Abstract
A three step synthesis of N-unsubstituted tetrazolo γ- and δ-lactams involving a key Ugi-4CR is presented. The compounds, otherwise difficult to access, are conveniently synthesized in overall good yields by our route. PDB analysis of the N-unsubstituted γ- and δ-lactam fragment reveals a strongly tri-directional hydrogen bond donor acceptor interaction with the amino acids of the binding sites.
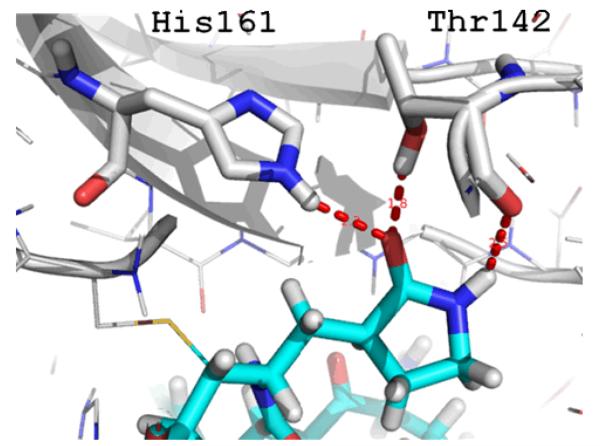
The γ-and δ-lactam moiety is an often found fragment in bioactive compounds; examples of compounds with a lactam fragment include the anti-Parkinson drug oxotremorin1 or the anti-rhinoviral and - enteroviral rupintrivir.2 Generally, the lactam nitrogen can be either unsubstituted or substituted which influences its hydrogen bonding profile in the receptor binding site. For example in the crystal structure of rupintrivir with the human rhinovirus 3C protease, the γ-lactam-N forms a short hydrogen bond to the Thr142 backbone carbonyl, whereas the δ-lactam-O forms two short contacts to side chain His161-NH and the Thr142-OH, respectively (Fig. 1).3 In our ongoing efforts in structure- and computational-based design of bioactive compounds we were interested in a short and versatile synthesis of N-unsubstituted γ- and δ-lactams with potential multiple hydrogen bond interactions.4 Multicomponent reactions (MCR) were found to be an excellent tool to rapidly access a large and versatile drug-like chemical space to address the corresponding biological space.5 A convenient and versatile synthesis of N-substituted γ- and δ-lactams using convergent Ugi-type MCR’s was recently introduced by Marcos et al. and refined by others.6-8 However no MCR-based synthesis of N-unsubstituted γ- and δ-lactams has been described up to date. Clearly many synthetic approaches towards N-unsubstituted γ- and δ-lactams are described, however these contain limitations regarding variability and length of the synthetic routes.9
Fig. 1.
Triforcated hydrogen bonding interactions (red dotted lines) of an N-unsubstituted γ-lactam (cyan sticks) with some protease amino acids in the example of rupintrivir (PDB ID 1CQQ).
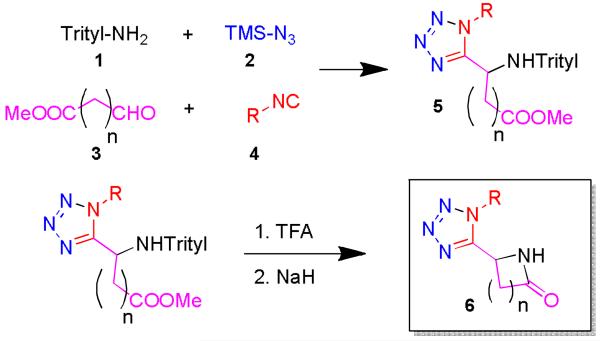
Ammonia is one of the few amine components which does not regularly give convincing results in the Ugi MCRs.10-13 Therefore we recently introduced tritylamine as an ammonia surrogate in the Ugi tetrazole MCR.14 This reaction forms the core of our design of a synthetic pathway towards N-unsubstituted γ- and δ-lactams (Scheme 1). The first step comprises the Ugi tetrazole MCR using tritylamine, followed by cleavage of the trityl group. The formed primary amine should easily undergo cyclisation to form the target γ- and δ-lactam compounds.
