Abstract
Background
Mortality in hospitalized, febrile patients in Sub-Saharan Africa is high due to HIV-infected, severely immunosuppressed patients with opportunistic co-infection, particularly disseminated tuberculosis (TB) and cryptococcal disease. We sought to determine if a positive lateral flow assay (LFA) result for urine lipoarabinomannan (LAM) and cryptococcal antigenuria was associated with mortality.
Methods
351 hospitalized, HIV-positive adults with symptoms consistent with TB and who were able to provide both urine and sputum specimens were prospectively enrolled at Mulago National Referral Hospital in Uganda as part of a prospective accuracy evaluation of the lateral flow Determine TB LAM test. Stored frozen urine was retrospectively tested for cryptococcal antigen (CRAG) using the LFA. We fitted a multinomial logistic regression model to analyze factors associated with death within 2 months after initial presentation.
Results
The median CD4 of the participants was 57 (IQR: 14–179) cells/µl and 41% (145) were microbiologically confirmed TB cases. LAM LFA was positive in 38% (134), 7% (25) were CRAG positive, and 43% (151) were positive for either test in urine. Overall, 21% (75) died within the first 2 months, and a total of 32% (114) were confirmed dead by 6 months. At 2 months, 30% of LAM or CRAG positive patients were confirmed dead compared to 15.0% of those who were negative. In an adjusted model, LAM or CRAG positive results were associated with an increased risk of death (RRR 2.29, 95% CI: 1.29, 4.05; P = 0.005).
Conclusions
In hospitalized HIV-infected patients, LAM or CRAG LFA positivity was associated with subsequent death within 2 months. Further studies are warranted to examine the impact of POC diagnostic ‘test and treat’ approach on patient-centered outcomes.
Background
In sub-Saharan Africa (SSA), tuberculosis (TB) is responsible for considerable morbidity and mortality [1]. Hospitalized patients in SSA continue to suffer high mortality (range 30–45%); the majority of these patients are HIV-infected and die from undiagnosed and untreated opportunistic infections [2]–[6]. Autopsy studies have shown that many of these severely immunosuppressed patients die of potentially treatable infections, particularly disseminated TB and cryptococcal infection, but the infectious diagnosis at autopsy was not clinically considered during hospitalization [7]. Co-infection with more than one opportunistic pathogen may also contribute to mortality [8]. Among hospitalized HIV-infected adults with fever and/or sepsis syndrome, significant rates of TB have been noted (often disseminated) as well as invasive fungal disease [9], [10].
Several studies have examined predictors of mortality in hospitalized HIV patients or among TB suspects and have found a significant proportion of patients have disseminated tuberculosis with mycobacteremia (range 13–42%) which may present with only fever progressing to septic shock [3], [9], [11]–[14]. Mycobacteremia doubled the risk of in-hospital death from 22% to 44% (OR1.97, 95% CI = 1.19, 3.27, P = 0.026) in a study of patients who fulfilled systemic inflammatory response syndrome (SIRS) criteria for sepsis [9]. Unfortunately, mycobacterial blood culture is slow as a diagnostic due to the 20 hour doubling time of Mycobacterium tuberculosis (Mtb). Mortality has also been associated with enzyme-linked immunoassay detection of urinary LAM, a glycolipid component of the Mtb cell wall that is excreted in urine [2], [15]; this suggests that urinary LAM may be detected in patients with disseminated disease and may be a good surrogate marker for mycobacteremia as well as death.
In the pre-antiretroviral therapy era, cryptococcal disease was one of the leading contributors to death in HIV-infected adults [16] and remains a significant opportunistic infection in SSA [17], [18]. Cryptococcal antigenemia is an independent predictor of death in patients with low CD4 T cell counts about to initiate ART [19]–[21]. In a survey of bacterial and fungal infections in hospitalized, HIV-infected adults in Tanzania, 11% were found to have Cryptococcus neoformans infection [10], and TB and cryptococcal infection were both important causes of hospitalized illness and death.
Rapid point-of-care tests (POC) that fulfill the ASSURED criteria (affordable, sensitive, specific, user-friendly, robust/rapid, equipment free, deliverable to those who need the test/no refrigeration requirement) are very attractive in resource-limited settings [22]. Recently, several new POC lateral flow assays have been developed that have high sensitivity in this population of immunosuppressed HIV-infected patients. The urinary lipoarabinomannan (LAM) lateral flow assay (LFA) (Determine TB LAM Ag, Alere, Waltham, MA, USA) is a point-of-care test that has the highest sensitivity in patients with disseminated TB [23]–[26]. Among patients with CD4 T cell counts less than 100 cells/µl, the sensitivity ranged from (52%–59%), with uniformly high specificity (>94%). In another South African study of mostly hospitalized patients suspected to have extrapulmonary and disseminated TB, the sensitivity in patients with a CD4 T cell count <100 cells/µl was 82.6%, specificity 93% [27]. The cryptococcal antigen LFA (Immuno-Mycologics, Inc. Norman, OK, USA) is specific and easy to use [28], and in urine, the sensitivity was 91% compared to serum. We sought to determine risk factors for early death (within 2 months) in hospitalized HIV-infected adults in Uganda, with a particular focus on understanding whether lateral flow assays for TB and cryptococcal disease diagnosis can identify patients at risk for early death.
