Abstract
Objective
To determine frequency, anatomic site, and host factors associated with asymptomatic shedding of herpes simplex virus after initial episodes of genital herpes.
Design
Cohort study with follow-up for a median of 63 weeks.
Setting
Referral clinic.
Patients
Women (306) with first episode of herpes; 43 had primary herpes simplex virus type 1, and 227 and 36 had primary and nonprimary herpes simplex virus type 2, respectively.
Measurements
Cultures were obtained for herpes simplex virus every 4 to 6 weeks at times in which genital lesions and symptoms were not present.
Main Results
Asymptomatic shedding was detected among 11.9%, 18.3%, and 22.9% of women with primary herpes simplex virus type 1, primary HSV type 2, and nonprimary HSV type 2, respectively. Among patients with type 2 infection, previous type 1 antibody was associated with a lower rate of asymptomatic vulvar shedding. Asymptomatic cervical shedding was 3 times more frequent during the first three months after resolution of primary type 2 disease than during later time periods. In contrast, the rate of symptomatic recurrent herpes did not change over time.
Conclusions
Asymptomatic genital herpes simplex type 2 is more common than type 1. Asymptomatic genital shedding occurs more often during the first 3 Months after acquisition of primary type 2 disease than during later periods. Patients with HSV type 2 should be advised of this high early rate of asymptomatic shedding and of potential transmission to sexual partners.
Contact with infectious virus shed asymptomatically into the genitourinaiy tract is an important factor in the transmission of HSV to sexual partners and neonates (1-6). Little is known of the natural history of asymptomatic shedding of HSV in the genitourinary tract: whether shedding patterns change over the course of infection and what host or virologic factors influence the frequency, anatomic site or sites, and time course of asymptomatic reactivation of disease. We have prospectively followed a cohort of women with culture and serologically proven first-episode genital herpes with sequential viral cultures and routine clinical observations (7-11). We report the data on asymptomatic reactivation of HSV among these women.
Methods
Study Group
We prospectively followed all women who presented to the University of Washington Viral Disease Research Clinic within 7 days of the onset of first-episode genital HSV. Patients were referred from private practitioners and from clinics at affiliated institutions. All patients followed for at least 60 days after the resolution of clinical first-episode genital herpes were enrolled in a study of the natural history of genital herpes. Between 1978 and 1988, 306 women with serologic and culture evidence of first-episode genital herpes were enrolled. These women represented 78% of those with first episodes of genital herpes referred and fully evaluated at the clinic during this period. Reasons for exclusion from this study were acute and convalescent sera that indicated that the patient had recurrent genital herpes (9%), inadequate sera or viral typing data to classify the patient (10%), or less than 60 days of follow-up after resolution of infection (3%). Of these 306 women, 175 (57%) were followed for at least 1 year from resolution of their first episode of genital herpes; This included 65% of the patients with primary type 1, 54% with primary type 2, and 69% with nonprimary type 2 genital herpes. The demographic characteristics of those who were followed for shorter periods (< 1 year) compared with longer (> 1 year) were similar. In addition, the duration, distribution of genital lesions, and severity of the first episode as determined by length of viral shedding and lesion healing, as well as rates of subsequent symptomatic recurrence, were similar in those who were followed for less than 1 year compared with those who remained in follow-up.
At enrollment, informed consent for prospective follow-up was obtained; a standardized interview and genital examination were done; cultures of the lesions, cervix, and vulva were taken, all lesions were diagrammed, and serum was obtained as previously described (8-11). During the initial episode of disease, patients were followed every other day until all lesions healed completely. After the initial episode was resolved, patients were asked to return to the clinic at 4- to 6-week intervals for routine visits and when symptomatic episodes recurred (8). At each visit an interim sexually transmitted disease and clinical history was obtained, genital and pelvic examinations were done, and cultures of the cervix and vulvar area were obtained as previously described (12). For symptomatic recurrences of genital lesions, dates of onset and healing of lesions were recorded. Of the 2572 symptomatic recurrences experienced by this group during follow-up, 1322 (51%) were observed in our clinic and 1250 (49%) were reported historically by the patient.