Scheme 1.
Devised synthetic pathway to tetrazolo N-unsubstituted γ- and δ-lactams
The Ugi tetrazole synthesis was initially performed under Ugi azide conditions with tritylamine (1), azidotrimethylsilane (2), phenylethylisonitrile and various aldehyde esters to check scope and limitations.15 The aliphatic aldehydes 3a-b were prepared according to previous described methods starting from the acid chloride (Scheme 2.).16
Scheme 2.
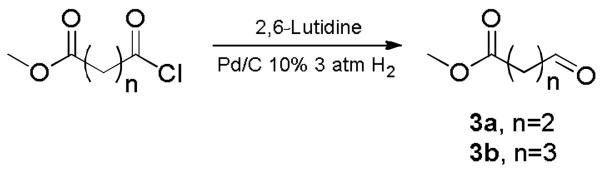
The two aldehydes utilized in the Ugi tetrazole synthesis.
Due to the steric hindrance of the trityl moiety, the Schiff-base condensation step of the Ugi reaction only proceeds smoothly under microwave irradiation.14 Moreover it was found that only aliphatic aldehydes such as 3a and 3b gave good yields varying between 40 and 80%. The used number of aldehydes was bigger with even n=4 and a cyclopropyl moiety in the chain. The Ugi tetrazole reaction was also for these aldehydes successful, however, the subsequent cyclisation gave problems, not yielding the desired lactams. Aromatic aldehydes were investigated and only in some reactions a moderate yield was observed.14 Aryl ester aldehydes did not yield any product at all.
Product diversity by using four other isonitriles (4) (Table 1.) showed yields comparable with phenylethylisocyanide. Deprotection conditions in order to cleave the trityl from the amine requires at least 2 equivalents of TFA. Full conversion into the amine is achieved within seconds monitored by TLC. Simple purification was performed by pouring the reaction mixture directly onto a 5 cm silica gel filter wetted with heptane:EtOAc (1:1) removing the impurities by washing with 50 mL heptane:EtOAc. Then the product was eluted from the filter using 50 mL CH2Cl2:MeOH (1:1) to obtain the pure amine. Subsequent cyclisation gave difficulties. Generally Et3N or potassium carbonate is sufficient to cyclize comparable molecules, however it was found that the resulting TFA salt needed to be liberated using a strong base prior to cyclisation. The main disadvantage of using a strong base is that this results in saponification of the initial ester and thus preventing cyclisation at all. Sodium hydride was found to be a suitable base giving in most cases only a minor conversion to amino acids and reasonable to very good yields for the γ- and δ-lactams (Table 1).
Table 1.
Cyclization of the Ugi 4-CR to yield the isoindolone scaffold.
|
|
||||
|---|---|---|---|---|---|
| entry | R(4) | n | Ugi (%) a | entry | cyclisation(%) b |
| 5a |
|
2 | 73 | 6a | 55 |
| 5b |
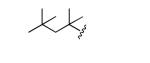
|
2 | 50 | 6b | 89 |
| 5c |
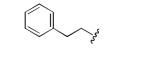
|
2 | 75 | 6c | 32 |
| 5d |
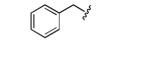
|
2 | 56 | 6d | 72 |
| 5e |
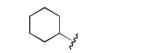
|
2 | 40 | 6e | 95 |
| 5f |
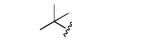
|
3 | 78 | 6f | 76 |
| 5g |
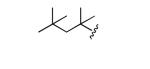
|
3 | 62 | 6g | 42 |
| 5h |
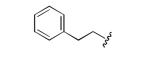
|
3 | 43 | 6h | 40 |
| 5i |
|
3 | 68 | 6i | 99 |
| 5j |
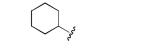
|
3 | 41 | 6j | 79 |
TFA, CH2Cl2, RT. 1 min.