Methods
Study Participants
506 TB suspects were prospectively enrolled at the Infectious Disease Institute (IDI) HIV clinic or at Mulago National Referral Hospital in Kampala, Uganda between January, 2011 and November 2011 as part of a study to evaluate the accuracy of a lateral flow immunochromatographic test (Determine TB LAM Ag) to detect mycobacterial LAM in urine [29]. All subjects were documented to be HIV-positive, at least 18 years of age, and suspected to have active TB with at least one of the following: cough, fever, night sweats, weight loss. Patients who had taken more than 2 days of TB treatment in the 60 days prior to screening or who were unwilling or unable to provide a urine specimen were excluded. This analysis focused on the subset of 351 hospitalized patients who were able to provide both sputum and urine samples for the main diagnostic accuracy study.
Procedures
All patients had demographic details recorded and underwent a standardized questionnaire related to the signs and symptoms of TB at enrollment. Two sputum specimens were collected for direct smear stained with the auramine O method and examined by fluorescence microscopy (FM), and then were processed using standardized protocols in the mycobacteriology lab. After sodium hydroxide and N-acetyl-L-cysteine decontamination, centrifuged pellets underwent FM smear, then solid (Lowenstein-Jensen slant) and liquid (Mycobacterial Growth Indicator Tube [MGIT], Becton Dickinson, Sparks, MD) mycobacterial culture. Excess pellet material was stored frozen. Mycobacterial cultures that were positive for acid fast bacilli underwent Capilia TB (Tauns Laboratory, Inc., Kamishima, Izunokuni, Japan) testing for Mtb complex identification. The frozen centrifuged remaining pellet was also analyzed using Xpert MTB/RIF according to the manufacturer's instructions. Whole blood was cultured for mycobacteria in the MYCO/F LYTIC (Becton and Dickinson; blood inoculation volume 3 ml) or BacT/ALERT MB (BioMerieux, Marcy-l'Etoile, France; inoculation volume 5 ml) tubes. CD4 T cell count and chest x-ray were also obtained at enrollment. Urine was collected for urine Determine TB-LAM lateral flow assay. For this assay, 60 µl was pipetted onto the sample pad. According to the manufacturer's instructions, the strip was read 25 minutes later by an independent technician who compared the test strips with the reference card provided by the manufacturer and graded the result from 1+ to 5+. If the positive control bar was not visible, then the strip was invalid and the test repeated. The remainder of the urine was heated to 95°C for 30 minutes using a boiling water bath or dry heating block, centrifuged at 10,000 rpm for 15 minutes, and supernatants pipetted off and frozen. Frozen thawed urine was also batch tested using the FDA-approved cryptococcal antigen (CRAG) LFA (Immy Inc., Norman, Oklahoma, US) according to the manufacturer's instructions. Briefly, 40 µL of urine was placed in an Eppendorf tube. The CRAG LFA dipstick was placed in the tube and allowed to incubate at room temperature for 10 minutes. The qualitative results were interpreted as follows: a single control line was negative, two lines visible were interpreted as positive, and if the control line failed to develop, the test was invalid and was repeated.
Participants had a scheduled two-month follow-up study visit if their initial urinary LAM test was positive and all other mycobacterial testing was negative. All other patients had a review of their medical records and were contacted (by phone call or home visit) at 2 months and then at 6 months to determine their final TB status (dead, alive, unknown, diagnosed and treated for TB).
TB status definitions
Participants who had Mtb cultured from any specimen were defined as confirmed TB. Possible TB was defined as a patient who had no culture positive, plus one or more of the following criteria: sputum smear microscopy positive but no sputum culture positive for Mtb, empiric TB treatment with documented clinical improvement, diagnosis of active TB per a non-study clinician, death reported to be due to active TB per medical source. Patients were classified as having no evidence of TB if they did not meet the criteria for either of the above.
Statistical Analysis
Descriptive statistics were used to characterize the hospitalized study population. We fitted a multinomial logistic regression model for a three-category outcome of dead, alive or status unknown at 2 months, with the alive status as the reference category. We first fitted this model to be consistent with a low-resource scenario where no other tests were available on admission (such as CD4 T cell count) except the LAM LFA, the cryptococcal LFA, and direct sputum smear. The multinomial regression model was fitted with the main predictor as a combination of LAM or CRAG result. This provided the relative risk ratio of being a) dead or b) lost to follow-up compared to those who were alive as predicted by the LAM positivity and adjusted for baseline characteristics. In a secondary analysis, we adjusted for CD4 category. We also constructed Kaplan-Meier curves of survival probability versus time-to-death by LAM positivity, with Cox model estimation of hazard ratios. Participants who could not be traced at 2 months were considered lost to follow-up on the day after enrollment.
Ethics Statement
The study was approved by the institutional review boards in Uganda, Johns Hopkins University, and Boston Medical Center. Written informed consent was obtained from all study subjects prior to enrollment into the study.