Asymptomatic shedding was defined as the isolation of HSV from an internal or external genital site at a visit at which neither the patient nor examining clinician noted symptoms or signs of genital herpes. Such visits are termed routine visits. An independent review of the charts was done for all routine visits at which HSV was isolated to ensure that no genital lesions were noted by either the patient or clinician. All episodes of asymptomatic shedding were confirmed by this independent review. Patient follow-up for this analysis was limited to the time before institution of suppressive acyclovir for those patients who received this therapy.
Antibody Status and Viral Typing at Enrollment
Enrollment and convalescent sera at 6 weeks after initial infection were assayed for antibodies to HSV type 1 and HSV type 2 by Western blot (13). Patients were classified as having primary genital herpes if they lacked antibodies to HSV type 1 and HSV type 2 in their acute-phase sera. Patients were classified as having nonprimary first-episode genital HSV type 2 if HSV type 1 antibodies were present in their acute-phase sera on Western blot. All such patients included in this report had HSV type 2 isolated from their genital lesions and serocon-verted to HSV type 2 in their convalescent-phase sera. All patients with primary HSV type 1 or HSV type 2 serocon-verted in convalescent-phase sera to the viral type isolated at the time of their first visit.
Statistical Analysis
Demographic characteristics were compared between groups using the chi-square test for categorical outcomes and the Wilcoxon rank-sum test for continuous data. For descriptive purposes, both the percentage of women in whom an asymptomatic culture was detected and the percentage of cultures in which HSV was detected are presented. The former percentage represents a minimum value for the percentage of patients who shed virus because the rate of detection of at least one positive culture increases with the number of cultures obtained (14). Because women were sampled more than once, a random effects model for logistic regression was employed, using the software package EGRET (15). This model accounts for the correlation between cultures taken from the same women and for the variable number of cultures among women. The model was used to evaluate differences in shedding rates between viral groups and to evaluate the relationship between time after resolution of the first episode and the subsequent frequency of asymptomatic shedding. Symptomatic recurrence rates for each woman were expressed as the average number of recurrences per month of follow-up.
Results
Patient Group
Of the 306 women with symptomatic first-episode genital herpes, primary HSV type 2 infection was the clinical and laboratory diagnosis in 227 (74%), primary genital HSV type 1 infection in 43 (14%), and first-episode nonprimary HSV type 2 in 36 (12%) (Table 1). The median age of women was 24 years, 91% were single, 92% were white, 55% used birth control pills, and 20% used barrier forms of contraception. There were no statistically significant differences among women with primary HSV type 2, primary HSV type 1, or nonprimary HSV type 2 in marital status, race, age of first intercourse, number of lifetime sexual partners, or past frequency of other sexually transmitted diseases. As expected, patients with HSV type 1 antibodies in their enrollment sera reported a higher frequency of previous oral-labial lesions than women who were seronegative for HSV. Of interest, 25 initially seronegative patients (1 with primary HSV type 1 and 24 with primary HSV type 2) reported a history of previous oral HSV, indicating either that the medical history was inaccurate for defining oral-labial HSV or that HSV type 1 antibodies were possibly lost over time. Overall, 32% of the women were enrolled into treatment trials of systemic antiviral chemotherapy for their initial episode of disease; 27% received oral acyclovir, and 5% received intravenous acyclovir for 10 and 5 days, respectively. Because previous studies have shown no effect of systemic acyclovir at the doses used in our patients on subsequent reactivation rates and because patients did not use antiviral chemotherapy during follow-up, these patients were included in our subsequent analyses (9, 11, 16-19).