NaH, THF, RT, 4h
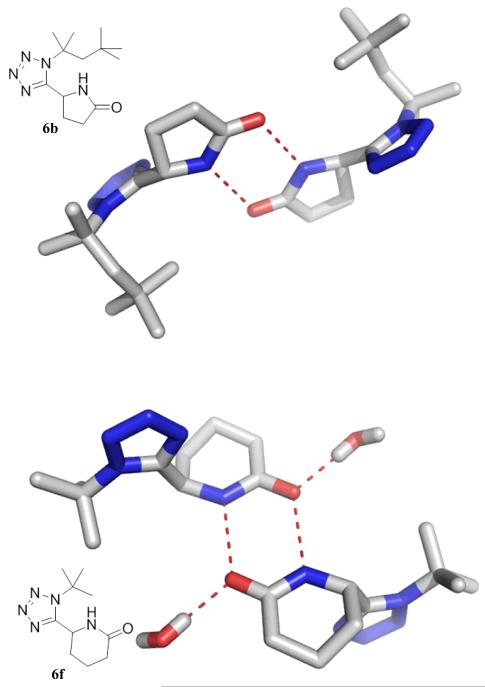
We could grow four crystals of γ- (6b, 6e) and δ-lactams (6f, 6j) suitable for single crystal structure determination (Fig. 2). Interestingly the lactam amide group in all cases show intermolecular hydrogen bonding involving the amide NH and the carbonyl CO. Five-membered γ-lactam 2 shows an intermolecular hydrogen bonding with a neighbouring γ-lactam amide with a distance between the heavy atoms of 2.8 Å. Six-membered δ-lactam 6, however, exhibits a trifurcated hydrogen bonding network involving a neighbouring δ-lactam and a co-crystallized water molecule. Thus the oxygen atom shows two hydrogen bonds along the lone pair electrons.
Fig. 2.
Intermolecular hydrogen bond network of exemplary γ- and δ-lactams in the solid state. Molecule 6a shows a pair wise hydrogen bonding with a neighbour molecule, while 6f shows a bifurcated hydrogen bonding pattern including a neighbour molecule and a water molecule.
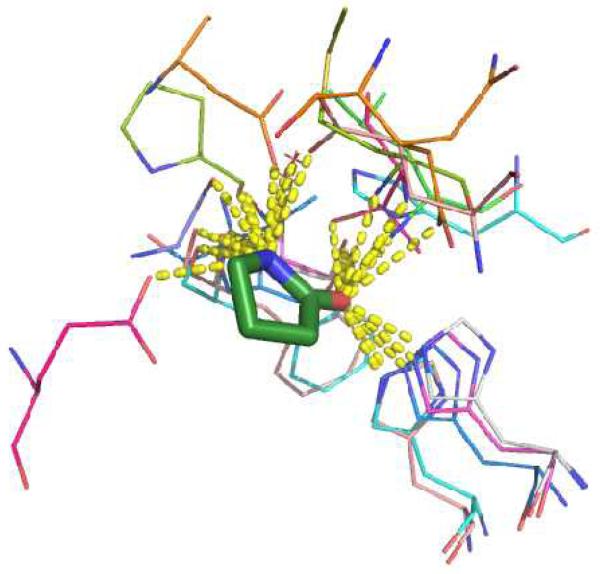
The γ- and δ-lactam moiety is present in many bioactive molecules and therefore we also decided to analyse the moieties in the protein data bank (PDB).17 Structure search (April 24th 2013) on γ-lactams yielded a total of 817 structures containing this moiety; δ-lactams resulted in 37 structure hits. After removing structures with ≥ 95% structure similarity, the results were reduced to 281 structures and 27 structures respectively. Of those structures, only the N-unsubstituted lactam rings were included in the analysis. In addition, all lactam structures that were part of the protein and photosynthesis related structures were also excluded due to redundancy of the ligands. Although 37 and 11 structures, respectively for the γ- and δ-lactams, are not sufficient for a statistical analysis, it well provides the basis for a trend. Key findings include: 1) for the majority of structures, the lactam amide group undergoes a triforcated hydrogen bond network involving the carbonyl oxygen twice and the amide NH once. 2) the γ-lactams interact with the histidine side chains most frequently through the carbonyl oxygen. Analysis of the structures show that the histidines are, all but one, part of viral proteases. In addition, the amide-nitrogen polar interactions are also similar for these structures. 3) the δ-lactams possess more hydrogen bonding interaction possibilities as many amino acid residues are found in close proximity to the lactam moiety. In figure 3, ten random structures (3D23, 3EWJ, 3QZR, 3RHK, 3TNT, 3UR9, 3DPM, 1H0V, 3JUC and 3Q3Y) are aligned showing the amino acid residues/molecules with which the γ-lactam moiety shows polar interactions.
