-6 >mes-2. It has been shown that mes-2 and mes-6 mutations reciprocally abrogate each other’s protein localizations; mes-3 mutations have a somewhat intermediate effect on MES-2 or MES-6, and mes-4 has no observable effect (Holdeman et al., 1998; Korf et al., 1998). Whether these observations indicate a functional hierarchy among the MES factors remains to be determined.
Our initial analysis of non-maternal-effect mutations that result in desilencing is consistent with the hypothesis that a failure in germline-specific silencing mechanisms leads to dramatic defects in germline function. We have isolated mutations defining at least three different genes showing a zygotic requirement for germline silencing. All of these mutations have conditional germline defects that are correlated with desilencing of reporter arrays in the germline (W. G. K., unpublished results). We expect that these genes will define new components of the silencing apparatus distinct from the mes genes, in that the requirement for their function is zygotic rather than maternal.
In all cases in which we have observed desilencing of the let-858∷gfp transgene in the germline, this activity was only apparent in later larvae and adults. No activity of the transgene was observed in earlier larvae, when the bulk of germ cells are mitotic and cellularized. The lack of observed desilencing at earlier stages could reflect properties intrinsic to the let-858∷gfp reporter. Alternatively, this could reflect an underlying difference in requirements for silencing in proliferative (mitotic) versus non-proliferative (meiotic) germline. The existence of mes-independent silencing mechanisms that operate in proliferating tissue might explain the failure of mes mutants to desilence in the distal mitotic region of the adult germline (see Fig. 2).
The generational aspects of gene silencing in the germline remain puzzling. In previous examinations of the activity of repetitive transgene arrays, we observed a progressive silencing in the germline occurring over several generations (Kelly et al., 1997). The mes mutations exhibit a surprising delay of a generation before a sterile phenotype is observed (Capowski et al., 1991). In the case of mes-4, we have seen see an additional unexpected effect: (M+Z−) mutants desilence germline gene expression in the generation before cytological defects are observed. These observations suggest this is an epigenetic phenomenon: in this case, the existence of metastable expression states that can be maintained or can change independently of the primary structure of the genome.
What are the natural targets of germline-specific silencing? One possibility is that genes responsible for somatic differentiation may be subject to silencing in the germline. This mechanism might be required as part of maintaining an undifferentiated germline (Denis and Lacroix, 1993). Germline cells may be particularly sensitive to the differentiating influences of transcription factors. The germline is a highly transcriptionally active, undifferentiated syncytium and may thus require specific repressive mechanisms that act on large numbers of genes. Alternatively, a germline-specific repressive mechanism might act globally on the entire genome. Specific mechanisms (e.g. insulators, locus control regions) could then be used to allow activation of critical genes (Dillon and Grosveld, 1994).
It is likely that the germline of C. elegans uses a combination of different mechanisms to prevent somatic gene expression. In germline blastomeres of the early embryo, a global mechanism blocking almost all (or perhaps all) transcription by RNA polymerase II has been shown to exist (Seydoux et al., 1996; Seydoux and Dunn, 1997). The factor responsible for this suppression, PIE-1, is absent in later stages (Mello et al., 1996). Thus the protection of the germline can be divided into (at least) two separate phases: the early embryonic phase in which virtually all new gene expression is blocked in a pie-1 dependent mechanism, and a later phase in which gene expression occurs but context-dependent silencing effects (and perhaps other mechanisms) maintain germline identity.
The suppression of somatic activity need not be an exclusive role (or even a major role) for the silencing process. Timing of germline-specific gene expression must be critical, given the rapid changes and signaling events that accompany the progression of immature germ cells to oocytes and sperm (Schedl, 1997). Blocking the inappropriate transcription of genes responsible for germline functions could be a critical partner to the post-transcriptional control mechanisms that have been described (Anderson and Kimble, 1997; Seydoux, 1996).
An additional role for C. elegans germline silencing in the control of transposons and viruses has been suggested (Kelly et al., 1997). The transposons that have been described for C. elegans tend to be immobile in the germline (Emmons and Yesner, 1984). This could conceivably involve a mechanism that recognizes the repeated nature of such elements. We are currently testing the connection between germline repeat silencing and transposon activity by measuring the mobility of transposable elements in the germline of mutants with silencing defects.
Our analysis of germline transgene silencing and of the germline-specific effects of the mes mutations leaves open the question of whether C. elegans possesses a soma-specific silencing mechanism similar to the Polycomb system in Drosophila. Three observations are of interest in this regard. First, C. elegans has a homeotic gene cluster similar to the Polycomb-regulated Antennapedia and Bithorax clusters in Drosophila (Salser and Kenyon, 1994). Second, a soma-specific chromodomain-containing protein has been described in C. elegans. The chromodomain is a conserved motif originally identified as a region of homology between Polycomb and the heterochromatin protein HP1 in Drosophila; the C. elegans chromobox gene cec-1 is described by Agostoni et al. (1996). Third, certain transgenes expressed in somatic tissue are silenced in repetitive sequence contexts (Okkema et al., 1993; Mello and Fire, 1995; J. Hsieh, S. Xu, and A. F., unpublished results.). It will be interesting to determine whether these define one or a variety of somatic silencing mechanisms.