Table 1. Demographic and Clinical Characteristics of the Initial Attack of Genital Herpes among Women Prospectively Followed for Symptomatic and Asymptomatic Recurrences.
| Characteristic | Primary Type 1 (n = 43) |
Primary Type 2 (n = 227) |
Nonprimary First-Episode Type 2 (n = 36) |
|---|---|---|---|
| Mean, age y (25% to 75% range) |
22 (20-27) |
24 (21-28) |
24 (21-27) |
| Single, % | 90 | 92 | 89 |
| White, % | 90 | 93 | 92 |
| History of oral herpes, % | 2 | 11 | 44 |
| Median duration of first-episode genital herpes, d (25% to 75%) |
18 (14-21.5) |
18 (14-25) |
15 (11-23) |
| Use of systemic acyclovir during first episode, % | 40 | 30 | 39 |
| Median follow-up, wk (25% to 75%) |
82 (32-193) |
59 (31-138) |
83 (43-112) |
| Median number of days routine cultures were obtained (25% to 75%) |
9 (5-18) |
9 (4-15) |
7.5 (5-14.5) |
| Women with asymptomatic shedding in the first year of follow-up, n(%) |
|||
| Any genital site | 4 (10) | 40 (18) | 8 (23) |
| Cervix | 2 (5) | 34 (16) | 6 (17) |
| Vulva | 2 (5) | 27 (3) | 2 (6) |
The median duration of follow-up after the first episode resolved was 63 weeks, and the median number of routine visits was nine (range, 0 to 124 visits). All but nine women (one with genital HSV type 1 and eight with primary genital HSV type 2) had at least one routine visit.
Association between Asymptomatic Reactivation, Viral Type, and Previous Herpes Simplex Virus Type 1 Antibody Status
Asymptomatic shedding of HSV from the genital tract was documented during follow-up on 90 (3.7%) of the 2401 routine follow-up visits (Table 2). Among the 297 women with at least one routine visit, asymptomatic shedding from the genitourinary tract during the first year after resolution of their first episode of genital herpes was documented in 10% of the women with genital HSV type 1, 18% of those with primary HSV type 2, and 23% of those with nonprimary first-episode genital herpes virus type 2 (see Table 1).
Table 2. Correlation between Viral Type and Previous Herpes Simplex Virus Type 1 Antibody Status and Subsequent Frequency of Asymptomatic Reactivation of Herpes Simplex Virus from the Genitourinary Tract after First-Episode Genital Herpes*.
| Asymptomatic Shedding | Primary Type 1 | Primary Type 2 | Nonprimary Initial Type 2 |
|---|---|---|---|
| n/N (%) | |||
| Days of asymptomatic cervical viral shedding† | 2/328 (0.6) | 54/1749 (3.1) | 6/228 (2.6) |
| Days of asymptomatic vulvar viral shedding‡ | 2/308 (0.6) | 36/1540 (2.3) | 2/236 (0.8) |
| Asymptomatic shedding at both cervix and vulva at same visit§ | 0/303 (0) | 16/1483 (1.1) | 1/218 (0.5) |
| Days with asymptomatic shedding from any genital site† | 4/335 (1.2) | 78/1820 (4.3) | 8/246 (3.3) |
Analyses confined to “routine” visits in the first year after acquisition of infection. HSV = herpes simplex virus.
P < 0.05 for comparison between primary HSV-2 and primary HSV-1 or nonprimary HSV-2 and primary HSV-1.
P < 0.05 for comparison between patients with primary HSV-2 and other patients with initial episodes of genital herpes.
Among days when both sites were cultured.
The overall rate of asymptomatic shedding from any site, as measured by the percentage of routine cultures that were positive, was significantly higher among women with both primary genital HSV type 2 infection (4.3%) and nonprimary initial genital HSV type 2 infection (3.3%) than among women with primary HSV type 1 infection (1.2%) (Table 2) (P < 0.05 using the random effects model). Women with primary and nonprimary HSV type 2 infection had significantly higher rates of Symptomatic viral shedding from the cervix (3.1% and 2.6%, respectively) than women with primary HSV type 1 (0.6%) (P < .05 for comparison between both primary and nonprimary HSV type 2 and primary HSV type 1). In addition, asymptomatic vulvar shedding was hgher among women with primary HSV type 2 infection (2.3%) than among those with primary genital HSV type 1 (0.6%) or nonprimaiy genital HSV type 2 (0.8%) (P < 0.05 for comparison between patients with primary HSV type 2 and both other groups). Asymptote shedding rates did not differ between women who Reived acyclovir therapy during their first episodes and those who did not. For example, among patients with primary HSV type 2 enrolled in our placebo-controlled trials of systemic acyclovir, asymptomatic shedding frequencies were 7.1% among patients treated with acyclovir compared with 6.1% among patients treated with placebo (P > 0.2).
