Abstract
Purpose of review
Briefly summarize the epidemiologic findings of selected lifestyle factors for prostate cancer progression, metastasis, or death, with a focus on behaviors after diagnosis where possible. We conclude by providing guidance on lifestyle practices that physicians may wish to prioritize for discussion with their patients.
Recent findings
Growing, but still limited, evidence suggests that lifestyle factors after prostate cancer diagnosis may impact prostate cancer-specific and overall morality. In particular, smoking and obesity may increase risk of disease progression and mortality, while engaging in vigorous physical activity or brisk walking and consuming a diet rich in vegetables (particularly tomato sauce and cruciferous) and vegetable fats may lower risk.
Summary
Patients should be counseled not to use tobacco products; to engage in daily physical activity; to minimize sedentary behavior; to consume plenty of healthy fats (i.e. fish, nuts, vegetable oils, soybeans, avocados, flaxseed) and vegetables; to focus on getting nutrients from foods rather than supplements; and to limit refined grains, sugars, processed meat, and high-fat dairy.
Keywords: prostate cancer, diet, physical activity, survival
INTRODUCTION
More than 2.6 million men in the United States are prostate cancer survivors who may potentially improve their prognosis by adopting healthier lifestyle habits. 1 Our research group and others have evaluated whether lifestyle factors after diagnosis are associated with risks of recurrence or prostate cancer-specific mortality.
Given the heterogeneity of prostate cancer and the advent of prostate specific antigen (PSA) screening, it is increasingly important that epidemiologic research focus on the outcomes of aggressive prostate cancer, prostate cancer progression, or prostate cancer death, rather than simply overall prostate cancer incidence. Risk factors for total incident prostate cancer may differ from those for aggressive and fatal disease,2 and combining all incident prostate cancer cases can lead to incorrect conclusions. In this report, we briefly summarize the epidemiologic findings of selected lifestyle factors for prostate cancer progression, metastasis or death, with a focus on behaviors after diagnosis where possible. We conclude by providing guidance on lifestyle practices that physicians may wish to prioritize for discussion with their patients.
Smoking & Prostate Cancer
Data are highly suggestive that there is a greater risk of death from prostate cancer in smokers than in nonsmokers and that, among men who have prostate cancer, there is a greater risk of aggressive prostate cancer (e.g. worse stage or grade) in smokers than in nonsmokers.3 Furthermore, smokers have a higher risk of prostate cancer progression, independent of stage and grade.3
Several studies reported that smoking is associated with progression of the disease after diagnosis, including the development of biochemical recurrence, metastasis, and hormone refractory prostate cancer. With 22 years of follow-up and many outcomes in the Health Professionals Follow-up Study (HPFS; 524 prostate cancer-specific deaths and 878 biochemical recurrences), we reported that current smoking was associated with an approximate 60% increased risk of prostate cancer mortality and biochemical recurrence (hazard ratios (HR) 1.61, 95% confidence interval (CI) 1.11–2.32 and 1.61, 95% CI 1.16–1.22, respectively).4 Compared to current smokers, those who had quit smoking for ≥10 years, or who had quit for <10 years but smoked <20 pack-years, had prostate cancer-specific mortality risks similar to never smokers, while those who had quit for <10 years and had smoked ≥20 pack-years had risks similar to current smokers. Two smaller studies also reported a positive association between smoking and prostate cancer-specific mortality, based on few prostate cancer deaths.5,6 In summary, men with prostate cancer should be counseled to quit smoking and provided with referrals to obtain smoking cessation support.
