Abstract
Background
Adjuvant chemotherapy reduces recurrences of non-small cell lung cancer (NSCLC). To determine which patients need adjuvant chemotherapy, we assessed factors associated with time to relapse (TTR).
Methods
In 230 resected stages I-II NSCLCs we correlated immunohistochemistry (IHC) scores for factors associated with cell growth rate, growth regulation, hypoxia, cell survival, and cell death with TTR.
Results
With a median follow-up of 82 (1-158) months for those alive and relapse-free at last follow-up, median time to recurrence was not reached. The 2- and 5-year probabilities of maintaining freedom from recurrence were 80.7% (95% confidence interval [CI]:(75.3%, 86.4%)) and 74.6% (95% CI:(68.6%, 81.2%)), respectively. TTR curves flattened at an apparent cure rate of 70%. In multicovariate Cox models, factors correlating with shorter TTR were membranous carbonic anhydrase IX (mCAIX) staining (any vs none, hazard ratio [HR]=2.083, p=0.023) and node stage (N1 vs N0, HR=2.591, p=0.002). mCAIX scores correlated positively with tumor size, grade, squamous histology, necrosis, mitoses, Ki67, p53, nuclear DNMT1 and cytoplasmic SHARP2, and correlated inversely with papillary histology, EGFR mutation (trend), CTR1, and cytoplasmic HIF-1α, VEGF, DNMT1, and ERCC1.
Conclusion
Nodal stage and mCAIX IHC were the strongest independent predictors of shorter TTR in resected NSCLCs. mCAIX correlated with tumor size, markers of tumor proliferation and necrosis, and tumor genetic characteristics, and paradoxically correlated inversely with the hypoxia markers HIF-1α and VEGF. Presence of mCAIX could help determine patients with high-risk of recurrence who might require adjuvant chemotherapy.
Keywords: carbonic anhydrase IX, non-small cell lung cancer
Introduction
Lung cancer is the world's leading cause of cancer death,1 and US lung cancer 5-year relative survival rate has only increased from 12% in 1975–77 to 16% currently.2 The poor prognosis of lung cancer is due in part to a high proportion of patients presenting initially with advanced disease, but even patients with operable early stage disease are at moderately high risk of relapse.3 Adjuvant chemotherapy reduces the probability of relapse after lung cancer resection, but we are currently not able to accurately determine which patients actually need adjuvant therapy. In patients with resected stages I and II non-small cell lung cancer (NSCLC), we assessed association with time to relapse (TTR) and overall survival (OS) of various tumor markers related to tumor growth rate, growth control, tumor cell survival and death, and hypoxia. In defining clinically important biomarkers to predict tumor biological behavior, OS is a more precise endpoint than TTR but it has the major disadvantage that it is affected by several factors unrelated to tumor biology, including patient age, comorbidities and therapy details.4 Hence, our primary objective was to define factors associated with TTR (defined as the time from surgery to tumor recurrence, with patients censored at time of last follow-up, death from other causes, or development of a new primary malignancy (in lung or any other site) that was associated with metastases, if they remained free of evidence of recurrence of their initial primary lung cancer at that time).
Materials and Methods
Using an IRB-approved laboratory protocol, we accessed the University of Texas/MD Anderson Cancer Center Lung SPORE Tissue Bank and selected resected archival formalin-fixed, paraffin-embedded (FFPE) tumor samples from 230 stage I-II NSCLC patients with squamous cell (n=87) or adenocarcinomas (n=143) who had given informed consent at the time of tissue collection and who had not received any adjuvant or neoadjuvant chemotherapy or radiotherapy.
Tissue microarrays were prepared using standard methods:5 a biopsy needle was inserted into each FFPE tumor specimen 3 times to obtain 3 tissue cores, each measuring 1 mm in diameter by 2–3 mm long. Based on prior assessment of H&E slides, the TMA cores were obtained preferentially from areas with high tumor cell content and with minimal necrosis or fibrosis, with one sample taken from around the center of the tumor, one from the periphery, and one from an intermediate area. Serial sections were cut from the TMA constructed from these cores and mounted on glass slides. Slides were deparaffinized in Xylene for 10 minutes 3 times. The tissue sections were hydrated in graded ethanols 100%, 90%, 70% and 50% for 5 minutes each time. Heat-induced epitope retrieval was performed in a Dako antigen retrieval bath at 121°C for 30 minutes and 90°C for 10 minutes using a Decloaking chamber (Biocare), followed by a 30 min cool down. Prior to antibody immunostaining, endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 30 min. In order to block non-specific antibody binding, tissue sections were incubated with 10% FBS serum in TBSt for 30 min. Incubation with primary antibodies is as presented in Supplementary Table 1. This was followed by incubation with Envision plus labeled polymer, anti-rabbit-HRP antibody (Dako) for 30 min at room temperature. Staining development was performed with DAB, with timed monitoring using a positive control sample. The slides were then counterstained with hematoxylin, dehydrated, cleared and mounted.
Experienced lung pathologists (MIN and IIW, blinded for patient outcome) then manually recorded percent of tumor cells staining with 0 (absent), 1+ (mild), 2+ (moderate) and 3+ (strong) intensity. As appropriate, factor staining intensity was assessed for one or more of tumor cell nucleus, cytoplasm and membrane. Immunohistochemistry (IHC) scores (0–300) were then calculated for each relevant cell region by multiplying stain intensity (0–3) by percent tumor cells staining with each intensity. For each tumor specimen, results from the 3 cores were then averaged. If for a given patient results for only a single core were evaluable by IHC, then that single value was used, while the results for 2 cores were averaged if only 2 of the 3 cores were evaluable.
Molecular factors that we assessed included nuclear factors (p53, p21WAF1/CIP1 and Ki67), cytoplasmic factors (Cyclo-oxygenase-2 [COX2] and Decoy Receptor-2 [DcR2]); nuclear and cytoplasmic factors (Copper Transporter-1 [CTR1], DNA Methyltransferase 1 [DNMT1], Hypoxia Inducible Factor-1α [HIF1α], Retinoblastoma [Rb], phospho-Rb, Enhancer-of-Split-and-Hairy-Related Protein [SHARP2, also known as “differentially expressed in chondrocytes protein-1”/DEC1], Survivin, Vascular Endothelial Growth Factor [VEGF], p14ARF, p16INK4 and Excision Repair Cross-Complementing Rodent Repair Deficiency, Complementation Group 1 [ERCC1]); and cytoplasmic and membrane Carbonic Anhydrase IX (CAIX) and Transforming Growth Factor-β (TGF-β). We also defined number of apoptotic cells (by TUNEL assay) per 10 high-powered fields, and for a subset of patients we had information on mutation status for epidermal growth factor receptor (EGFR) and Kras genes.
Tumor specimens were assessed by pathologists IIW and MIN for tumor histopathologic type, percent of adenocarcinomas made up of acinar, lepidic, mucinous, papillary, solid and micropapillary regions, number of mitoses per 10 high-powered fields, presence of necrosis, and invasion of pleura or lymphovascular structures. Number of nodes examined, number of positive nodes, tumor diameter, and pathologic stage were also recorded.
Statistical Methods
Patient gender, age, race, and smoking history information was recorded and summarized by descriptive statistics. Continuous biomarkers were summarized by mean, standard deviation, median and range. The difference of biomarkers between/among patient characteristic groups was tested by Wilcoxon rank sum test or Kruskal-Wallis test, when appropriate. Correlation between biomarkers was assessed using Spearman correlation analysis. Two experienced clinicians (DJS and CB) reviewed patient records and scans to assess TTR and overall survival (OS) from surgery.