High Rate of Asymptomatic Shedding in the First 3 Months after Primaiy Genital Herpes Simplex Type 2 Infection
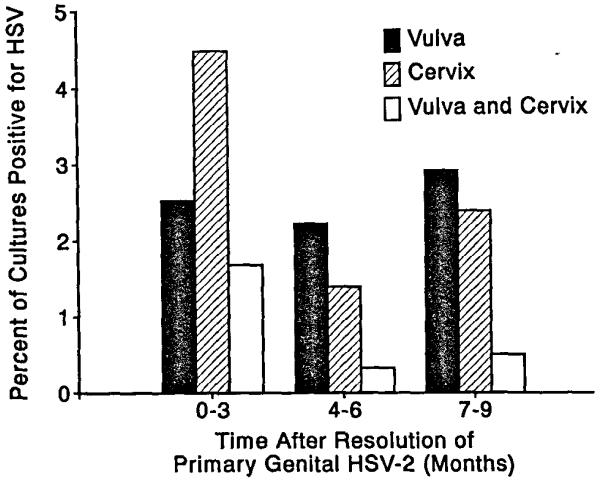
Asymptomatic shedding from ccrvical sites was detected more frequently in the time intervals soon after resolution of the initial episode in patient with primary genital herpes simplex type 2 infection (P < 0.005 using time as a covariate in the random effects model). For example, HSV was isolated from the cervix in 3.1% of the days sampled during the first year compared with 1.3% of days sampled in the subsequent years (Table 3). In the first 3 months after resolution of the primary episode, asymptomatic ccrvical shedding was dctccted in 4.4% of the days sampled compared with 1.4% during the second two quarters of the full year (Figure 1). Among women with at least one routine visit in the first 3 months after acquisition of primary HSV type 2, 17% shed virus at least once during this period compared to 7% during the 4 to 6 and 7 to 9 month periods, respectively. Among women with primary HSV type 2, the frequency of asymptomatic viral shedding from the vulvar area was 2.3% in the first year after acquisition compared with 1.5% during months 13 to 36. These latter differences did not, however, achieve statistical significance. When data from all genital sites were combined, shedding rates were significantly higher in the first year (4.3% of cultures) compared with subsequent periods (2.3 and 2.1% of cultures in the second and third years, respectively) (Table 3, P < 0.005).
Table 3. Rate of Asymptomatic Shedding Over Time among Women with Primary Simplex Virus Type 2 Infection.
| Site | Time from End of Primary Episode |
||
|---|---|---|---|
| 0 to 12 months |
13 to 24 months |
25 to 36 months |
|
|
|
|||
| n/N (%) | |||
| Cervical Site* | 54/1749 (3.1) | 6/464 (1.3) | 3/226(1.3) |
| Vulvar Site | 36/1540 (2.3) | 7/448 (1.6) | 3/224 (1.3) |
| Days with any genital site |
78/1820 (4.3) | 11/482 (2.3) | 5/234 (2.1) |
P ≤ 0.005; random effects model using time as a covariate.
Figure 1. Decreasing frequency of asymptomatic shedding of HSV type 2 after acquisition of primary genital HSV type 2.
Data represent total number of days in which viral cultures were taken during months 1 to 9 after resolution of genital herpes among the women with primary genital HSV type 2.
Fifteen women had 22 separate episodes of asymptomatic shedding of HSV from two anatomic sites on the same day (concurrent shedding). All 15 women had HSV type 2 infections; 14 of these 15 women had primary HSV type 2 infection. During the first year of follow-up, 11 women had 17 episodes of concurrent shedding; 15 of these 17 episodes occurred within the first 3 months after acquisition of infection. Two women had multiple episodes of concurrent shedding; one woman had five successive episodes of concurrent shedding of HSV type 2 within a month of resolution of her primary infection, whereas a second woman had two episodes in the week after resolution of her primary episode of infection.