Physical Activity & Prostate Cancer
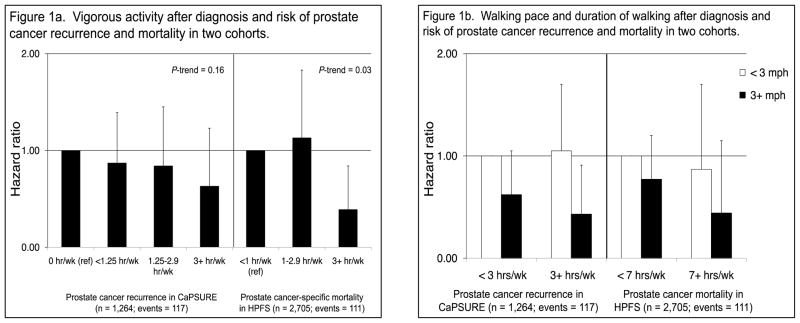
Aerobic exercise may reduce prostate cancer progression and prostate cancer-specific death by influencing energy metabolism, inflammation, oxidative stress, and androgen receptor signaling pathways. Accumulating evidence from prospective cohort studies suggests that physical activity, specifically vigorous activity (i.e. activities that require an energy expenditure ≥6 times the resting metabolic rate, such as jogging or bicycling), is associated with reduced risk of advanced, aggressive, and fatal prostate cancer. Our group was the first to report on physical activity after diagnosis in relation to prostate cancer-specific and total mortality in the HPFS.7 We observed that ≥3 hours/week of vigorous activity after diagnosis vs. <1 hour/week was significantly inversely associated with not only total mortality (which was anticipated), but also with an approximate 60% reduction in risk of prostate cancer-specific mortality (p-trend: 0.03; Figure 1a), independent of clinical, demographic, and other lifestyle factors. We observed similar results among 1,455 men in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE™) examining risk of prostate cancer progression, although the results were not statistically significant (Figure 1a). In CaPSURE, progression was primarily measured by biochemical recurrence or undergoing secondary treatment; these results were very compelling as there was less potential for reverse causation (men reducing their activity levels due to illness from disease progression) as physical symptoms of prostate cancer progression that may cause a decrease in physical activity are unlikely to precede biochemical recurrence.
FIGURE 1.
FIGURE 1a. Vigorous activity after diagnosis and risk of prostate cancer recurrence and mortality in two cohorts.
FIGURE 1b. Walking pace and duration of walking after diagnosis and risk of prostate cancer recurrence and mortality in two cohorts.
Abbreviations: CaPSURE, Cancer of the Prostate Strategic Urologic Research Endeavor; HPFS, Health Professionals Follow-up Study; miles per hour, mph. Error bars represent the upper bound of the 95% confidence intervals.
We also observed a benefit of brisk walking in both cohorts (Figure 1b), with the association reaching statistical significance for the outcome of progression in CaPSURE. While compelling and complementary, these studies warrant confirmation in distinct cohorts; and it remains to be elucidated what biological mechanisms underlie these findings. Together, these data suggest that an aspect of engaging in relatively vigorous physical activity and/or having higher cardiorespiratory fitness may protect against prostate cancer progression.
Body Size and Prostate Cancer
Increasing evidence suggests that obesity (either before or at the time of diagnosis) is strongly associated with prostate cancer progression and prostate cancer-specific mortality, independent of lifestyle or clinical factors. For example, among 2,546 men diagnosed with localized prostate cancer in the Physicians’ Health Study, a one-unit increase in pre-diagnostic body mass index (BMI) was associated with ≈10% increase in risk of prostate cancer-specific mortality, and BMI≥30 kg/m2 was associated with a nearly 2-fold increased risk of prostate cancer death (relative risk (RR) = 1.95; 95% CI:1.17, 3.23).8 A meta-analysis of six studies in prostate cancer patients reported that a 5 kg/m2 increase in BMI increased risk of prostate cancer-specific mortality by 20% and biochemical recurrence by 21%.9
Diet & Prostate Cancer
While some foods and nutrients assessed prior to diagnosis have been associated with advanced, metastatic, or fatal prostate cancer,10 few studies have examined post-diagnostic intake of foods and nutrients in relation to risk of prostate cancer recurrence and prostate cancer-specific mortality.
Fruit and Vegetables
In the first study to examine the relation of post-diagnosis diet with risk of progression using data from the HPFS, we reported a 20% lower risk of progression (mostly biochemical progression) per two servings/week increase of tomato sauce (Ptrend=0.04).11 In contrast, there was no association between post-diagnostic tomato sauce intake and risk of prostate cancer progression in CaPSURE.12 Studies are underway to clarify these findings for the more clinically relevant outcome of prostate cancer death.