Acknowledgments
We thank R. Holdeman, I. Korf, and S. Strome, G. Seydoux, M. Montgomery, K. Liu, L. Timmons, S. Dymecki, S. Kostas, B. Harfe, S. Xu and T. Schedl for their help and suggestions. This work was supported by U.S. Public Health Service grants R01-GM37706 and F32-HD07794. Some strains were obtained from the C. elegans stock center, supported by the NIH division of research resources.
References
- Agostoni E, Albertson D, Wittman C, Hill F, Tobler H, Muller F. cec-1, a soma-specific chromobox-containing gene in C. elegans. Dev Biol. 1996;178:316–326. doi: 10.1006/dbio.1996.0221. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kimble J. mRNA and translation C. elegans II. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1996. pp. 185–208. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski EE, Martin P, Garvin C, Strome S. Identification of grandchildless loci whose products are required for normal germ-line development in the nematode. Caenorhabditis elegans. Genetics. 1991;129:1061–1072. doi: 10.1093/genetics/129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis H, Lacroix J. The dichotomy between germ line and somatic line, and the origin of cell mortality. Trends Genetics. 1993;9:7–11. doi: 10.1016/0168-9525(93)90065-P. [DOI] [PubMed] [Google Scholar]
- Dillon N, Grosveld F. Chromatin domains as potential units of eukaryotic gene function. Curr Op Gen Dev. 1994;4:260–264. doi: 10.1016/s0959-437x(05)80053-x. [DOI] [PubMed] [Google Scholar]
- Emmons SW, Yesner L. High frequency excision of transposable element Tc1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984;36:599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Garvin C, Holdeman R, Strome S. The phenotype of mes-2, mes-3, mes4, and mes-6, maternal-effect genes required for survival of the germline in C. elegans, is sensitive to chromosome dosage. Genetics. 1997;148:1–20. doi: 10.1093/genetics/148.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C. elegans. Nuc Acids Res. 1994;22:1762–1763. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman R, Nehrt S, Strome S. MES-2, a maternal protein essential for viability of the germline in C. elegans, is homologous to a Drosophila polycomb group protein. Development. 1998;125:2457–2467. doi: 10.1242/dev.125.13.2457. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed C. elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA. The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Ann Rev Genetics. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- Kimble J, Hirsh D. The post-embryonic lineage of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- Korf I, Fan F, Strome S. The polycomb group in C. elegans and maternal control of germline development. Development. 1998;125:2469–2478. doi: 10.1242/dev.125.13.2469. [DOI] [PubMed] [Google Scholar]
- Martin DIK, Whitelaw E. The vagaries of variegating transgenes. BioEssays. 1996;18:919–923. doi: 10.1002/bies.950181111. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Fire A. DNA transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Meth Cell Biol. Vol. 48. 1995. pp. 451–482. [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. Sequence requirements for myosin gene expression and regulation in C. elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Birchler JA. Cosuppression in Drosophila: gene silencing of alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell. 1997;90:479–490. doi: 10.1016/s0092-8674(00)80508-5. [DOI] [PubMed] [Google Scholar]
- Paulsen J, Capowski EE, Strome S. Phenotypic and molecular analysis of mes-3, a maternal-effect gene required for proliferation and viability of the germ line in C. elegans. Genetics. 1995;141:1383–1398. doi: 10.1093/genetics/141.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenefisch JD, DeLong L, Meyer BJ. Genes that implement the hermaphrodite mode of dosage compensation in Caenorhabditis elegans. Genetics. 1989;121:57–76. doi: 10.1093/genetics/121.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T. C elegans II. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. Developmental genetics of the germline; pp. 241–269. [PubMed] [Google Scholar]
- Sabl JF, Henikoff S. Copy number and orientation determine the susceptibility of a gene to silencing by nearby heterochromatin in Drosophila. Genetics. 1996;142:447–458. doi: 10.1093/genetics/142.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser SJ, Kenyon C. Patterning C. elegans: homeotic cluster genes, cell fates and cell migrations. Trends in Genetics. 1994;10:159–164. doi: 10.1016/0168-9525(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Schnabel H, Schnabel R. An organ specific differentiation gene, pha-1, from Caenorhabditis elegans. Science. 1990;250:686–688. doi: 10.1126/science.250.4981.686. [DOI] [PubMed] [Google Scholar]
- Seydoux G. Mechanisms of translational control in early development. Curr Op Gen Dev. 1996;6:555–561. doi: 10.1016/s0959-437x(96)80083-9. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Mello CC, Pettitt J, Wood WB, Priess JR, Fire A. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382:713–716. doi: 10.1038/382713a0. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development. 1997;124:2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]