Symptomatic Recurrences during Follow-up
Among the 175 women followed for at least 1 year, 18 of 28 (64%) of those with primary HSV type 1, 103 of 122 (84%) of those with primary HSV type 1, and 24 of 25 (96%) of those with nonprimary first-episode HSV type 2 subsequently developed an external genital recurrence during the first year of follow-up. The median recurrence rate among those with primary HSV type 1 was 0.06 recurrences compared with 0.29 and 0.34 among those with primary and nonprimary HSV type 2 (P < 0.01 for comparison of HSV type 1 and HSV type 2). Unlike asymptomatic shedding, the frequency of symptomatic recurrences remained constant in each of the three patient groups during each quarter of the first year of follow-up (median, 0 recurrences per month for primary type 1 and 0.33 recurrences per month for both primary and nonprimary type 2). To evaluate potential association between frequent symptomatic recurrences and frequent asymptomatic shedding, we compared the rates of shedding with recurrence rates for each patient effect of We were unable to detect any correlation between symptomatic recurrences and asymptomatic shedding.
Discussion
Our data show several unexpected differences in time course and host factors associated with asymptomatic versus symptomatic reactivation of HSV in the genital tract. The frequency of asymptomatic but not symptomatic reactivation after primary HSV type 2 infection decreased over the course of foliow-up. Among patients with primary genital herpes type 2, asymptomatic reactivation was observed more frequently in the first 3 months after acquisition than later in the disease course. Previous HSV type 1 infection appeared to decrease the frcqucncy of subsequent asymptomatic reactivation of HSV type 2 from the vulva, but had no discernible effect on the frequency of symptomatic recurrent genital HSV type 2 lesions.
Women with genital HSV type 1 infections were less likely to show subsequent asymptomatic shedding than women with genital HSV type 2 infections. We and others have shown that genital HSV type 1 infections are less likely to recur symptomatically than those with genital HSV type 2 infections (7, 16, 19). The data from this study, which represent the largest reported group of women with primary HSV type 1, confirm our earlier findings and extend the differences in reactivation rates between HSV type 1 and HSV type 2 to include asymptomatic reactivations of infection. Our findings suggest that the risk of exposure to HSV type 1 via sexual activity, whether from contact with genital lesions or unwitting contact with asymptomatic shedding, is less than that of HSV type 2 infection. As such, subtyping of HSV isolates from genital lesions of women with first-episode infections provides the clinician and patient with an important prognostic risk factor for predicting subsequent asymptomatic and symptomatic genital shedding (20, 21).
A major clinical finding of our analysis was the influence that time had on asymptomatic reactivation after primary HSV type 2. Asymptomatic reactivation was approximately two to three times more frequent in the first 3 months after resolution of primary first-episode type 2 infections than later in the time course. Among women with primary HSV type 2, 4.4% of days sampled during the 3 months immediately after acquisition of infection were associated with asymptomatic cervical reactivation of disease compared with 1.4% during subsequent periods. In contrast, the frequency of symptomatic reactivation was similar during the early compared with later time points of infection. It should be recognized, however, that asymptomatic and symptomatic reactivation occurred during all periods sampled. Although our current cohort exhibited only a few asymptomatic shedding events late after first-episode disease, the number of days sampled during later intervals were small, and our previous studies and those of other groups clearly indicate that asymptomatic reactivation of HSV in the genital region can occur in those who have longstanding infection of the genitourinary tract (14, 22-27).
Our data also suggest a potentially protective effects of type 1 antibody for asymptomatic vulvar but not cervical shedding of HSV type 2. The number of observed asymptomatic vulvar shedding events among women with diagnoses other than primary herpes simplex type 2 was low (two in each group), making it difficult to achieve adequate statistical power to define differences between groups. Further studies are necessary to define the effect of type 1 antibody on asymptomatic shedding of HSV type 2 from various sites.