Results
Patient characteristics
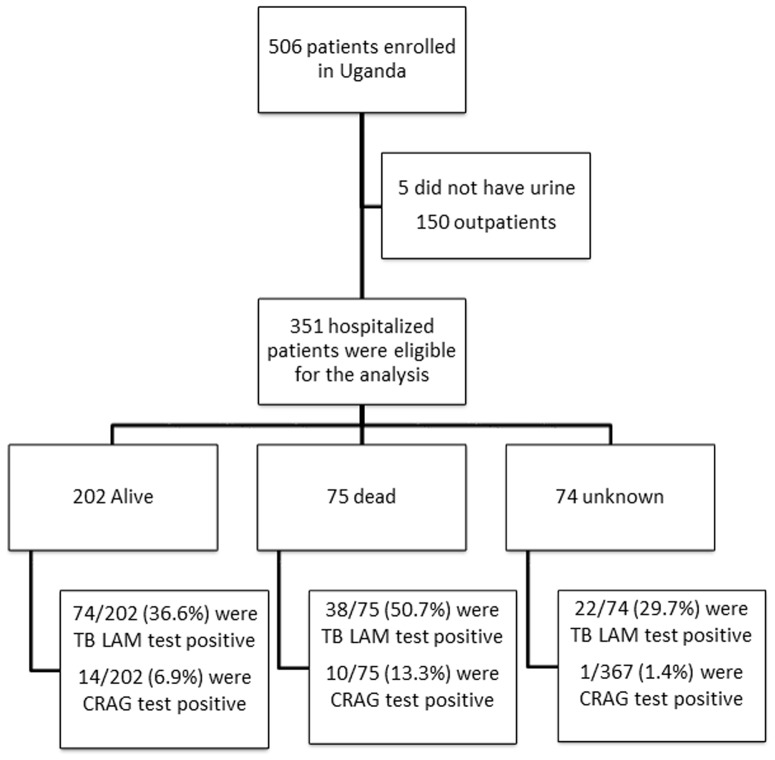
Table 1 shows the characteristics of the 351 hospitalized participants included in this analysis. (Figure 1) Of these, 64.4% (226) were women and the median age was 31 years (IQR: 27–38). The median CD4 was 57 (IQR: 14–179) cells/µl; 47.6% (167) of participants had a CD4<50 cells/µl and 37.6% (132) of participants were on antiretroviral therapy at enrollment. The majority (96.9%) of patients had cough of any duration; only 62.7% had cough more than 14 days. Fevers (94.1%) and weight loss (96.6%) in the previous 4 weeks were present in the majority of the patients.
Table 1. Baseline (enrollment) clinical characteristics and laboratory testing results of the hospitalized study population Uganda (N = 351).
| Characteristic | Total N = 351 (% or IQR) | Alive N = 202 (% or IQR) | Dead N = 75 (% or IQR) | Unknown status N = 74 (% or IQR) |
| Female | 226 (64.4) | 123(60.9) | 53(70.7) | 50(67.6) |
| Median age in years (SD) | 31(27,38) | 32(7.9) | 30(8.8) | 31(7.7) |
| Median Karnofsky score (SD) | 60 (5.9)) | 60(5.6) | 60(6.2) | 60(6.1)) |
| 80–100 | 113 (32.2 | 71(35.2) | 15(20) | 27(36.5) |
| 50–70 | 238 (67.8) | 131(64.9) | 60(80) | 47(63.5 |
| Median CD4 (IQR) cells/mm3+ | 57 (14,179) | 73(18,200) | 22(8,72) | 90.5(18,195) |
| CD4 counts (cells/mm3) <50 | 167 (47.6) | 89(44.1) | 47(62.7) | 31(41.9) |
| CD4 counts (cells/mm3) 50–99 | 42 (12.0) | 23(11.4) | 11(14.7) | 8(10.8) |
| CD4 counts (cells/mm3) 100–199 | 62 (17.7) | 37(18.3) | 7(9.3) | 18(24.3) |
| CD4 counts (cells/mm3) ≥200 | 75 (21.4) | 50(24.8) | 8(10.7) | 17(22.9) |
| On antiretroviral therapy at enrollment | 132 (37.6) | 81(40.1) | 26(34.7) | 25(33.8) |
| On cotrimoxazole prophylaxis at enrollment | 299 (85.2) | 176(87.1) | 67(89.3) | 56(75.7) |
| Cough for more than 14 days | 220(62.7) | 124(61.4) | 49(65.3) | 47(63.5) |
| Fevers-within past 4 weeks | 330 (94.1) | 189(93.6) | 71(94.7) | 157(77.7) |
| Night sweats-within past 4 weeks | 274 (78.1) | 157(77.7) | 56(74.7) | 61(82.4) |
| Weight loss-within past 4 weeks | 339 (96.6) | 192(95.1) | 74(98.6) | 73(98.7) |
| Chest X-ray compatible with TB | 207(59.0) | 118(58.4) | 43(57.3) | 46(62.1) |
| TB treatment ever prescribed | 50 (14.3) | 37(18.3) | 6(8) | 7(9.5) |
| Respiratory specimen smear positive for acid fast bacilli | 45 (12.8) | 27(13.3) | 12(16.0) | 6(8.1) |
| Respiratory specimen culture positive for Mtb* | 133 (37.9) | 72(35.6) | 29(38.7) | 32(43.2) |
| Respiratory specimen culture positive for Mtb but all smears negative | 53 (15.1) | 30(14.8) | 11(14.7) | 12(16.2) |
| Blood culture positive for Mtb** | 69 (19.7) | 37(18.3) | 19(25.3) | 13(17.6) |
| Blood culture positive for non-tuberculous mycobacteria | 0 | 0 | 0 | 0 |
| Blood culture positive for Mtb but no respiratory culture positive for Mtb | 12 (3.4) | 9(4.4) | 3(4.0) | 0 |
| Any culture positive for Mtb | 145 (41.3) | 81(40.1) | 32(42.7) | 32(43.2) |
| Lateral LAM flow positive ≥2+ | 90 (25.6) | 45(22.2) | 28(37.3) | 17(23.0) |
| Lateral LAM flow any band positive | 134 (38.2) | 74(36.6) | 38(50.7) | 22(29.7) |
| Cryptococccal antigen test positive | 25 (7.1) | 14(6.9) | 10(13.3) | 1(1.4) |
| LAM or CRAG antigen test positive | 151 (43) | 83 (41.1) | 45 (60.0) | 23 (31.1) |