For the purposes of this study, TTR was defined as the time from surgery to relapse or last follow up (LFU), with censoring at LFU, at death from other causes, or at diagnosis of a second primary associated with metastases if the patient was clinically relapse-free from their initial NSCLC. For example, if a patient was diagnosed with colorectal cancer, with intraabdominal nodal and liver metastases, this was interpreted for the purposes of this study as representing metastatic colorectal cancer unless there were compelling clinical, radiological or histopathologic data to indicate that it did instead indicate recurrent lung cancer, and the patient was censored for lung cancer recurrence at the time of diagnosis of the colorectal cancer. Similarly, while development of more than one lung nodule was generally interpreted as recurrence of the original lung cancer, development of a solitary lung nodule or mass that appeared clinically and radiologically to be more likely a new lung cancer primary was coded as a new primary rather than a recurrence. For example, a nodule or mass developing near a resection margin was coded as a recurrence of the original lung cancer while development of a single spiculated nodule in a different lobe was interpreted as a new primary. The investigators were blinded with respect to biological markers when making these designations.
Patients who died of other apparent causes and who had not had clinically suspected or confirmed relapse by the time of death were censored for TTR at their last relapse-free follow-up if they had not had sufficient evaluation shortly prior to their death to conclude with reasonable clinical certainty that they were free of relapse at the time of death. Again, this designation was done in a blinded fashion. We recognized that in both this designation and in the designation of recurrence vs new primary we would miss some recurrences, but chose to err on the side of being more certain of recurrence vs being more certain about lack of recurrence.
TTR and OS were estimated using the Kaplan-Meier method, and the log-rank test was employed to compare TTR between mCAIX staining groups. Cox models were used to assess factors associated with TTR. Only the significant factors with p<0.05 from univariate Cox model were included in the multicovariate Cox model, and backward selection was used to eliminate the ones that were not significant. P-value less than 0.05 was considered as significant. The analysis was performed using SAS 9.3(SAS Institute Inc., Cary, NC, USA). Factors associated with TTR in multicovariate analyses were then tested for their association with OS.
Results
The median age of the 230 patients was 68.2 (range, 33.5 – 90.3) years, the majority were Caucasian (214/230, 93.0%), 122 (53.0%) were female and 108 were male (47.0%), 92 (40.0%) were current smokers, 103 (44.8%) were former smokers and 35 (15.2%) were never smokers, and 182 (79.1%) had stage I and 48 (20.9%) had stage II NSCLC. Patient characteristics are outlined in Supplementary Table 2 and tumor IHC scores are presented in Supplementary Table 3. Median (range) follow-up for TTR was 82 (1-158) months for those who were alive and remained relapse-free at last follow-up. Median (range) follow-up for OS for those who remained alive at last follow-up was 80 (1-158) months. A total of 52 patients had confirmed recurrence and 113 patients died. TTR for the population is presented in Supplementary Figure 1, and OS is presented in Supplementary Figure 2, with the median TTR not being reached and with median OS being 79 months. The 2- and 5-year probabilities of maintaining freedom from recurrence were 80.7% (95% confidence interval [CI]:(75.3%, 86.4%)) and 74.6% (95% CI:(68.6%, 81.2%)), respectively. When TTR was replotted as an exponential decay (log-linear) curve and subjected to nonlinear regression analysis as previously described,6 those on the terminal flat portion of the curve (the “cured” fraction) constituted 70% of the overall population, and the estimated half-life to relapse for the 30% relapsing was 20 months (Supplementary Figure 3).
In Table 1 are presented univariate Cox model analyses for impact of patient and tumor variables on TTR. Nodal stage (N1 vs N0), higher number of nodes positive, larger tumor diameter, and pathological stage (II vs I) each correlated with short TTR. Among the IHC markers, TTR was significantly shorter if any membrane CAIX (mCAIX) staining was detected compared to patients whose tumors had no detectable mCAIX staining (Figure 1). The 24- and 60-month probabilities of freedom from recurrence were 75.1% (95% CI: (67.3%, 83.7%)) and 66.0% (95% CI: (57.3%, 76.0%)), respectively, for patients with any mCAIX staining; and 87.5% (95% CI: (80.2%, 95.5%)) and 84.4% (95% CI: (76.4%, 93.4%)), respectively, for patients with negative mCAIX staining (p=0.014, log-rank test).
Table 1.
Univariate Cox Model Analysis for Time to Relapse
| Covariate | Estimate | Stand Error | Hazard Ratio | HR 95% CI | p |
|---|---|---|---|---|---|
| Histology: Adenocarcinoma vs Squamous | −0.187 | 0.285 | 0.830 | 0.474–1.452 | 0.513 |
| Gender: Male vs Female | 0.153 | 0.277 | 1.165 | 0.676–2.007 | 0.582 |
| Age | 0.026 | 0.014 | 1.027 | 0.999–1.055 | 0.062 |