Fig. 3.
Above: Alignment of several PDB structures showing the polar interactions for 10 γ-lactam containing ligands. The ligand γ-lactam moiety is shown as green sticks, the interacting receptor amino acids as colored lines and the hydrogen bonding as yellow dotted lines.
Conclusions
We have designed a fast and reliable synthetic route to 5- and 6-membered unsubstituted tetrazololactams using a key azido-Ugi reaction, followed by a deprotection and cyclisation step. Analysis of the scaffold in the protein data bank indicates that the lactam-NH can undergo multiple and strong H-bonds. Moreover the scaffold is underused in medicinal chemistry and thus provides multiple chances for drug design.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details in chemistry, NMR, SFC-MS of all described compounds and crystallographic data of 4 compounds; CCDC 961190 (6b), CCDC 961191 (6e), CCDC 961188 (6f) and CCDC 961189 (6j). See DOI: 10.1039/c000000x/
Notes and references
- 1.Tang C, Castoldi AF, Costa LG. Biochem. Mol. Biol. Int. 1993;29:1047. [PubMed] [Google Scholar]; (b) Zhang X, et al. Antiviral Red. 2013;97:264–269. doi: 10.1016/j.antiviral.2012.12.029. [DOI] [PubMed] [Google Scholar]; Chem. 2006;118:1–5. [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:1. [Google Scholar]
- 2.Zhang X, et al. World J. Gastroenterol. 2010;16:201. doi: 10.3748/wjg.v16.i2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews DA, et al. PNAS. 1999;96:11000. doi: 10.1073/pnas.96.20.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koes D, et al. PLoS ONE. 2012;7:e32839. doi: 10.1371/journal.pone.0032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dömling A. Chem. Rev. 2006;106:17. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 6.Marcos CF, Marcaccini S, Menchi G, Pepino R, Torroba T. Tetrahedron Lett. 2008;49:149. [Google Scholar]
- 7.Gunawan S, Petit J, Hulme C. ACS Comb. Sci. 2012;14:160–163. doi: 10.1021/co200209a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolyarenko VY, Evdokimov AA, Shishkin VI. Mendeleev Commun. 2013;23:108. [Google Scholar]
- 9.Ghosh AK, Leshchenko-Yashchuk S, Anderson DD, Baldridge A, Noetzel M, Miller B, Tie Y, Wang Y, Koh Y, Weber IT, Mitsuya HJ. Med. Chem. 2009;52:3902. doi: 10.1021/jm900303m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pick R, Bauer M, Kazmaier U, Hebach C. Synlett. 2005:0757. [Google Scholar]
- 11.Kazmaier U, Hebach C. Synlett. 2003:1591. [Google Scholar]
- 12.Sung K, Chen FL, Huang PC. Synlett. 2006:2667. [Google Scholar]
- 13.Thompson MJ, Chen BJ. Org. Chem. 2009;74:7084. doi: 10.1021/jo9014529. [DOI] [PubMed] [Google Scholar]
- 14.Zhao T, Boltjes A, Herdtweck E, Dömling A. Org. Lett. 2013;15:639. doi: 10.1021/ol303348m. [DOI] [PubMed] [Google Scholar]
- 15.Ugi I, Steinbruckner C. Angew. Ed. 1960;72:267. [Google Scholar]
- 16.Treilhoe M, Couderec FJ. Labelled. Cpd. Radiopharm. 2001;44:737. [Google Scholar]
- 17.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. Nucleic Acids Res. 2000;28:235. doi: 10.1093/nar/28.1.235. www.pdb.org. Citations here in the format Name A, Name B, Name C. Journal Title. 2000;35:3523. Name A, Name B, Name C. Journal Title. 2000;35:3523.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.