A benefit with increased intake of cruciferous vegetables was reported in CaPSURE, where men in the highest vs. lowest quartile of post-diagnostic intake of cruciferous vegetables had a statistically significant 59% decreased risk of prostate cancer progression (Ptrend=0.003).13 There is evidence from in vitro and in vivo studies suggesting anti-carcinogenic effects of metabolites of cruciferous vegetables, including glucosinolates, isothiocyanates, and indoles, which supports the biologic plausibility of a benefit of consuming cruciferous vegetables for men with prostate cancer.14–17 However, additional research is needed from randomized controlled trials in humans to determine whether this association is causal.
Legumes
Meta-analyses of observational studies examining soy intake and incident prostate cancer have generally suggested an inverse association, although the data are predominantly driven by studies conducted in Asian populations where the intake of soy products is substantially higher than U.S. or European populations.18,19 A recent randomized masked clinical trial examining a soy protein supplement vs. placebo reported null results for the outcome of biochemical recurrence; this study was conducted among primarily Caucasian men at high risk for recurrence post-surgery.20
Fish
In the first study to examine the relation of post-diagnosis diet with risk of progression using data from the HPFS, we observed a 17% lower risk (Ptrend=0.006) of progression per two servings/week increase of fish intake.12 However, there was no association between fish intake after diagnosis and risk of prostate cancer progression in CaPSURE. 21 Studies are underway to clarify these findings for the more clinically relevant outcome of prostate cancer death.
Additionally, a few large prospective studies with long follow-up have reported on an inverse association (i.e., benefit) between fish consumption (before diagnosis of prostate cancer) and the subsequent risk of prostate cancer death.22,23 Furthermore, among men scheduled for surgery for prostate cancer, an intervention comprised of four to six weeks of a low-fat + fish oil supplemented diet was associated with lower prostate tumor proliferation biomarkers (i.e., cell cycle progression score and ki67, assessed in their radical prostatectomy specimens) compared to assignment to a Western diet.24,25
Red and Processed Meat
There is limited evidence to suggest that greater intake of meat (red and processed meat) after diagnosis may increase risk of prostate cancer progression or death. In HPFS and CaPSURE, processed red meat was positively associated with risk of prostate cancer death and recurrence (respectively), but neither association was statistically significant. Unprocessed red meat intake after diagnosis was not associated with risk of prostate cancer death or recurrence in the HPFS or CaPSURE, respectively.26 Nevertheless, given that cardiovascular disease is the number one cause of death among men diagnosed with localized prostate cancer, it is prudent to advise men to limit intake of processed red meat after diagnosis.
This is also supported by Strom et al, who reported a 2-fold increased risk of biochemical recurrence with greater saturated fat consumption among 390 men who underwent radical prostatectomy for organ-confined prostate cancer at diagnosis.27 Recently, we reported that replacing 10% of energy intake from animal fat with vegetable fat after diagnosis of non-metastatic prostate cancer was associated with a significant 34% lower risk of death from all-causes (p-value: <0.001). Moreover, replacing 10% of calories from carbohydrate with vegetable fat was associated with a 29% lower risk of lethal prostate cancer (Pvalue=0.04).28
Coffee
While data are limited and not entirely consistent, observational studies suggest a benefit for greater consumption of coffee and risks of all cause mortality, as well as advanced or lethal prostate cancer and prostate cancer recurrence (Note, coffee intake was assessed before diagnosis and studies have yet to specifically address the question of coffee intake after diagnosis).29–39
Multivitamins, Supplements, and Prostate Cancer
At this time, there is no strong evidence that any single supplement or multivitamin may offer protection again prostate cancer (incidence, progression, or death); and in fact caution is warranted in the usage of several supplements (e.g. selenium, vitamin E, beta-carotene). 40–45 For example, among ~4400 men initially diagnosed with localized prostate cancer, we observed more than 2.5-fold increased risk of prostate cancer death among men who reported taking ≥140 ug/day of supplemental selenium compared to those taking none (RR 2.60, 95% CI,1.44–4.70).46
Combined Healthy Lifestyle Practices
Though the data are limited and mainly focused on assessments of behaviors pre-diagnosis, research suggests that practicing multiple healthy lifestyle habits in combination may offer protection against developing lethal prostate cancer. Our group has reported that engaging in 5–6 healthy behaviors before diagnosis (i.e., not smoking or quitting smoking ≥10 y ago, having a BMI < 30 kg/m2, engaging in vigorous physical activity or brisk walking, and consuming greater amounts of tomatoes and dark (fatty) fish and less processed meat) was associated with nearly a 40% decreased risk of lethal prostate cancer compared to men who reported only one or none of these behaviors (HR: 0.61; 95% CI: 0.42–0.88, Ptrend=0.0009) in the HPFS. This finding was confirmed in a distinct population of men (Physicians Health Study).47 Additionally, data from a small pilot trial of 30 men with early stage, low grade prostate cancer on active surveillance who adopted comprehensive lifestyle changes (low-fat, whole-food, plant-based diet supplemented with soy, fish oil, vitamin E+selenium; moderate exercise, support group, meditation/stress reduction) indicated alterations in the tumor microenvironment and circulating telomerase levels that were suggestive of cancer protection (benefit), after three months.48,49 While provocative, this trial was small and did not have a randomized control group. Future randomized controlled trials in larger populations of men with prostate cancer are warranted to confirm these findings and directly address whether behavior change after diagnosis can improve survival outcomes.