| Race: Caucasian vs others | 0.544 | 0.721 | 1.723 | 0.419–7.086 | 0.451 |
| Smoking: Ever vs Never | 0.653 | 0.471 | 1.922 | 0.764–4.834 | 0.165 |
| Smoking: Current vs Never | 0.637 | 0.496 | 1.890 | 0.716–4.993 | 0.199 |
| Former vs Never | 0.668 | 0.490 | 1.951 | 0.746–5.098 | 0.173 |
| Nodes: N1 vs N0 | 1.096 | 0.302 | 2.991 | 1.655–5.406 | 0.0003 |
| No. nodes positive | 0.383 | 0.106 | 1.467 | 1.191–1.808 | 0.0003 |
| Tumor diameter (cm) | 0.123 | 0.058 | 1.131 | 1.009–1.267 | 0.035 |
| Pathologic Stage: II vs I | 0.945 | 0.302 | 2.573 | 1.425–4.647 | 0.002 |
| CAIX, membrane* | 0.002 | 0.001 | 1.002 | 0.999–1.004 | 0.244 |
| CAIX, cytoplasm* | 0.002 | 0.002 | 1.002 | 0.999–1.005 | 0.249 |
| COX2, cytoplasm* | 0.001 | 0.001 | 1.001 | 0.998–1.004 | 0.502 |
| CTR1, cytoplasm* | 0.001 | 0.002 | 1.001 | 0.998–1.005 | 0.436 |
| CTR1, nuclear* | 0.000 | 0.002 | 1.000 | 0.997–1.004 | 0.873 |
| DNMT1, cytoplasm* | −0.002 | 0.002 | 0.998 | 0.993–1.002 | 0.256 |
| DNMT1, nuclear* | 0.004 | 0.004 | 1.004 | 0.996–1.012 | 0.352 |
| DcR2, cytoplasm* | 0.004 | 0.002 | 1.004 | 0.999–1.008 | 0.134 |
| ERCC1, cytoplasm* | −0.002 | 0.002 | 0.998 | 0.993–1.002 | 0.343 |
| ERCC1, nuclear* | −0.001 | 0.002 | 0.999 | 0.995–1.002 | 0.558 |
| HIF1α, cytoplasm* | 0.001 | 0.002 | 1.001 | 0.998–1.004 | 0.544 |
| HIF1α, nuclear* | 0.007 | 0.006 | 1.007 | 0.995–1.019 | 0.245 |
| Rb, cytoplasm* | −0.003 | 0.002 | 0.997 | 0.993–1.002 | 0.224 |
| Rb, nuclear* | 0.001 | 0.002 | 1.001 | 0.997–1.004 | 0.738 |
| SHARP2, cytoplasm* | −0.003 | 0.003 | 0.997 | 0.992–1.002 | 0.219 |
| SHARP2, nuclear* | 0.002 | 0.002 | 1.002 | 0.999–1.005 | 0.262 |
| SURVIVIN, cytoplasm* | 0.002 | 0.002 | 1.002 | 0.998–1.005 | 0.426 |
| SURVIVIN, nuclear* | 0.002 | 0.001 | 1.002 | 0.999–1.005 | 0.24 |
| TGFβ, membrane* | −0.004 | 0.003 | 0.996 | 0.991–1.002 | 0.16 |
| TGFβ, cytoplasm* | −0.004 | 0.002 | 0.996 | 0.991–1.000 | 0.065 |
| VEGF, cytoplasm* | −0.002 | 0.002 | 0.998 | 0.994–1.003 | 0.434 |
| VEGF, nuclear* | 0.005 | 0.008 | 1.005 | 0.989–1.021 | 0.556 |
| p14ARF, cytoplasm* | −0.003 | 0.004 | 0.997 | 0.990–1.004 | 0.431 |
| p14ARF, nuclear* | 0.005 | 0.006 | 1.005 | 0.994–1.016 | 0.385 |
| p16INK4, cytoplasm* | −0.002 | 0.001 | 0.998 | 0.996–1.001 | 0.268 |
| p16INK4, nuclear* | −0.001 | 0.001 | 0.999 | 0.997–1.002 | 0.513 |
| phospho Rb, cytoplasm* | 0.000 | 0.004 | 1.000 | 0.991–1.008 | 0.923 |
| phospho Rb, nuclear* | 0.001 | 0.002 | 1.001 | 0.996–1.005 | 0.751 |
| p21WAF1/CIP1, nuclear* | 0.003 | 0.002 | 1.003 | 0.999–1.008 | 0.109 |
| p53, nuclear* | −0.001 | 0.001 | 0.999 | 0.997–1.002 | 0.491 |
| Ki67, nuclear* | 0.002 | 0.002 | 1.002 | 0.998–1.005 | 0.377 |
| CAIX, membrane, >0 vs 0 | 0.776 | 0.323 | 2.172 | 1.154–4.090 | 0.016 |
| CAIX, cytoplasm, >0 vs 0 | 0.520 | 0.521 | 1.681 | 0.605–4.672 | 0.319 |
| COX2, cytoplasm, >0 vs 0 | 0.192 | 0.474 | 1.212 | 0.478–3.072 | 0.685 |
| CTR1, nuclear, >0 vs 0 | 0.091 | 0.313 | 1.095 | 0.593–2.022 | 0.772 |
| DNMT1, cytoplasm, >0 vs 0 | −0.200 | 0.310 | 0.818 | 0.446–1.503 | 0.518 |
| DNMT1, nuclear, >0 vs 0 | 0.480 | 0.311 | 1.617 | 0.880–2.972 | 0.122 |
| DcR2, cytoplasm, >0 vs 0 | 0.393 | 0.598 | 1.482 | 0.459–4.788 | 0.511 |
| ERCC1, cytoplasm, >0 vs 0 | −0.274 | 0.291 | 0.760 | 0.430–1.345 | 0.346 |
| ERCC1, nuclear, >0 vs 0 | −0.367 | 0.289 | 0.693 | 0.393–1.221 | 0.204 |
| HIF1α, cytoplasm, >0 vs 0 | −0.160 | 0.521 | 0.852 | 0.307–2.366 | 0.759 |
| HIF1α, nuclear, >0 vs 0 | 0.036 | 0.341 | 1.037 | 0.532–2.021 | 0.916 |
| Rb, cytoplasm, >0 vs 0 | 0.468 | 1.011 | 1.597 | 0.220–11.58 | 0.643 |
| Rb, nuclear, >0 vs 0 | 0.343 | 0.368 | 1.409 | 0.686–2.896 | 0.351 |
| SHARP2, cytoplasm, >0 vs 0 | 0.216 | 0.723 | 1.241 | 0.301–5.123 | 0.766 |
| SHARP2, nuclear, >0 vs 0 | −0.190 | 0.524 | 0.827 | 0.296–2.311 | 0.717 |
| SURVIVIN, nuclear, >0 vs 0 | −0.194 | 0.722 | 0.824 | 0.200–3.391 | 0.789 |
| TGFβ, membrane, >0 vs 0 | 0.198 | 0.285 | 1.219 | 0.697–2.130 | 0.488 |
| TGFβ, cytoplasm, >0 vs 0 | −0.038 | 0.316 | 0.963 | 0.519–1.788 | 0.905 |
| VEGF, nuclear, >0 vs 0 | 0.138 | 0.357 | 1.148 | 0.571–2.309 | 0.699 |
| p14ARF, cytoplasm, >0 vs 0 | −0.157 | 0.308 | 0.854 | 0.468–1.561 | 0.609 |
| p14ARF, nuclear, >0 vs 0 | −0.133 | 0.595 | 0.875 | 0.273–2.811 | 0.823 |
| p16INK4, cytoplasm, >0 vs 0 | −0.342 | 0.286 | 0.711 | 0.405–1.246 | 0.233 |
| p16INK4, nuclear, >0 vs 0 | −0.335 | 0.287 | 0.715 | 0.408–1.254 | 0.242 |
| phospho Rb, cytoplasm, >0 vs 0 | 0.295 | 0.318 | 1.343 | 0.720–2.505 | 0.354 |
| phospho Rb, nuclear, >0 vs 0 | 0.002 | 0.338 | 1.002 | 0.517–1.941 | 0.996 |
| p21WAF1/CIP1, nuclear, >0 vs 0 | −0.103 | 0.474 | 0.902 | 0.356–2.286 | 0.829 |
| p53, nuclear, >0 vs 0 | −0.076 | 0.281 | 0.927 | 0.535–1.607 | 0.787 |
| Ki67, nuclear, >0 vs 0 | −0.085 | 0.522 | 0.919 | 0.331–2.555 | 0.871 |
As a continuous variable.
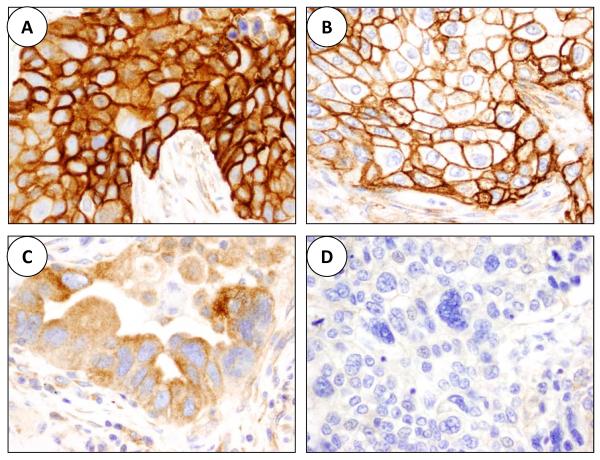
Figure 1.
Microphotographs illustrating CAIX immunohistochemistry expression in malignant cells of lung cancer tissue specimens (×40). A, combined CAIX strong membrane and cytoplasmic expressions in a squamous cell carcinoma. B, strong CAIX membrane expression in a squamous cell carcinoma. C, moderate CAIX cytoplasmic expression in an adenocarcinoma. D, lack of CAIX expression in a poorly differentiated adenocarcinoma.