| Xpert MTB/RIF test positive | 93 (26.5) | 54(26.7) | 23(30.7) | 16(21.6) |
Number alive = 202 patients, number dead at 2 months = 75 patients, number unknown status at 2 months = 74 patients, CI = confidence intervals, N = number, IQR = interquartile range, SD = standard deviation, TB = tuberculosis, Mtb = Mycobacterium tuberculosis, LAM = lipoarabinomannan, CRAG = cryptococcal antigen.
*2 results missing.
**7 results missing.
of the patients confirmed alive and 2 of the dead patients at 2 months had CD4 count missing.
Figure 1. Study participant flow chart.
TB and the performance sensitivity of Determine TB LAM for TB disease and urine CRAG in hospitalized patients
Table 1 also shows the Mtb test results of the hospitalized participants. Overall 41.3% (145) were microbiologically confirmed TB cases by either respiratory specimen or blood culture. CXR was available for review for 310 participants; 58.9% of participants had an abnormal CXR consistent with TB. LAM LFA was positive with any band intensity in 134 patients (38.2%) with a sensitivity and specificity for detecting TB given the culture-confirmed TB and “no TB” status of 62.1% (90/145) and 81.1% (150/185) respectively (Table S1 in File S1). Sensitivity and specificity of sputum smear for TB were (31.0% (45 of 145) and 100%); and sputum culture (91.7% and 99.5%). Of the 69 patients with MYCO/F LYTIC blood culture positive for Mtb, 61(88.4%) were urinary LAM positive at any band intensity.
Twenty-five (7.0%) patients were CRAG positive in urine. 151(43%) were positive for either test of which 8 (2%) were positive for both tests.
Mortality
Table 1 also shows the vital status of the 351 participants in this analysis; 75 (21.4%) died within the first 2 months (9 weeks), 202 (57.6%) were known to be alive at 2 months and 74 (21.1%) were not contactable (unknown status). The baseline characteristics of the non-contactable group were similar to those of the ‘alive’ group. At 6 months, 114 (32%) participants were confirmed to have died.
Of the LAM positive patients, 28.4% (38/134) died by 2 months (22 unknown status), compared to 17.1% (37/217) of the LAM negative patients (52 unknown status). (Table S1 in File S1). Among those with culture-confirmed TB from any site, 28% of those that were LAM-positive died, compared to only 13% of those that were LAM-negative (p = 0.035). Among those in whom no TB diagnosis was made, 34% of those that were LAM-positive died compared to 19% of those that were LAM-negative (p = 0.053). (Table S1 in File S1) Many of the LAM-positive patients with confirmed TB who died were also Xpert MTB/RIF positive (18 of 25). (Figure 2) None of the 12 LAM-positive patients who did not have microbiologically confirmed TB and died were diagnosed by Xpert MTB/RIF. Among all patients at six months, 40% of those that were LAM positive were confirmed to have died, compared to only 28% of the LAM negative patients (p = 0.049). Of the patients with confirmed TB, 39% of those that were LAM-positive died by 6 months compared to 20% of the LAM negative (p = 0.016). (Table S2 in File S1).
Figure 2. Venn diagram showing the proportions of patients confirmed dead at 2 months (n = 75) who had positive sputum and/or blood cultures (“confirmed TB”), Xpert MTB/RIF positive, and Determine TB LAM LFA positive.