We documented asymptomatic shedding in 17.6% of our patients during the first year after first-episode genital herpes. The incidence and prevalence rates of viral shedding and asymptomatic reactivation that we report here are likely to be minimal ones in that the frequency of asymptomatic viral shedding is a function of the number of days that patients are sampled. In a previous study, for example, asymptomatic shedding was detected in five of six patients sampled for more than 50 days and less frequently among patients sampled less intensively (14). Studies by Barton and colleagues (22), Stenzel-Poore and colleagues (24), and Straus and colleagues (23) reported asymptomatic shedding rates of 50% (4 of 8), 45% (5 of 11 women), and 12% (2 of 17) of patient samples, respectively. Further study is needed to determine whether all persons with genital HSV type 2 shed asymptomatically, especially in the first 3 months after acquisition.
Clinical Implications
Our study has several implications concerning the counseling and management of patients with genital herpes. Our data indicate that the risk for asymptomatic reactivation is greatest in the period shortly after resolution of primary infection. We have also previously shown that neonatal transmission rates at and shortly after first-episode infection appear to be higher than later in the disease course (28). Patients should be counseled concerning the potential high rate of transmission of genital HSV, especially HSV type 2 infection, during this period (1, 4, 5). Patient education programs to help patients identify symptomatic episodes of disease and to instruct patients about the potential high rate of asymptomatic reactivation shortly after resolution of first-episode genital herpes are needed. Our data provide a rationale for recommending the routine use of condoms to prevent transmission of disease during this high-risk period.
Acknowledgments
Grant Support: By National Institutes of Health grants AI-20381 and AI-30731. Dr. Koelle is supported by a training award from the American Social Health Association.
Footnotes
Current Author Addresses: Dr. Koelle: Virology Office, D536 Children’s Hospital CH-82, 4800 Sand Point Way North East, P.O. Box C-5371, Seattle, WA 98105.
Dr. Benedetti: Department of Biostatistics, ZH-15, University of Washington, Seattle, WA 98195
Dr. Langenberg: Kaiser Permanente, 280 West MacArthur Boulevard, Oakland, CA 94611
Dr. Corey: University of Washington, Virology Division, 1200 12th Avenue South, Room 9301, Seattle, WA 98144.
References
- 1.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, et al. Frequency of acquisition of first-episode genital infection with HSV from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985;12:33–9. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Stone KM, Brooks CA, Guinan ME, Alexander ER. National surveillance for neonatal HSV infections. Sex Transm Dis. 1989;16:152–6. doi: 10.1097/00007435-198907000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Whnlty RJ, Corey L, Arvin A, Lakeman FD, Sumaya CV, Wright PF, et al. Changing presentation of HSV infection in neonates. J Infect Dis. 1988;158:109–16. doi: 10.1093/infdis/158.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Mem GJ, Coombs RW, Ashley R, Jourden J, Remington M, Winter C, et al. Transmission of genital herpes in couples with one symptomatic and one asymptomatic partner: a prospeclive study. J Infect Dis. 1988;157:1169–77. doi: 10.1093/infdis/157.6.1169. [DOI] [PubMed] [Google Scholar]
- 5.Rooney JF, Felser JM, Ostrove JM, Straus SE. Acquisition of genital herpes from an asymptomatic sexual partner. N Engl J Med. 1986;314:1561–4. doi: 10.1056/NEJM198606123142407. [DOI] [PubMed] [Google Scholar]
- 6.Barton SE, Davis JM, Moss VW, Tyms AS, Munday PE. Asymptomatic shedding and subsequent transmission of genital HSV. Genitourin Med. 1987;63:102–5. doi: 10.1136/sti.63.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305:315–9. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- 8.Corey L, Adams HG, Brown ZA, Holmes KK. Genital HSV infections: clinical manifestaiions, course, and complicalions. Ann Intern Med. 1983;98:958–72. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 9.Mertz GJ, Critchfow CW, Benedetti J, Reichman RC, Dolin R, Connor J, et al. Double-blind placebo-controlled trial of oral acyclovir in first-episode HSV infection. JAMA. 1984;252:1147–51. [PubMed] [Google Scholar]