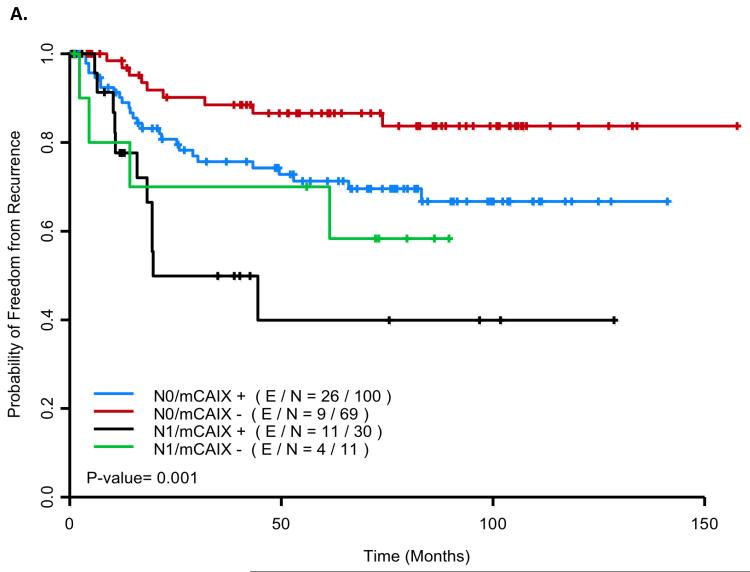
In multicovariate Cox model analysis, nodal stage (N1 vs N0) (HR=2.561 (95% CI: 1.409–4.766), p=0.002) and presence vs absence of mCAIX staining (HR=2.083 (95% CI: 1.104–3.929), p=0.023) emerged as independent prognostic variables for TTR (Table 2). If tumor diameter was forced into the model (HR=1.068 (95% CI: 0.928–1.231), p=0.36) mCAIX remained important (HR=1.923 (95% CI: 0.999–3.705), p=0.05). Figure 2a presents TTR curves for patients with vs without detectable mCAIX staining and with vs without node involvement. The 60-month probability of freedom from recurrence for mCAIX negative/N0 patients, mCAIX positive/N0 patients, mCAIX negative/N1 patients, and mCAIX positive/N1 patients was 0.866 (95% CI (0.783, 0.957)), 0.713 (95% CI (0.621–0.818)), 0.70 (95% CI (0.467, 1.0)) and 0.399 (95% CI (0.213, 0.748), respectively.
Table 2.
Multicovariate Cox Model Analysis for Time to Relapse
| Variable | Parameter Estimate | Standard Error | p-value | Hazard Ratio | 95% Hazard Ratio CI | |
|---|---|---|---|---|---|---|
| Nodes (N1 vs N0) | 0.952 | 0.311 | 0.002 | 2.591 | 1.409 | 4.766 |
| CAIX. Membrane, >0 vs 0 | 0.734 | 0.324 | 0.023 | 2.083 | 1.104 | 3.929 |
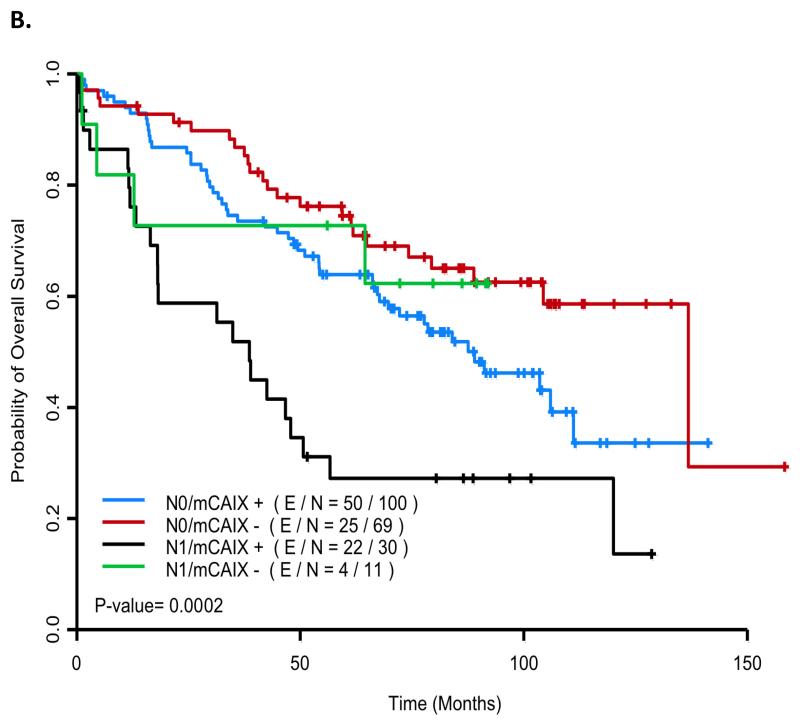
Figure 2.
Kaplan Meier curves for relapse and survival by nodal and membrane carbonic anhydrase IX (mCAIX) staining status (E/N: no. events/no. cases). Figure 2a: Time to Relapse: The 60-month probability of freedom from recurrence was 0.866 (95% CI (0.783, 0.957)), 0.713 (95% CI (0.621–0.818)), 0.70 (95% CI (0.467, 1.0)) and 0.399 (95% CI (0.213, 0.748), for N0/mCAIX negative (red), N0/mCAIX positive (blue), N1/mCAIX negative (green) and N1/mCAIX positive (black) patients, respectively. Figure 2b: Overall Survival: The 60-month probability of being alive was 0.745 (95% CI (0.647, 0.858)), 0.639 (95% CI (0.0.550, 0.742)), 0.727 (95% CI (0.506, 1.0)) and 0.272 (95% CI (0.149, 0.497) for N0/mCAIX negative (red), N0/mCAIX positive (blue), N1/mCAIX negative (green), and N1/mCAIX positive (black) patients, respectively.
Five N0 patients (3 stage IA patients with tumors measuring 1.6–2.2 cm and 2 stage IB patients, both with tumors measuring 4.5 cm) were judged clinically and radiologically to have developed second primary cancers rather than recurrences. Of these, 2 were negative for mCAIX on IHC and 3 were positive. If they were excluded from the analyses, TTR continued to be significantly associated with mCAIX (HR=1.903 (95% CI: 1.114–3.250), p=0.0185). None of the 5 had their new primaries compared to their old ones histopathologically.
We then assessed characteristics that correlated with mCAIX IHC scores. In Table 3 are median mCAIX scores for different patient groups. mCAIX scores were significantly higher in squamous cell carcinomas than adenocarcinomas, and less differentiated tumors than better differentiated tumors, with a trend toward higher scores (p=0.074) in patients with EGFR wild type tumors compared to those with EGFR mutations. There was also a trend towards higher mCAIX scores in patients with higher stage tumors (p=0.063 for N0 vs N1).
Table 3.