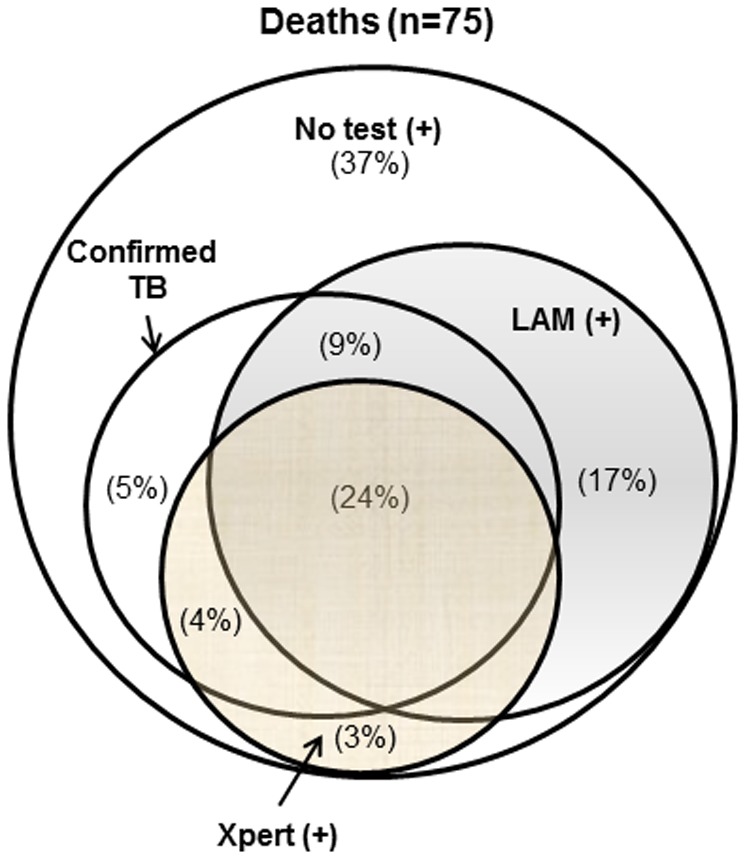
Mutually exclusive proportions of patients are shown in each compartment. 17% of the patients who died were LAM positive, but were neither Xpert nor culture confirmed. 24% of the patients who died were positive by all assays.
Mortality proportions overall and stratified by TB status are presented in the supplemental Tables S1 (2 month mortality) and S2 (6 month mortality). Of the LAM positive patients, 28.4% (38/134) and 40% (54/134) died by 2 and 6 months, respectively, compared to 17.1% (37/217) and 28% (60/217) of the LAM negative patients (P-values<0.05). Among the confirmed TB patients, LAM alone (P-value = 0.035) or LAM or CRAG positive results (P-value<0.001) were associated with an increased risk of death at 2 months. The same results were observed for death at 6 months (LAM positive result, P-value = 0.016 and LAM or CRAG positive results, P-value<0.001).
Many of the urine LAM-positive patients with confirmed TB who died were also sputum Xpert MTB/RIF positive (18 of 25). (Figure 2) None of the 12 LAM-positive patients who did not have microbiologically confirmed TB and died were diagnosed by sputum Xpert MTB/RIF.
The Kaplan-Meier plot by LAM positivity is shown in Figure 3. Patients whose vital status was unknown at 2 months were considered lost to follow-up on day 2. An unadjusted hazard ratio for LAM positivity was 1.67 (P-value = 0.025).
Figure 3. Kaplan-Meier survival curves of LAM negative patients (solid line), and LAM positive patients (dashed line).
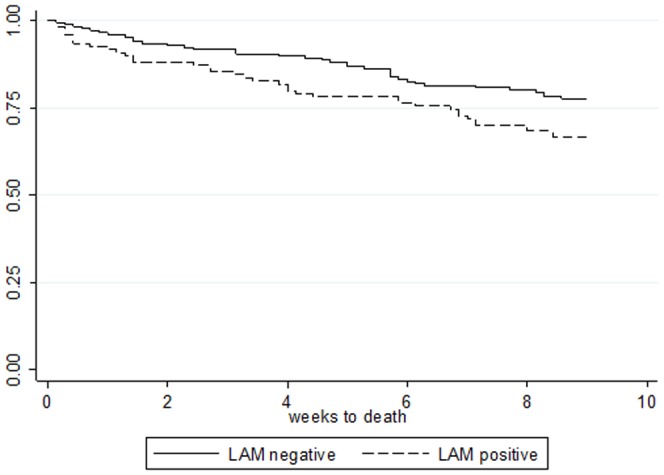
Participants who could not be traced at 2 months were considered lost to follow-up on the day after enrollment.
Adjusted multinomial model for factors associated with death within 2 months
The multinomial regression model-adjusted relative risk ratio results for 2-month mortality status are given in Table 2. Relative risk ratios were adjusted for information readily attainable in most hospital settings on admission including gender, age, baseline direct smear positivity and ART status. A LAM or CRAG positive result had a RRR of 2.29(95% CI: 1.29, 4.05; P = 0.005). In a secondary analysis adding CD4 T cell count categories to the model, the RRR was attenuated likely due to the interaction between LAM and CD4 results, although there is still a trend toward significance (Table S3 in File S1). Only the lowest CD4 category (<50 cells/µL) remained as an independent, significant predictor of death (RRR 2.71, 95% CI: 1.14, 6.44, P-value 0.02), although LAM or CRAG positive results trends towards significant in the multinomial regression analysis (RRR 1.75 (0.94–3.23) P-value = 0.08). (Table 2)
Table 2. Adjusted relative risk ratios (RRR), 95% confidence intervals (CI), and P-values for risk factors for death at 2 months*.