CONCLUSION
Growing, but still limited, evidence suggests that lifestyle factors after prostate cancer diagnosis may impact prostate cancer-specific and overall morality. In particular, smoking and obesity may increase risk of disease progression and mortality, while engaging in vigorous physical activity or brisk walking and consuming a diet rich in vegetables (particularly tomato sauce and cruciferous) and vegetable fats may lower risk. Based on these findings and extensive data from cardiovascular disease research, we suggest the following recommendations for men with prostate cancer.
For Patients- Lifestyle Practices for Reducing Risk of Prostate Cancer Progression
Do not use tobacco products.
Engage in daily physical activity. If able, engage in a level of activity that raises your heart rate and breathing such that you can only speak a sentence at a time. Start with small increments and build up to 3 or more hours of activity per week. Bouts of activity lasting at least 10 minutes count toward your weekly goal.
Minimize sedentary behavior (e.g. watching TV, driving, using a computer). If you must sit for extended periods of time, take frequent short breaks to stand up and, ideally, walk around.
Consume plenty of healthy fats such as that in dark (fatty) fish (i.e. sablefish, salmon, trout, herring, sardines), nuts, vegetable oils (i.e. olive oil, canola oil), soybeans, avocados, flaxseed.
Focus on getting nutrients from your diet, rather than supplements.
Eat plentiful amounts of vegetables daily (target >= 5 servings or >=2.5 cups daily),50,51 including cruciferous vegetables and cooked tomatoes.
At each meal, try to have at least 2/3 of your plate comprised of vegetables, fruits, whole grains, or beans.50,51
Avoid processed and refined grains/flours/sugars. Keep WHITE off your plate - bread, pasta, rice, cereal, cream sauces, cakes, and more.
Limit processed meat and dairy, particularly high-fat dairy. Choose unprocessed lean protein sources such as fish and poultry without skin.
KEY POINTS.
Engaging in vigorous physical activity or brisk walking after diagnosis of prostate cancer may reduce risk of disease progression, prostate cancer-specific mortality, and all-cause mortality.
Substituting vegetable fats for animal fats and carbohydrates after prostate cancer diagnosis may reduce risk of all-cause mortality.
There is no strong evidence that any supplement is beneficial for men with prostate cancer; caution is warranted for many supplements (e.g. selenium, vitamin E, beta-carotene).
Footnotes
CONFLICTS OF INTEREST
None declared. The authors receive funding from the National Institutes of Health, Department of Defense, and Prostate Cancer Foundation.