Membrane carbonic anhydrase IX in different patient groups
| Group | N | mCAIX* (mean ± standard deviation, median) | P value |
|---|---|---|---|
|
| |||
| Histopathologic Type: | |||
| Adenocarcinoma | 133 | 60.4 ± 91.1, 0 | <0.0001 |
| Squamous cell carcinoma | 77 | 118.0 ± 104.5, 103 | |
|
| |||
| Nodal Stage: | |||
| N0 | 169 | 75.6 ± 97.1, 30 | 0.063 |
| N1 | 41 | 106.2 ± 108.4, 60 | |
|
| |||
| Pathological Stage: | |||
| I | 164 | 75.3 ± 96.6, 30 | 0.077 |
| II | 46 | 103.9 ± 109.2, 60 | |
|
| |||
| Differentiation: | |||
| Well | 47 | 63.2 ± 99.4, 0 | 0.043 |
| Moderate | 87 | 76.6 ± 96.2, 20 | |
| Poor | 75 | 97.9 ± 103.6, 60 | |
|
| |||
| EGFR: | |||
| Mutation | 12 | 27.5 ± 56.5, 0 | 0.074 |
| Wild type | 129 | 72.3 ± 95.4, 20 | |
|
| |||
| KRAS: | |||
| Mutation | 12 | 82.1 ± 107.2, 35 | 0.88 |
| Wild type | 128 | 66.5 ± 91.7, 18.33 | |
|
| |||
| Gender: | |||
| Female | 110 | 79.6 ± 102.4, 20 | 0.3879 |
| Male | 100 | 83.7 ± 97.6, 42.5 | |
|
| |||
| Race: | |||
| Caucasian | 194 | 77.3 ± 96.2, 30 | 0.090 |
| Other | 16 | 133.5 ± 130.2, 72.5 | |
|
| |||
| Smoking History: | |||
| Current | 84 | 81.0 ± 97.4, 37.5 | 0.605 |
| Former | 94 | 87.3 ± 104.3, 42.5 | |
| Never | 32 | 66.1 ± 94.4, 15 | |
mCAIX range for each category was 0–300
In Table 4 are correlations between mCAIX scores and other continuous variables. mCAIX scores correlated directly with tumor diameter, cytoplasmic SHARP2 scores, number of mitoses, tumor necrosis, and with scores for cytoplasmic SHARP2, nuclear Ki67, nuclear DNMT1, and nuclear p53 scores, and correlated inversely with percent of tumor that had papillary characteristics (among adenocarcinomas) and with scores for cytoplasmic HIF1α, cytoplasmic VEGF, cytoplasmic DNMT1, nuclear and cytoplasmic CTR1, cytoplasmic ERCC1, nuclear and cytoplasmic p16, and nuclear p14. mCAIX scores did not correlate with apoptosis or with scores for nuclear HIF1α, nuclear VEGF, SHARP2, or other pro-cell-survival or tumor suppressor gene-related factors.
Table 4.
Factors correlating with membrane carbonic anhydrase IX score
| Factor* | N | Spearman coefficient | P value |
|---|---|---|---|
| Tumor diameter | 204 | 0.243 | 0.0005 |
| Adenocarcinoma composition (% of tumor made up by each histopathologic subtype): | |||
| % acinar | 133 | 0.121 | 0.167 |
| % lepidic | 133 | −0.126 | 0.148 |
| % mucinous | 133 | 0.018 | 0.836 |
| % papillary | 133 | −0.212 | 0.014 |
| % solid | 133 | 0.094 | 0.282 |
| % micropapillary | 133 | −0.067 | 0.44 |
| Hypoxia-associated markers: | |||
| nHIF1α | 208 | −0.016 | 0.818 |
| cHIF1α | 208 | −0.153 | 0.027 |
| nVEGF | 188 | 0.120 | 0.100 |
| cVEGF | 188 | −0.143 | 0.051 |
| nSHARP2 | 187 | −0.044 | 0.551 |
| cSHARP2 | 187 | 0.255 | 0.0004 |
| Proliferation-associated markers: | |||
| Mitoses | 209 | 0.203 | 0.003 |
| nKi67 | 200 | 0.212 | 0.003 |
| nDNMT1 | 201 | 0.192 | 0.006 |
| cDNMT1 | 201 | −0.240 | 0.0006 |
| nCTR1 | 198 | −0.208 | 0.003 |
| cCTR1 | 198 | −0.228 | 0.001 |
| Cell death- or survival-associated markers: | |||
| Necrosis | 209 | 0.260 | 0.0001 |
| Apoptosis | 195 | 0.060 | 0.408 |
| cDcR2 | 191 | 0.006 | 0.929 |
| nSURVIVIN | 205 | 0.052 | 0.463 |
| cSURVIVIN | 205 | −0.074 | 0.294 |
| cTGFbeta | 203 | −0.135 | 0.055 |
| mTGFbeta | 203 | −0.125 | 0.077 |
| DNA repair and inflammation markers: | |||
| nERCC1 | 203 | −0.049 | 0.49 |
| cERCC1 | 203 | −0.151 | 0.031 |
| cCOX2 | 174 | 0.061 | 0.42 |
| Tumor Suppressor Genes and Related Molecules: | |||
| nP53 | 209 | 0.157 | 0.024 |
| nP21 | 196 | 0.081 | 0.256 |
| nRB | 204 | −0.029 | 0.677 |
| cRB | 204 | −0.125 | 0.074 |
| nPhosphoRB | 176 | 0.136 | 0.071 |
| cPhosphoRB | 176 | −0.018 | 0.814 |
| nP16 | 202 | −0.14 | 0.043 |
| cP16 | 202 | −0.14 | 0.041 |
| nP14 | 209 | −0.135 | 0.051 |
| cP14 | 209 | −0.09 | 0.191 |
c: cytoplasmic m: membrane n: nuclear
IHC data for mCAIX were evaluable for all 3 cores for 63.2% of patients, for 2 cores for 23.4%, and for only 1 core for 13.4% of patients. Using data from patients in whom at least 2 cores were evaluable for mCAIX, we also assessed impact of heterogeneity of tumor mCAIX IHC expression on TTR. Whether just considering patients with 3 evaluable cores (37% mCAIX negative for all cores, 45% mCAIX positive for all cores and 19% heterogeneous with 1–2 cores negative and the remaining positive for mCAIX), or also adding in patients with just 2 evaluable cores (38% mCAIX negative in both cores, 45% positive in both cores and 17% positive in one and negative in the other), the TTR curve for heterogeneous patients was intermediate between the TTR curves for homogeneously negative patients and for homogeneously positive patients (p=0.0471, log-rank test for trend). If we separately assessed the patients with only a single evaluable core, 47% were negative for mCAIX and 53% were positive. Confidence intervals were wide, but TTR was significantly worse for positive patients than for negative patients (HR=6.413 (95% CI: 1.363–30.17), p=0.0187).
In patients with at least 2 evaluable cores, the proportion of patients with mCAIX heterogeneity was similar for patients with adenocarcinomas vs squamous carcinomas (17% vs 16%), while the proportion of patients with all cores being positive was lower in adenocarcinomas than in squamous carcinomas (35% vs 60%, p=0.0007). Similarly, although only 6% of patients with no necrosis noted in their samples had heterogeneity between cores compared to 17% of patients with at least some degree of tumor necrosis, this difference was not significant (p=0.49), while the proportion of patients who had all cores positive for mCAIX was higher in those with necrosis than in those without necrosis (48% vs 21%, p=0.0287).
While we were primarily interested in TTR, we then assessed whether the factors associated with TTR in multicovariate Cox models were also associated with OS. When nodal stage and mCAIX were taken together as the only factors considered for a multicovariate OS model, the HR for N1 vs N0 was 1.918 (95% CI (1.223, 3.008), p=0.0133) and the HR for mCAIX positive vs negative was 1.762 (95% CI (1.142, 2.720), p=0.0105). The 60-month probability of OS for mCAIX negative/N0 patients, mCAIX positive/N0 patients, mCAIX negative/N1 patients, and mCAIX positive/N1 patients was 0.745 (95% CI (0.647, 0.858)), 0.639 (95% CI (0.0.550, 0.742)), 0.727 (95% CI (0.506, 1.0)) and 0.272 (95% CI (0.149, 0.497), respectively (Figure 2b).
Discussion
In determining who should be considered for adjuvant therapies, it helps to be able to define those patients who are at highest risk of tumor relapse. In this study, we found that for patients with resected stage I-II NSCLC, there appeared to be a plateau on the TTR curve, with approximately 70% of patients projected to be on this “cured” plateau. TTR differs from “relapse-free survival” since our patients were censored if they died of other apparent causes, while either relapse or death from any causes is counted as a relapse-free survival event. We wished to maximize the probability of defining factors associated with tumor biology without contamination from factors associated with death from comorbidities or second primary malignancies. Patients who died of uncertain causes without recent reevaluation of relapse status were censored at the time of last evaluation for relapse if they were relapse-free at that time. Our approach (designed to improve biological and clinical relevance of our assessments) decreased statistical power by decreasing the number of evaluable “events” and by decreasing the length of follow-up for censored patients, and would have missed any relapses occurring after last follow-up.