| Risk Factor | Adjusted RRR of death* (95% CI) | P-value | Adjusted RRR of unknown status* (95% CI) | P-value |
| Female (reference) | 1 | 1 | ||
| Male | 0.63(0.35,1.15) | 0.13 | 0.76(0.43,1.35) | 0.35 |
| Age<30 years (reference) | 1 | 1 | ||
| Age 30–40 years | 0.71(0.38,1.15) | 0.27 | 1.18(0.66,2.09) | 0.58 |
| Age>40 years | 0.89(0.41,1.95) | 0.78 | 0.59(0.24,1.47) | 0.26 |
| Sputum smear negative (reference) | 1 | 1 | ||
| Sputum smear positive | 0.86(0.39,1.90) | 0.70 | 0.64(0.24,1.69) | 0.37 |
| No antiretroviral therapy at enrollment (reference) | 1 | 1 | ||
| On antiretroviral therapy at enrollment | 0.82(0.46,1.45) | 0.49 | 0.74(0.42,1.31) | 0.31 |
| No cough more than 14 days (reference) | 1 | 1 | ||
| Cough>14 days | 1.15(0.6,2.03) | 0.63 | 1.11(0.63,1.94) | 0.72 |
| LAM or CRAG antigen test positive | 2.29(1.29,4.05) | 0.005 | 0.71(0.39,1.28) | 0.25 |
CI = confidence intervals, RRR = relative risk ratio, SD = standard deviation, Mtb = Mycobacterium tuberculosis, LAM = lipoarabinomannan, CRAG = cryptococcal antigen.
*N = 350 with complete records, Multinomial logistic regression model adjusted for gender, age, baseline direct smear positivity, currently on ART. Alive group as reference.
Discussion
In hospitalized patients, a urinary LAM positive result is associated with death in an adjusted multinomial regression analysis. One-third of the hospitalized, HIV-infected participants in this study died within 6 months of enrollment. These mortality rates are consistent with those previously reported by others [4]–[6], [30] and have not changed appreciably since the beginning of the HIV epidemic in resource-limited settings in SSA. The reasons for this high mortality rate are multifactorial including late HIV presentation, antiretroviral treatment failure, and delays in diagnosis leading to the high acuity of illness in hospitalized patients. Among TB suspects with cough and in a broader category of febrile HIV-positive patients, many studies have documented Mtb infection and mycobacteremia as well as cryptococcal infection as prominent causes of illness and death [3], [9]–[13]. The timely application of new, urine-based lateral flow tests for both TB disease and cryptococcal infection that do not require electricity or technical expertise to perform may avert mortality by accelerating the time to diagnosis.
Among culture-confirmed TB cases who died, LAM testing had additive value to sputum based Xpert testing; 78% were diagnosed by LAM LFA (28% were Xpert negative), and 66% by Xpert (14% were LAM LFA negative). This corroborates the previously published increased sensitivity of sputum smear plus LAM, and Xpert MTB/RIF plus LAM for TB cases overall [24], [31]–[34]. Interestingly, 28% of participants that were LAM-positive died within 2 months compared to 17% of those that were LAM-negative (p = 0.035); all LAM positive cases classified as “no evidence of TB” who died were Xpert negative. Based on our own data and others, urinary LAM is most sensitive in patients with disseminated TB disease [35] who are least likely to be positive by sputum Xpert MTB/RIF and often have atypical symptoms (e.g. fever only) which is often not recognized as TB with concomitant treatment delays [13], [14], [33]. LAM LFA may be more sensitive for an in-patient population with higher mortality risk and may explain why implementation of on-site Xpert MTB/RIF did not impact 2-month mortality in a study done in the same hospital [36].
In our study, 7% of the patients were cryptococcal antigen positive without overt signs of meningitis which is similar to previously reported rates in hospitalized patients in Uganda [30] and not significantly different from the prevalence in ART initiation cohorts (CD4<100 cells/µl) in SSA (Uganda 8.2% [21], South Africa 8.8% [20]). The serum cryptococcal antigen test has high sensitivity and specificity; the urine assay has 91% sensitivity compared to the serum test in published comparative data [28]. Cryptococcal antigenemia is a known independent risk factor for death [19], [20], and pre-emptive treatment with fluconazole can impact outcomes [37]. Testing prior to ART initiation is cost effective using the latex agglutination cryptococcal test and even more cost effective with the new, less expensive lateral flow assay [21], [38].
Our study had several strengths including the prospective collection and testing of urine with LAM LFA in an HIV-infected hospitalized population suspected to have TB in a setting outside of South Africa where many TB diagnostic studies are implemented. Because mortality was not a primary endpoint of the parent study, however, not all patients could be located at 2 months despite rigorous tracing exercises. In addition, we do not have reliable data on which patients received TB treatment which could have altered their outcomes. Secondly, one-third (44/134) of the LAM positive patients were positive at the lowest band intensity. In previous studies, the lowest 1+ band intensity has been difficult to interpret and has had lower specificity than band intensities 2+ and greater [25]. With respect to CRAG detection, the retrospective analysis of previously boiled, freeze-thawed urine could have further decreased the sensitivity of the assay in addition to the use of urine. CRAG titers in urine are considerably lower (22-fold) compared to serum or plasma [28] though this was not clinically significant since the LFA was positive in serum, plasma, and urine in 61 of 62 patients.