References
- 1.Surveillance E, and End Results Program. SEER Stat Facts Sheets: Prostate Cancer. National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/prost.html. [Google Scholar]
- 2.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.General S. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Vol. 2014. Rockville, MD: Office of the Surgeon General; 2014. [Google Scholar]
- 4.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011 Jun 22;305(24):2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniell HW. A worse prognosis for smokers with prostate cancer. J Urol. 1995 Jul;154(1):153–157. [PubMed] [Google Scholar]
- 6.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008 Feb;19(1):25–31. doi: 10.1007/s10552-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 7.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011 Feb 20;29(6):726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008 Nov;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011 Apr;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005 Nov 10;23(32):8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 11.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Causes Control. 2006 Mar;17(2):199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 12.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer causes & control : CCC. 2006 Mar;17(2):199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 13*.Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012 Jul 1;131(1):201–210. doi: 10.1002/ijc.26348. This report, along with reference 28 highlights the evidence underlying our recommendations for consuming more vegetables (cruciferous in particular) and vegetable fats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr. 2008 May;47 (Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS, Chung FL, Richie JP, Jr, et al. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995 Dec;4(8):877–884. [PubMed] [Google Scholar]
- 16.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007 Mar;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shertzer HG, Senft AP. The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug Metabol Drug Interact. 2000;17(1–4):159–188. doi: 10.1515/dmdi.2000.17.1-4.159. [DOI] [PubMed] [Google Scholar]
- 18.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutrition and Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 19.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. The American journal of clinical nutrition. 2009 Apr;89(4):1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 20*.Bosland MC, Kato I, Zeleniuch-Jacquotte A, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA : the journal of the American Medical Association. 2013 Jul 10;310(2):170–178. doi: 10.1001/jama.2013.7842. This article, along with reference 45 provides, data from randomized clinical trials of nutritional supplements and multivitamins and report null results for prostate cancer specific outcomes. These in part underly the recommendation that no specific supplement should be recommended at this time for prostate cancer secondary prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. The American journal of clinical nutrition. 2010 Mar;91(3):712–721. doi: 10.3945/ajcn.2009.28474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008 Nov;88(5):1297–1303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham TM, Fujino Y, Kubo T, et al. Fish intake and the risk of fatal prostate cancer: findings from a cohort study in Japan. Public health nutrition. 2009 May;12(5):609–613. doi: 10.1017/S1368980008003182. [DOI] [PubMed] [Google Scholar]
- 24.Aronson WJ, Kobayashi N, Barnard RJ, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer prevention research. 2011 Dec;4(12):2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galet C, Gollapudi K, Stepanian S, et al. Effect of a low-fat fish oil diet on proinflammatory eicosanoids and cell-cycle progression score in men undergoing radical prostatectomy. Cancer prevention research. 2014 Jan;7(1):97–104. doi: 10.1158/1940-6207.CAPR-13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate specific antigen-era: incidence and survival. Cancer Prev Res (Phila) 2011 Sep 19; doi: 10.1158/1940-6207.CAPR-11-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strom SS, Yamamura Y, Forman MR, Pettaway CA, Barrera SL, DiGiovanni J. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer. 2008 Jun 1;122(11):2581–2585. doi: 10.1002/ijc.23414. [DOI] [PubMed] [Google Scholar]
- 28*.Richman EL, Kenfield SA, Chavarro JE, et al. Fat Intake After Diagnosis and Risk of Lethal Prostate Cancer and All-Cause Mortality. JAMA internal medicine. 2013 Jun 10;:1–8. doi: 10.1001/jamainternmed.2013.6536. This report, along with reference 13 highlights the evidence underlying our recommendations for consuming more vegetables (cruciferous in particular) and vegetable fats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Discacciati A, Orsini N, Andersson SO, et al. Coffee consumption and risk of localized, advanced and fatal prostate cancer: a population-based prospective study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013 Jul;24(7):1912–1918. doi: 10.1093/annonc/mdt105. This article, along with references 33 and 39, provides novel data regarding the emerging hypothesis that coffee consumption may offer benefits for overall mortality, as well as protect against prostate cancer-specific progression and mortality. [DOI] [PubMed] [Google Scholar]