In keeping with the well-established impact of stage on outcome,3 node involvement emerged as the most important predictor of relapse. Presence of membrane staining for CAIX was the only other factor that correlated with outcome in multicovariate analysis. While mCAIX correlated with both tumor size and with Ki67, mCAIX correlated independently with TTR while size and Ki67 did not.
CAIX mRNA7,8 and protein (by IHC)9–12 are frequently expressed in resected NSCLCs, with CAIX expression being particularly common in squamous cancers.9,13 It is unknown whether this association of CAIX with squamous lung cancers is related to the frequent loss of the von Hippel-Lindau (VHL) tumor suppressor gene that is seen in NSCLC.14 VHL loss of heterozygosity (LOH)15 and methylation/downregulation16 are common in NSCLC, and occur in squamous cell carcinomas much more frequently than in adenocarcinomas. In other tumor types, mCAIX expression was noted in pheochromocytomas only if they were associated with VHL germline mutations,17 while in renal cell carcinomas, CAIX expression was perinecrotic in tumors with intact VHL systems but was diffuse in VHL-defective tumors.18
Tumor subtype may also be important. We found particularly low expression in lung adenocarcinomas with a high papillary component. Of interest, CAIX expression is also lower in papillary renal cell carcinomas than in clear cell kidney carcinomas,19,20 and CAIX expression also varies with subtype in ovarian21 and breast22–25 cancers.
CAIX catalyzes the reversible hydration of carbon dioxide to carbonic acid, and is upregulated in cancers to help maintain physiologic intracellular pH despite high glycolysis rates.26 While maintaining a physiologic intracellular pH, it acidifies the extracellular space.26 CAIX may promote a metastatic or invasive phenotype by reducing cell adhesion,27 increasing cell invasiveness28 and mobility and migration,27 inducing angiogenesis,27 and activating proteases.27
CAIX expression in normal tissues is generally substantially less than in tumors.7,8,12,13 Hypoxia leads to increased CAIX gene expression,29 transcription,30 and protein expression31,32 in several types of tumor cell lines, and low glucose and low bicarbonate both increase CAIX transcription and protein expression in hypoxic cells.30
In resected NSCLC, tumor oxygenation correlated negatively with CAIX IHC staining,33 although there has not been consistent correlation between tumor hypoxia and CAIX expression in vivo,32 and for at least some tumor types, there may be both hypoxia-driven and hypoxia-independent CAIX signaling pathways.34,35
In keeping with our findings, most (but not all36) other NSCLC studies assessing CAIX IHC11,13,37–39 or mRNA7,33,40 tumor expression or plasma levels37 have also reported an association of high expression with worse overall11,13,33,37,38,40 or disease-free7,13,33,39 survival, with the greatest negative impact on survival being noted in later stage NSCLCs and in squamous cell carcinomas.33 While we found the strongest correlation between mCAIX staining and TTR, others had previously noted perinuclear CAIX staining to be particularly important.11,38
High tumor cell CAIX expression has also been associated with worse prognosis in sarcomas,41,42 gliomas,28,43 neuroblastomas,44 papillary renal cell carcinomas,45 and carcinomas of the breast,23,24,46,47 head and neck,48 nasopharynx,49 ovary,21 cervix,50 and rectum,51 but CAIX expression did not correlate with outcome in meningiomas52 or in carcinomas of the pancreas53 or prostate,54 and was paradoxically associated with improved outcome in renal clear cell carcinomas.19,55,56 High CAIX levels in tumor stromal cells may also be associated with poor outcome.57,58
In our study, mCAIX expression correlated with tumor size, with markers of proliferation (including Ki67 and number of mitoses), with poor differentiation, and with necrosis (but not apoptosis), and we noted a trend (p=0.063) towards higher mCAIX expression in N1 vs N0 tumors. Other studies that included a variety of tumor types also noted a correlation of CAIX expression with tumor size,47,50 mitoses,44 Ki67,10,43,47 lack of differentiation,13,23,25,43,46,47,58 necrotic11,23,31,39,59 or perinecrotic areas,18,31,43,59,60 and higher stage,13,31,44 although some of these factors failed to correlate with CAIX expression in still other studies.9,20,21,46,50,55,61,62
CAIX and VEGF are both target genes of the transcription factor HIF-1α63,64 that is generally induced by hypoxia but that may also be induced by Src.64 While mCAIX expression in our patients did correlate with cytoplasmic expression of the hypoxia-inducible factor SHARP-2, it did not correlate with HIF-1α or VEGF nuclear expression, and paradoxically correlated inversely with HIF-1α and VEGF cytoplasmic expression. Results have been variable in other studies. In various tumor types, CAIX expression has correlated with HIF-1α expression in some studies,39,43,47,49,52,65 but not in others.61,65,66 HIF-1α may also lose its transcriptional ability (eg, through repression by p53) such that CAIX induction does not happen despite high HIF-1α expression.67 Furthermore, CAIX expression may correlate with HIF-1α expression in tumors in which the HIF-1α expression is perinecrotic, but not in tumors in which HIF-1α expression is diffuse throughout the tumor.60 Also, CAIX has a much longer half-life in tissues (about 38 hours30) than does HIF-1α, and HIF-1α expression will rapidly decrease in tumor areas that have low nutrient levels, while CAIX will persist due to its longer half-life.68 In addition, in the absence of hypoxia and HIF-1α, CAIX expression may be upregulated by high cell density via the PI3K pathway,69 and increased expression of CAIX in the absence of hypoxia may also occur with hypomethylation of the CAIX gene promoter.70
Similarly, some previous studies have found positive correlations between CAIX and VEGF expression,33,43,52,59 while others have not,34,50,71 and still others have found an inverse correlation between CAIX and VEGF expression,56 similar to what we found. Lack of a consistent correlation between CAIX and VEGF may be due in part to the fact that upon reoxygenation of tissues, VEGF mRNA declines rapidly, while CAIX mRNA expression persists for >72 hours.59
In addition to serving as a prognostic marker, CAIX could also potentially serve as a therapeutic target or as a predictive marker for efficacy of other therapies. Therapies targeting CAIX were effective in preclinical models,27 and some are in early stages of clinical investigation.72 Moreover, high CAIX expression in NSCLC may be associated with decreased efficacy of radiotherapy,9 and interference with CAIX strongly augments the efficacy of both radiotherapy and some chemotherapy agents in preclinical systems.28 Conversely, activity of targeted agents and cytokines may be augmented in renal cell carcinomas with high CAIX expression,73 and tumor uptake and efficacy of some chemotherapy agents that are weak acids could potentially be augmented by the acidic milieu promoted by CAIX.74
In summary, high CAIX expression is associated with poor prognosis across a wide range of tumor types, and we found that membrane CAIX expression in particular was associated with an increased risk of relapse in our stage I-II NSCLC patients. Based on these observations, it would be reasonable to assess mCAIX expression further as a prognostic factor in NSCLC, and given the range of studies that have found an association between CAIX expression and poor outcome in NSCLC, it would be reasonable in advanced NSCLC to assess efficacy of new investigational agents targeting CAIX.
Supplementary Material
Acknowledgments
Supported in part by Department of Defense grant # W81XWH-07-1-0306, UT-Lung SPORE P50CA070907 and Cancer Center Support Grant 5-P30 CA16672-32.
