Abstract
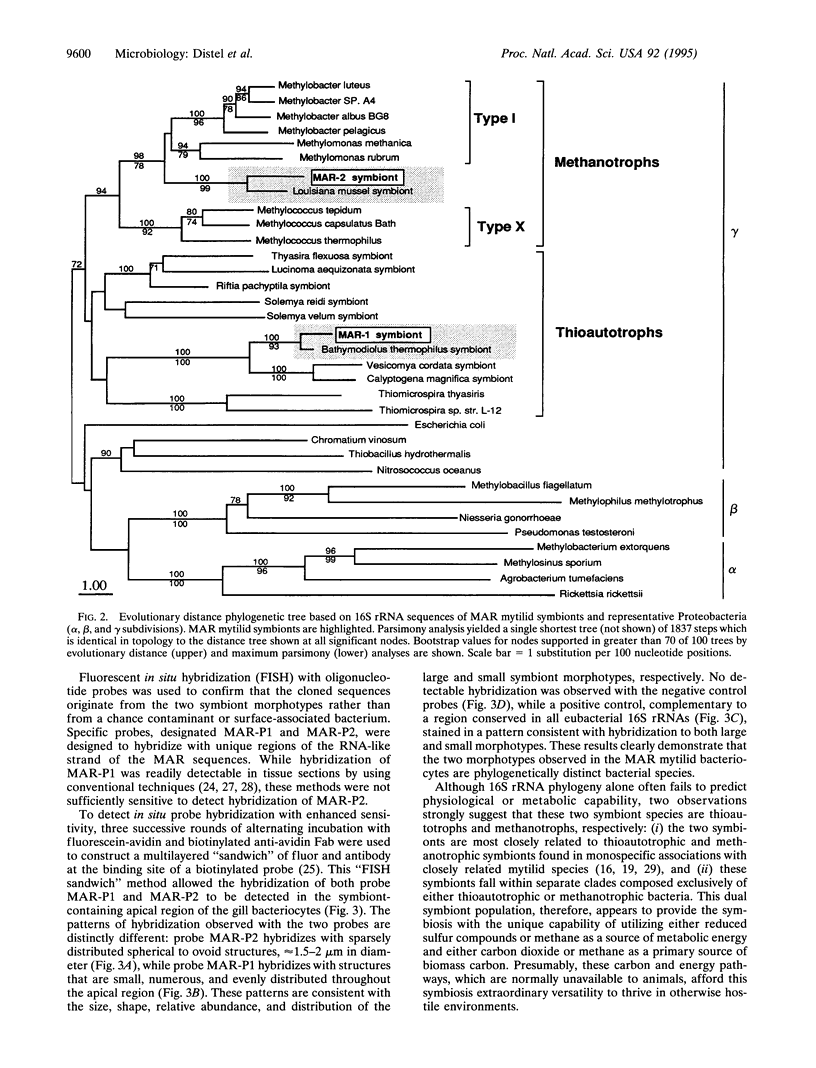
The coexistence of two phylogenetically distinct symbiont species within a single cell, a condition not previously known in any metazoan, is demonstrated in the gills of a Mid-Atlantic Ridge hydrothermal vent mussel (family Mytilidae). Large and small symbiont morphotypes within the gill bacteriocytes are shown to be separate bacterial species by molecular phylogenetic analysis and fluorescent in situ hybridization. The two symbiont species are affiliated with thioautotrophic and methanotrophic symbionts previously found in monospecific associations with closely related mytilids from deep-sea hydrothermal vents and hydrocarbon seeps.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavanaugh C. M., Wirsen C. O., Jannasch H. W. Evidence for methylotrophic symbionts in a hydrothermal vent mussel (bivalvia: mytilidae) from the mid-atlantic ridge. Appl Environ Microbiol. 1992 Dec;58(12):3799–3803. doi: 10.1128/aem.58.12.3799-3803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter-Mackie M. B. Single-base sequencing for rapid screening of plasmids for inserts with known mutations and correct orientation. Biotechniques. 1994 Jun;16(6):1026-8, 1030. [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Distel D. L., Cavanaugh C. M. Independent phylogenetic origins of methanotrophic and chemoautotrophic bacterial endosymbioses in marine bivalves. J Bacteriol. 1994 Apr;176(7):1932–1938. doi: 10.1128/jb.176.7.1932-1938.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel D. L., DeLong E. F., Waterbury J. B. Phylogenetic characterization and in situ localization of the bacterial symbiont of shipworms (Teredinidae: Bivalvia) by using 16S rRNA sequence analysis and oligodeoxynucleotide probe hybridization. Appl Environ Microbiol. 1991 Aug;57(8):2376–2382. doi: 10.1128/aem.57.8.2376-2382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel D. L., Lane D. J., Olsen G. J., Giovannoni S. J., Pace B., Pace N. R., Stahl D. A., Felbeck H. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16S rRNA sequences. J Bacteriol. 1988 Jun;170(6):2506–2510. doi: 10.1128/jb.170.6.2506-2510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J. A., Smith S. W., Cavanaugh C. M. Phylogenetic relationships of chemoautotrophic bacterial symbionts of Solemya velum say (Mollusca: Bivalvia) determined by 16S rRNA gene sequence analysis. J Bacteriol. 1992 May;174(10):3416–3421. doi: 10.1128/jb.174.10.3416-3421.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. The evolutionary origins of organelles. Trends Genet. 1989 Sep;5(9):294–299. doi: 10.1016/0168-9525(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Kim Y. W., Yasuda M., Yamagishi A., Oshima T., Ohta S. Characterization of the endosymbiont of a deep-sea bivalve, Calyptogena soyoae. Appl Environ Microbiol. 1995 Feb;61(2):823–827. doi: 10.1128/aem.61.2.823-827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Lim E. L., Amaral L. A., Caron D. A., DeLong E. F. Application of rRNA-based probes for observing marine nanoplanktonic protists. Appl Environ Microbiol. 1993 May;59(5):1647–1655. doi: 10.1128/aem.59.5.1647-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Maidak B. L., Larsen N., McCaughey M. J., Overbeek R., Olsen G. J., Fogel K., Blandy J., Woese C. R. The Ribosomal Database Project. Nucleic Acids Res. 1994 Sep;22(17):3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. W., Overbeek R., Woese C. R., Gilbert W., Gillevet P. M. The genetic data environment an expandable GUI for multiple sequence analysis. Comput Appl Biosci. 1994 Dec;10(6):671–675. doi: 10.1093/bioinformatics/10.6.671. [DOI] [PubMed] [Google Scholar]
- Unterman B. M., Baumann P., McLean D. L. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J Bacteriol. 1989 Jun;171(6):2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarda B., Amann R., Wallner G., Schleifer K. H. Identification of single bacterial cells using digoxigenin-labelled, rRNA-targeted oligonucleotides. J Gen Microbiol. 1991 Dec;137(12):2823–2830. doi: 10.1099/00221287-137-12-2823. [DOI] [PubMed] [Google Scholar]