- 30.Wilson KM, Balter K, Moller E, et al. Coffee and risk of prostate cancer incidence and mortality in the Cancer of the Prostate in Sweden Study. Cancer causes & control : CCC. 2013 Aug;24(8):1575–1581. doi: 10.1007/s10552-013-0234-9. [DOI] [PubMed] [Google Scholar]
- 31.Bosire C, Stampfer MJ, Subar AF, Wilson KM, Park Y, Sinha R. Coffee consumption and the risk of overall and fatal prostate cancer in the NIH-AARP Diet and Health Study. Cancer causes & control : CCC. 2013 Aug;24(8):1527–1534. doi: 10.1007/s10552-013-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z. Coffee consumption and risk of prostate cancer: a meta-analysis of prospective cohort studies. Carcinogenesis. 2014 Jan 13; doi: 10.1093/carcin/bgt482. [DOI] [PubMed] [Google Scholar]
- 33*.Discacciati A, Orsini N, Wolk A. Coffee consumption and risk of nonaggressive, aggressive and fatal prostate cancer--a dose-response meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013 Nov 24; doi: 10.1093/annonc/mdt420. This article, along with references 29 and 39, provides novel data regarding the emerging hypothesis that coffee consumption may offer benefits for overall mortality, as well as protect against prostate cancer-specific progression and mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geybels MS, Neuhouser ML, Stanford JL. Associations of tea and coffee consumption with prostate cancer risk. Cancer causes & control : CCC. 2013 May;24(5):941–948. doi: 10.1007/s10552-013-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geybels MS, Neuhouser ML, Wright JL, Stott-Miller M, Stanford JL. Coffee and tea consumption in relation to prostate cancer prognosis. Cancer causes & control : CCC. 2013 Nov;24(11):1947–1954. doi: 10.1007/s10552-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Kakizaki M, Sugawara Y, et al. Coffee consumption and the risk of prostate cancer: the Ohsaki Cohort Study. British Journal of Cancer. 2013 Jun 11;108(11):2381–2389. doi: 10.1038/bjc.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong S, Chen W, Yu X, Chen Z, Hu Q, Zhao J. Coffee consumption and risk of prostate cancer: an up-to-date meta-analysis. European journal of clinical nutrition. 2013 Dec 4; doi: 10.1038/ejcn.2013.256. [DOI] [PubMed] [Google Scholar]
- 38.Wilson KM, Kasperzyk JL, Rider JR, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. Journal of the National Cancer Institute. 2011 Jun 8;103(11):876–884. doi: 10.1093/jnci/djr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. The New England journal of medicine. 2012 May 17;366(20):1891–1904. doi: 10.1056/NEJMoa1112010. This article, along with references 29 and 33, provides novel data regarding the emerging hypothesis that coffee consumption may offer benefits for overall mortality, as well as protect against prostate cancer-specific progression and mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson KA, Wright ME, Subar A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. Journal of the National Cancer Institute. 2007 May 16;99(10):754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 41.Stevens VL, McCullough ML, Diver WR, et al. Use of multivitamins and prostate cancer mortality in a large cohort of US men. Cancer causes & control : CCC. 2005 Aug;16(6):643–650. doi: 10.1007/s10552-005-0384-5. [DOI] [PubMed] [Google Scholar]
- 42.Stratton J, Godwin M. The effect of supplemental vitamins and minerals on the development of prostate cancer: a systematic review and meta-analysis. Fam Pract. 2011 Jun;28(3):243–252. doi: 10.1093/fampra/cmq115. [DOI] [PubMed] [Google Scholar]
- 43.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA : the journal of the American Medical Association. 2011 Oct 12;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA : the journal of the American Medical Association. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA : the journal of the American Medical Association. 2012 Nov 14;308(18):1871–1880. doi: 10.1001/jama.2012.14641. This article, along with reference 20 provides, data from randomized clinical trials of nutritional supplements and multivitamins and report null results for prostate cancer specific outcomes. These in part underly the recommendation that no specific supplement should be recommended at this time for prostate cancer secondary prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenfield SA, DuPre N, Richman EL, Stampfer MJ, Giovannucci E, Chan JM. Selenium Supplementation and Lethal Prostate Cancer. Paper presented at: American Institute for Cancer Research; 2010; Washington DC. 2010. [Google Scholar]
- 47.Kenfield SA, Kasperzyk JL, Jahn JL, et al. American Association for Cancer Research, Advances in Prostate Cancer Research. San Diego, CA: AACR; 2014. Development and validation of a lifestyle score for prevention of lethal prostate cancer. [Google Scholar]
- 48.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008 Nov;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 49.Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8369–8374. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006 Sep-Oct;56(5):254–281. doi: 10.3322/canjclin.56.5.254. quiz 313–254. [DOI] [PubMed] [Google Scholar]
- 51.World Cancer Research Fund & American Institute for Cancer Research. Food, Nutrition, Physicial Activity, and the Prevention of Cancer: a Global Perspective. Washington D.C: American Institute for Cancer Research; 2007. [Google Scholar]