Footnotes
Presented in part at the 101st Annual Meeting of the American Association for Cancer Research, Washington, DC, April 17-21, 2010 (Abstract # 4648).
Disclosures: None of the authors have disclosures directly relevant to the contents of this manuscript
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 4.Stewart DJ, Kurzrock R. Fool's gold, lost treasures, and the randomized clinical trial. BMC Cancer. 2013;13:193. doi: 10.1186/1471-2407-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature medicine. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DJ, Behrens C, Roth J, Wistuba II. Exponential decay nonlinear regression analysis of patient survival curves: preliminary assessment in non-small cell lung cancer. Lung Cancer. 2011;71:217–23. doi: 10.1016/j.lungcan.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malentacchi F, Simi L, Nannelli C, et al. Alternative splicing variants of carbonic anhydrase IX in human non-small cell lung cancer. Lung Cancer. 2009;64:271–6. doi: 10.1016/j.lungcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Simi L, Venturini G, Malentacchi F, et al. Quantitative analysis of carbonic anhydrase IX mRNA in human non-small cell lung cancer. Lung Cancer. 2006;52:59–66. doi: 10.1016/j.lungcan.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Liu X, Guo W, Li X. Expression of carbonic anhydrase IX in NSCLC and its relationship with VEGF and Ki67 expression. Zhongguo Fei Ai Za Zhi. 2010;13:861–6. doi: 10.3779/j.issn.1009-3419.2010.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SJ, Rabbani ZN, Vollmer RT, et al. Carbonic anhydrase IX in early-stage non-small cell lung cancer. Clin Cancer Res. 2004;10:7925–33. doi: 10.1158/1078-0432.CCR-04-0636. [DOI] [PubMed] [Google Scholar]
- 11.Swinson DE, Jones JL, Richardson D, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol. 2003;21:473–82. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 12.Vermylen P, Roufosse C, Burny A, et al. Carbonic anhydrase IX antigen differentiates between preneoplastic malignant lesions in non-small cell lung carcinoma. Eur Respir J. 1999;14:806–11. doi: 10.1034/j.1399-3003.1999.14d14.x. [DOI] [PubMed] [Google Scholar]
- 13.Kon-no H, Ishii G, Nagai K, et al. Carbonic anhydrase IX expression is associated with tumor progression and a poor prognosis of lung adenocarcinoma. Lung Cancer. 2006;54:409–18. doi: 10.1016/j.lungcan.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 14.An Q, Liu Y, Huang J. Comparison of tumor suppressor gene deletion between squamous cell carcinoma and adenocarcinoma lung cancer in Chinese. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2001;23:470–2. [PubMed] [Google Scholar]
- 15.Ho WL, Chang JW, Tseng RC, et al. Loss of heterozygosity at loci of candidate tumor suppressor genes in microdissected primary non-small cell lung cancer. Cancer detection and prevention. 2002;26:343–9. doi: 10.1016/s0361-090x(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 16.Dmitriev AA, Kashuba VI, Haraldson K, et al. Genetic and epigenetic analysis of non-small cell lung cancer with NotI-microarrays. Epigenetics. 2012;7:502–13. doi: 10.4161/epi.19801. [DOI] [PubMed] [Google Scholar]
- 17.Pinato DJ, Ramachandran R, Toussi ST, et al. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. Br J Cancer. 2013;108:429–37. doi: 10.1038/bjc.2012.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–83. [PubMed] [Google Scholar]
- 19.Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int. 2007;100:556–60. doi: 10.1111/j.1464-410X.2007.07006.x. [DOI] [PubMed] [Google Scholar]
- 20.Takacova M, Bartosova M, Skvarkova L, et al. Carbonic anhydrase IX is a clinically significant tissue and serum biomarker associated with renal cell carcinoma. Oncology letters. 2013;5:191–7. doi: 10.3892/ol.2012.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choschzick M, Oosterwijk E, Muller V, et al. Overexpression of carbonic anhydrase IX (CAIX) is an independent unfavorable prognostic marker in endometrioid ovarian cancer. Virchows Arch. 2011;459:193–200. doi: 10.1007/s00428-011-1105-y. [DOI] [PubMed] [Google Scholar]
- 22.van der Groep P, Bouter A, Menko FH, van der Wall E, van Diest PJ. High frequency of HIF-1alpha overexpression in BRCA1 related breast cancer. Breast Cancer Res Treat. 2008;111:475–80. doi: 10.1007/s10549-007-9817-z. [DOI] [PubMed] [Google Scholar]
- 23.Chia SK, Wykoff CC, Watson PH, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–8. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 24.Kaya AO, Gunel N, Benekli M, et al. Hypoxia inducible factor-1 alpha and carbonic anhydrase IX overexpression are associated with poor survival in breast cancer patients. Journal of BUON : official journal of the Balkan Union of Oncology. 2012;17:663–8. [PubMed] [Google Scholar]
- 25.Choi J, Jung WH, Koo JS. Metabolism-related proteins are differentially expressed according to the molecular subtype of invasive breast cancer defined by surrogate immunohistochemistry. Pathobiology : journal of immunopathology, molecular and cellular biology. 2013;80:41–52. doi: 10.1159/000339513. [DOI] [PubMed] [Google Scholar]
- 26.Mees G, Vangestel C, Dierckx R, Pauwels P, Van Meerbeeck J, Van de Wiele C. Carbonic anhydrase IX expression correlates with FDG uptake by primary non-small cell lung cancer. Cancer Biother Radiopharm. 2010;25:149–54. doi: 10.1089/cbr.2009.0658. [DOI] [PubMed] [Google Scholar]
- 27.Gieling RG, Williams KJ. Carbonic anhydrase IX as a target for metastatic disease. Bioorganic & medicinal chemistry. 2013;21:1470–6. doi: 10.1016/j.bmc.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 28.Proescholdt MA, Merrill MJ, Stoerr EM, Lohmeier A, Pohl F, Brawanski A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol. 2012;14:1357–66. doi: 10.1093/neuonc/nos216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen BS, Hao J, Overgaard J, et al. Influence of oxygen concentration and pH on expression of hypoxia induced genes. Radiother Oncol. 2005;76:187–93. doi: 10.1016/j.radonc.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S. Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol. 2004;24:995–1004. [PubMed] [Google Scholar]
- 31.Beasley NJ, Wykoff CC, Watson PH, et al. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001;61:5262–7. [PubMed] [Google Scholar]
- 32.Mayer A, Hockel M, Vaupel P. Endogenous hypoxia markers: case not proven! Adv Exp Med Biol. 2008;614:127–36. doi: 10.1007/978-0-387-74911-2_15. [DOI] [PubMed] [Google Scholar]
- 33.Le QT, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–14. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 34.Goethals L, Debucquoy A, Perneel C, et al. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys. 2006;65:246–54. doi: 10.1016/j.ijrobp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ihnatko R, Kubes M, Takacova M, et al. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int J Oncol. 2006;29:1025–33. [PubMed] [Google Scholar]
- 36.Andersen S, Eilertsen M, Donnem T, et al. Diverging prognostic impacts of hypoxic markers according to NSCLC histology. Lung Cancer. 2011;72:294–302. doi: 10.1016/j.lungcan.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Ilie M, Mazure NM, Hofman V, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer. 2010;102:1627–35. doi: 10.1038/sj.bjc.6605690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swinson DE, Cox G, O'Byrne KJ. Coexpression of epidermal growth factor receptor with related factors is associated with a poor prognosis in non-small-cell lung cancer. Br J Cancer. 2004;91:1301–7. doi: 10.1038/sj.bjc.6602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SJ, Rabbani ZN, Dewhirst MW, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer. 2005;49:325–35. doi: 10.1016/j.lungcan.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Skrzypski M, Jassem E, Taron M, et al. Three-gene expression signature predicts survival in early-stage squamous cell carcinoma of the lung. Clin Cancer Res. 2008;14:4794–9. doi: 10.1158/1078-0432.CCR-08-0576. [DOI] [PubMed] [Google Scholar]