- 10.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital HSV infection. Influence of site of infection and viral type. N Engl J Med. 1987;316:1444–9. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- 11.Corey L, Fife KH, Benedetti JK, Winter CA, Fahnlander A, Connor JD, et al. Intravenous acyclovir for the treatment of primary genital herpes. Ann Intern Med. 1983;98:914–21. doi: 10.7326/0003-4819-98-6-914. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty WE, Kroffit S, Remington M, Giddings R, Winter C, Cent A, et al. Diagnosis of HSV by direct immunofluorescence and viral isolation from samples of external genital lesions in a high prevalence population. J Clin Microbiol. 1987;25:323–6. doi: 10.1128/jcm.25.2.323-326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-spccific immunodot enzyme assay for detecting antibodies to herpes simplex types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brock BV, Selke S, Benedetti J, Douglas JM, Jr, Corty L. Frequency of asymptomatic shedding of HSV in women with genital herpes. JAMA. 1990;263:418–21. [PubMed] [Google Scholar]
- 15.EGRET Reference Manual. Statistics and Epidemiology Research Corporation; Seattle, Washington: 1991. [Google Scholar]
- 16.Corey L, Mindel A, Fife KH, Sutherland S, Benedetti J, Adler MW. Risk of recurrence after treatment of lirsl-cpisode genital herpes with intravenous acyclovir. Sex Transm Dis. 1985;12:215–8. doi: 10.1097/00007435-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bryson VJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, et al. Treatment of first episodes of genital HSV infection wilh oral acyclovir. N Engl J Med. 1983;308:916–21. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- 18.Stone KM, Whittington WL. Treatment of genital herpes. Rev Infect Dis. 1990;12(Suppl 6):S610–9. doi: 10.1093/clinids/12.supplement_6.s610. [DOI] [PubMed] [Google Scholar]
- 19.Mindel A, Weller IV, Faherty A, Sutherland S, Flddian AC, Adler MW. Acyclovir in first attacks of genital herpes and prevention of recurrences. Genitourin Med. 1986;62:28–32. doi: 10.1136/sti.62.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson E, Schmidt OW, Goldstein LC, Nowinski RC, Corey L. Typing of clinical HSV isolates using mouse monoclonal antibodies to HSV types 1 and 2: comparison wilh type-specific rabbit anlisera and restriction endonuclease analysis of viral DNA. J Clin Microbiol. 1983;17:92–6. doi: 10.1128/jcm.17.1.92-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman TG, Roizman B, Adams G, Stover BH. Restriction endonuclease fingerprinting of HSV DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978;138:488–98. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- 22.Barton SE, Wright LK, Link CM, Munday PE. Screening lo dclecl asymptomatic shedding of herpes simplex virus (HSV) in women wilh recurrent genital HSV infection. Genitourin Med. 1986;62:181–5. doi: 10.1136/sti.62.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus SE, Seidlin M, Takiff HE, Rooney JF, Felser JM, Smith HA, et al. Effect of oral acyclovir treatment on symptomatic and asymptomatic virus shedding in recurrent genital herpes. Sex Transm Dis. 1989;16:107–13. doi: 10.1097/00007435-198904000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Stenzel-Poore MP, Hattick LM, Fendrick JL, Neuberg M, Storrs FJ, Hanifin JM. HSV shedding in genital secretions. Sex Transm Dis. 1987;14:17–22. doi: 10.1097/00007435-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Adam E, Kaufman RH, Mirkovic RR, Melnick JL. Persistence of virus shedding in asymptomatic women after recovery from genital herpes. Obstet Gynecol. 1979;54:171–3. [PubMed] [Google Scholar]
- 26.McCaughtty ML, Fleagle GS, Docherty JJ. Inapparent genital HSV infection in college women. J Med Virol. 1982;10:283–90. doi: 10.1002/jmv.1890100408. [DOI] [PubMed] [Google Scholar]
- 27.Guinan ME, MacCalman J, Kern ER, Overall JC, Jr, Spniance SL. The course of untreated recurrent genital herpes simplex infection in 27 women. N Engl J Med. 1981;304:759–63. doi: 10.1056/NEJM198103263041305. [DOI] [PubMed] [Google Scholar]
- 28.Brown ZA, Benedetti J, Ashley R, Burchett S, Selke S, Beny S, et al. Neonatal HSV infection in relalion to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–52. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]