Either LAM or CRAG LFA positive results were associated with mortality within 2 months (RRR 2.29, P = 0.005). Our data suggest that the LFA's are diagnosing disease-specific mortality which may be related to disease severity or undiagnosed, untreated disease due to a reference standard that does not capture all patients with true disease (TB sputum culture). Although POC CRAG and LAM may more rapidly diagnose infections that may lead to death in hospitalized patients and have the potential to accelerate timely treatment, the impact on patient centered outcomes needs to be prospectively tested when LAM LFA is used as a screening test in HIV-infected patients and patients are treated promptly. Prospective studies to investigate outcomes with empiric TB treatment as well as the Determine TB LAM test and treat strategies are on-going in sub-Saharan Africa [39]. One observational study from Brazil showed increased mortality with empiric TB treatment, although no weighted adjustment for selection bias was performed in this study [40].
Conclusions
In hospitalized HIV-positive adults, a positive urinary LAM POC LFA result is associated with mortality. When combined with the CRAG lateral flow POC LFA, the combination (i.e. either test positive) has even higher predictive value. Given the high rates of mortality in hospitalized patients despite the introduction of antiretroviral therapy for HIV, further assessment of the impact of these rapid diagnostics on averting mortality as components of a ‘test and treat’ algorithm is warranted.
Supporting Information
Supporting tables. Table S1, 2 month mortality and LAM/CRAG status stratified by TB categorization. Table S2, 6 month mortality and LAM/CRAG status stratified by TB categorization. Table S3, Adjusted relative risk ratios (RRR), 95% confidence intervals (CI), and P-values for risk factors for death at 2 months including CD4 category.
(DOCX)
Acknowledgments
The authors would like to thank the following individuals for their important contributions to the implementation of this study: John Mark Bwanika, Teddy Nalwoga, Willy Ssengooba, Francis Mumbowa, Carolyn Namaganda, Allan Buzibye, and Francis Kakooza in Kampala, Uganda, Molly Holshauser, Derek Armstrong, David Hom, Rachel Kubiak, and Mary Gaeddert in US. The authors and site team gratefully acknowledge the study participants for their willingness to participate in the study. The authors also thank Alere and Immy for their generous donation of the POC lateral flow assays.
Funding Statement
The project was funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract #HHSN2722000900050C, “TB Clinical Diagnostics Research Consortium.” Additional Support was provided to YCM by the Johns Hopkins University Center for AIDS Research (Grant Number 1P30AI094189 from the National Institute of Allergy And Infectious Diseases). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Corbett EL, Marston B, Churchyard GJ, De Cock KM (2006) Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 367: 926–937. [DOI] [PubMed] [Google Scholar]
- 2. Talbot E, Munseri P, Teixeira P, Matee M, Bakari M, et al. (2012) Test characteristics of urinary lipoarabinomannan and predictors of mortality among hospitalized HIV-infected tuberculosis suspects in Tanzania. PLoS ONE 7: e32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB (1998) Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 26: 290–296. [DOI] [PubMed] [Google Scholar]
- 4. Saleri N, Capone S, Pietra V, De Iaco G, Del Punta V, et al. (2009) Outcome and predictive factors of mortality in hospitalized HIV-patients in Burkina Faso. Infection 37: 142–147. [DOI] [PubMed] [Google Scholar]
- 5. Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, et al. (2011) Clinical characteristics and predictors of mortality in hospitalized HIV-infected Nigerians. J Infect Dev Ctries 5: 377–382. [DOI] [PubMed] [Google Scholar]
- 6. Munseri PJ, Talbot EA, Bakari M, Matee M, Teixeira JP, et al. (2011) The bacteraemia of disseminated tuberculosis among HIV-infected patients with prolonged fever in Tanzania. Scand J Infect Dis 43: 696–701. [DOI] [PubMed] [Google Scholar]
- 7. Cox JA, Lukande RL, Nelson AM, Mayanja-Kizza H, Colebunders R, et al. (2012) An autopsy study describing causes of death and comparing clinico-pathological findings among hospitalized patients in Kampala, Uganda. PLoS ONE 7: e33685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinson NA, Karstaedt A, Venter WD, Omar T, King P, et al. (2007) Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. Aids 21: 2043–2050. [DOI] [PubMed] [Google Scholar]
- 9. Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, et al. (2009) Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS ONE 4: e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, et al. (2011) Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 52: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacob ST, Banura P, Baeten JM, Moore CC, Meya D, et al. (2012) The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis: a prospective intervention study*. Crit Care Med 40: 2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, et al. (1998) A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol 19: 484–489. [DOI] [PubMed] [Google Scholar]
- 13. Lewis DK, Peters RP, Schijffelen MJ, Joaki GR, Walsh AL, et al. (2002) Clinical indicators of mycobacteraemia in adults admitted to hospital in Blantyre, Malawi. Int J Tuberc Lung Dis 6: 1067–1074. [PubMed] [Google Scholar]