- 41.Boeuf S, Bovee JV, Lehner B, Hogendoorn PC, Richter W. Correlation of hypoxic signalling to histological grade and outcome in cartilage tumours. Histopathology. 2010;56:641–51. doi: 10.1111/j.1365-2559.2010.03528.x. [DOI] [PubMed] [Google Scholar]
- 42.Maseide K, Kandel RA, Bell RS, et al. Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin Cancer Res. 2004;10:4464–71. doi: 10.1158/1078-0432.CCR-03-0541. [DOI] [PubMed] [Google Scholar]
- 43.Korkolopoulou P, Perdiki M, Thymara I, et al. Expression of hypoxia-related tissue factors in astrocytic gliomas. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol. 2007;38:629–38. doi: 10.1016/j.humpath.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Dungwa JV, Hunt LP, Ramani P. Carbonic anhydrase IX up-regulation is associated with adverse clinicopathologic and biologic factors in neuroblastomas. Hum Pathol. 2012;43:1651–60. doi: 10.1016/j.humpath.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Klatte T, Pantuck AJ, Said JW, et al. Cytogenetic and molecular tumor profiling for type 1 and type 2 papillary renal cell carcinoma. Clin Cancer Res. 2009;15:1162–9. doi: 10.1158/1078-0432.CCR-08-1229. [DOI] [PubMed] [Google Scholar]
- 46.Trastour C, Benizri E, Ettore F, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer. 2007;120:1451–8. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 47.Brennan DJ, Jirstrom K, Kronblad A, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. 2006;12:6421–31. doi: 10.1158/1078-0432.CCR-06-0480. [DOI] [PubMed] [Google Scholar]
- 48.Eckert AW, Lautner MH, Schutze A, et al. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J Oral Pathol Med. 2010;39:313–7. doi: 10.1111/j.1600-0714.2009.00829.x. [DOI] [PubMed] [Google Scholar]
- 49.Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–604. [PubMed] [Google Scholar]
- 50.Liao SY, Darcy KM, Randall LM, et al. Prognostic relevance of carbonic anhydrase-IX in high-risk, early-stage cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:452–8. doi: 10.1016/j.ygyno.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korkeila E, Sundstrom J, Pyrhonen S, Syrjanen K. Main effects and interactions of carbonic anhydrase IX, hypoxia-inducible factor-1alpha, ezrin and glucose transporter-1 in multivariate analysis for disease outcome in rectal cancer. Anticancer research. 2012;32:3299–303. [PubMed] [Google Scholar]
- 52.Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery. 2012;71:146–56. doi: 10.1227/NEU.0b013e3182567886. [DOI] [PubMed] [Google Scholar]
- 53.Chang DT, Chapman CH, Norton JA, et al. Expression of p16(INK4A) but not hypoxia markers or poly adenosine diphosphate-ribose polymerase is associated with improved survival in patients with pancreatic adenocarcinoma. Cancer. 116:5179–87. doi: 10.1002/cncr.25481. [DOI] [PubMed] [Google Scholar]
- 54.Weber DC, Tille JC, Combescure C, et al. The prognostic value of expression of HIF1alpha, EGFR and VEGF-A, in localized prostate cancer for intermediate- and high-risk patients treated with radiation therapy with or without androgen deprivation therapy. Radiat Oncol. 2012;7:66. doi: 10.1186/1748-717X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bui MH, Visapaa H, Seligson D, et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–6. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 56.Phuoc NB, Ehara H, Gotoh T, et al. Prognostic value of the co-expression of carbonic anhydrase IX and vascular endothelial growth factor in patients with clear cell renal cell carcinoma. Oncol Rep. 2008;20:525–30. [PubMed] [Google Scholar]
- 57.Brockton NT, Klimowicz AC, Bose P, et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral oncology. 2012;48:615–22. doi: 10.1016/j.oraloncology.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Kwon JE, Jung WH, Koo JS. The expression of metabolism-related proteins in phyllodes tumors. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:115–24. doi: 10.1007/s13277-012-0518-9. [DOI] [PubMed] [Google Scholar]
- 59.Turner KJ, Crew JP, Wykoff CC, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276–82. doi: 10.1038/sj.bjc.6600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vleugel MM, Greijer AE, Shvarts A, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–7. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juhasz M, Chen J, Lendeckel U, et al. Expression of carbonic anhydrase IX in human pancreatic cancer. Aliment Pharmacol Ther. 2003;18:837–46. doi: 10.1046/j.1365-2036.2003.01738.x. [DOI] [PubMed] [Google Scholar]
- 62.Hiraoka N, Ino Y, Sekine S, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer. 2010;103:1057–65. doi: 10.1038/sj.bjc.6605854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furlan D, Sahnane N, Carnevali I, et al. Up-regulation of the hypoxia-inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum Pathol. 2008;39:1483–94. doi: 10.1016/j.humpath.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Takacova M, Holotnakova T, Barathova M, Pastorekova S, Kopacek J, Pastorek J. Src induces expression of carbonic anhydrase IX via hypoxia-inducible factor 1. Oncol Rep. 2010;23:869–74. [PubMed] [Google Scholar]
- 65.Klatte T, Seligson DB, Riggs SB, et al. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:7388–93. doi: 10.1158/1078-0432.CCR-07-0411. [DOI] [PubMed] [Google Scholar]
- 66.Kuijper A, van der Groep P, van der Wall E, van Diest PJ. Expression of hypoxia-inducible factor 1 alpha and its downstream targets in fibroepithelial tumors of the breast. Breast Cancer Res. 2005;7:R808–18. doi: 10.1186/bcr1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vleugel MM, Shvarts D, van der Wall E, van Diest PJ. p300 and p53 levels determine activation of HIF-1 downstream targets in invasive breast cancer. Hum Pathol. 2006;37:1085–92. doi: 10.1016/j.humpath.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Sobhanifar S, Aquino-Parsons C, Stanbridge EJ, Olive P. Reduced expression of hypoxia-inducible factor-1alpha in perinecrotic regions of solid tumors. Cancer Res. 2005;65:7259–66. doi: 10.1158/0008-5472.CAN-04-4480. [DOI] [PubMed] [Google Scholar]
- 69.Kopacek J, Barathova M, Dequiedt F, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta. 2005;1729:41–9. doi: 10.1016/j.bbaexp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura J, Kitajima Y, Kai K, et al. Expression of hypoxic marker CA IX is regulated by site-specific DNA methylation and is associated with the histology of gastric cancer. Am J Pathol. 2011;178:515–24. doi: 10.1016/j.ajpath.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jubb AM, Pham TQ, Hanby AM, et al. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol. 2004;57:504–12. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stillebroer AB, Boerman OC, Desar IM, et al. European urology. 2012. Phase 1 Radioimmunotherapy Study with Lutetium 177-labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. [DOI] [PubMed] [Google Scholar]
- 73.Muriel Lopez C, Esteban E, Astudillo A, et al. Predictive factors for response to treatment in patients with advanced renal cell carcinoma. Investigational new drugs. 2012;30:2443–9. doi: 10.1007/s10637-012-9836-4. [DOI] [PubMed] [Google Scholar]
- 74.Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Critical reviews in oncology/hematology. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.