- 14. McDonald LC, Archibald LK, Rheanpumikankit S, Tansuphaswadikul S, Eampokalap B, et al. (1999) Unrecognised Mycobacterium tuberculosis bacteraemia among hospital inpatients in less developed countries. Lancet 354: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 15.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, et al. (2009) Diagnostic Accuracy of a Urine Lipoarabinomannan Test for Tuberculosis in Hospitalized Patients in a High HIV Prevalence Setting. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed]
- 16. French N, Gray K, Watera C, Nakiyingi J, Lugada E, et al. (2002) Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. Aids 16: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 17. Jarvis JN, Boulle A, Loyse A, Bicanic T, Rebe K, et al. (2009) High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. Aids 23: 1182–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530. [DOI] [PubMed] [Google Scholar]
- 19. Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, et al. (2007) Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 12: 929–935. [DOI] [PubMed] [Google Scholar]
- 20. Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, et al. (2009) Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 48: 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, et al. (2010) Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 51: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peeling RW, Holmes KK, Mabey D, Ronald A (2006) Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 82 Suppl 5: v1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawn SD, Dheda K, Kerkhoff AD, Peter JG, Dorman S, et al. (2013) Determine TB-LAM lateral flow urine antigen assay for HIV-associated tuberculosis: recommendations on the design and reporting of clinical studies. BMC Infect Dis 13: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawn SD, Kerkhoff AD, Vogt M, Wood R (2012) Clinical significance of lipoarabinomannan detection in urine using a low-cost point-of-care diagnostic assay for HIV-associated tuberculosis. AIDS 26: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 25.Peter JG, Theron G, van Zyl-Smit R, Haripersad A, Mottay L, et al. (2012) Diagnostic accuracy of a urine LAM strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. [DOI] [PMC free article] [PubMed]
- 26.Dorman S, Manabe Y, Nicol M, Nakiyingi L, Moodley M, et al. (2012) Accuracy of the Alere Determine TB-LAM Lateral Flow Test for Diagnosis of Tuberculosis in Adults with HIV Infection: Interim Results from a Multicenter Study. CROI 2012. Seattle.
- 27.Van Rie A, Jong E, Mkhwanazi M, Sanne I (2013) Diagnosting TB in those Hardest ot Diagnose: Urine Lipoarabionomannan for Suspects of Disseminated and Extrapulmonary TB. Conference on Retroviruses and Opportunistic Infections. Atlanta, GA. [Google Scholar]
- 28. Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, et al. (2011) Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis 53: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakiyingi L, Moodley VM, Manabe YC, Nicol MP, Holshouser M, et al. (2014) Diagnostic accuracy of a rapid urine lipoarabinomannan test for tuberculosis in HIV-infected adults. J Acquir Immune Defic Syndr.(epub) [DOI] [PMC free article] [PubMed]
- 30. Andama AO, den Boon S, Meya D, Cattamanchi A, Worodria W, et al. (2013) Prevalence and Outcomes of Cryptococcal Antigenemia in HIV-Seropositive Patients Hospitalized for Suspected Tuberculosis in Uganda. J Acquir Immune Defic Syndr 63: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lawn SD, Kerkhoff AD, Vogt M, Wood R (2012) Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 12: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Theron G, Pooran A, Peter J, van Zyl-Smit R, Kumar Mishra H, et al. (2012) Do adjunct tuberculosis tests, when combined with Xpert MTB/RIF, improve accuracy and the cost of diagnosis in a resource-poor setting? Eur Respir J 40: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, et al. (2012) Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis 54: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah M, Ssengooba W, Armstrong D, Nakiyingi L, Holshouser M, et al. (2014) Comparative performance of urinary lipoarabinomannan assays and Xpert MTB/RIF in HIV-infected individuals with suspected tuberculosis in Uganda. AIDS. [DOI] [PMC free article] [PubMed]
- 35. Wood R, Racow K, Bekker LG, Middelkoop K, Vogt M, et al. (2012) Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC Infect Dis 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon C, Cattamanchi A, Davis JL, Worodria W, den Boon S, et al. (2012) Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS ONE 7: e48599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rajasingham R, Meya DB, Boulware DR (2012) Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr 59: e85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Micol R, Tajahmady A, Lortholary O, Balkan S, Quillet C, et al. (2010) Cost-effectiveness of primary prophylaxis of AIDS associated cryptococcosis in Cambodia. PLoS One 5: e13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawn SD, Ayles H, Egwaga S, Williams B, Mukadi YD, et al. (2011) Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis 15: 287–295. [PubMed] [Google Scholar]
- 40. de Albuquerque MD, Coimbra I, Batista JD, Maruza M, Ximenes RA, et al. (2014) Empirical treatment for TB in HIV: lessons from a cohort study of people living with HIV treated in Recife, Brazil. BMC Public Health 14: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting tables. Table S1, 2 month mortality and LAM/CRAG status stratified by TB categorization. Table S2, 6 month mortality and LAM/CRAG status stratified by TB categorization. Table S3, Adjusted relative risk ratios (RRR), 95% confidence intervals (CI), and P-values for risk factors for death at 2 months including CD4 category.
(DOCX)