Abstract
The role of Panton-Valentine leukocidin (PVL) in Staphylococcus aureus infections is controversial. We used a mouse model of skin infection to compare the virulence of methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) strains with different levels of PVL production. Differences in PVL production were not associated with mutations in the genes lukS-PV and lukF-PV. However, MSSA and MRSA strains that produced high levels of PVL caused larger skin abscesses, higher bacterial burdens, and more tissue inflammation than did low-PVL-producing strains. Together, these data suggest that (1) the effect of PVL on the pathogenesis of staphylococcal infection may depend on the level of toxin produced and (2) many strains of MSSA that cause soft-tissue infections produce higher levels of PVL than do MRSA strains.
Community-acquired (CA) methicillin-resistant Staphylococcus aureus (MRSA) is an emerging pathogen with an evolving epidemiology. Several virulence factors contribute to the pathogenicity of S. aureus. One that may have particular importance is Panton-Valentine leukocidin (PVL), a pore-forming cytotoxin that results in leukocyte lysis. Although the direct role of PVL in the pathogenesis of CA-MRSA–mediated infections is unclear [1, 2], several studies have reported an association between PVL and skin and soft-tissue infections (SSTIs), necrotizing pneumonia, and osteomyelitis [3, 4]. PVL has not been commonly found in methicillin-susceptible S. aureus (MSSA) strains in the United States, and its role has not been comprehensively examined. Among SSTIs, PVL-positive MSSA strains have a wide range of prevalence (from 12% to 81% [4, 5]). A recent study suggested that the presence of PVL was associated with the need for surgical drainage [6, 7] in both MSSA- and MRSA-mediated SSTIs.
Despite the clinical evidence, conflicting views on the role of PVL in the pathogenesis of S. aureus infection remain prevalent. One study comparing the virulence of genetically modified strains [2] concluded that PVL is not required for the pathogenesis of S. aureus sepsis or skin abscess formation in murine models. In contrast, another study [8] demonstrated with different strains that PVL promotes staphylococcal lung infection in mice. Also, some studies have found that when PVL is injected intradermally into rabbits or mice, it induces severe inflammatory lesions, leading to capillary dilation and skin necrosis [9, 10]. This discrepancy among the results of different studies prompted us to further investigate the PVL-positive MRSA and MSSA strains that had been collected for a cohort study of patients with S. aureus–mediated SSTIs in the Bronx, New York. These strains were characterized with respect to PVL production, and a mouse model of SSTI was used to compare the virulence of the different strains. We conclude that the quantity of PVL production is highly variable and is associated with differences in virulence among both MSSA and MRSA strains. We propose that some of the discrepancy among the results regarding the contribution of PVL to virulence may be due to differences in PVL production. An unexpected finding was the high concentrations of PVL produced by MSSA strains, raising the concern that high producers may be emerging that could also cause pneumonia in influenza virus–infected patients [11].
Methods
S. aureus isolates collected in 3 hospitals in the Bronx, New York, were identified according to standard laboratory methods by the microbiology laboratory of Montefiore Medical Center. DNA was extracted from all S. aureus wound (n = 32) and blood (n = 4) isolates by using a Qiagen DNeasy Blood and Tissue kit. PVL genes were detected by polymerase chain reaction (PCR) with specific primers, as described else where [12]. Pulsed-field gel electrophoresis (PFGE) was performed on all PVL-producing strains, including 4 MRSA strains from blood isolates. Plugs were generated from S. aureus cultures that were incubated overnight and digested with SmaI prior to performing PFGE, as described elsewhere [4]. PFGE patterns were compared with published patterns and classified accordingly. To compare the genes lukS-PV and lukF-PV, they were amplified with the following primers: lukS-Pvl-F, 5′-AAA-GAAAGGAAATGATTTTTATGG-3′; lukS-Pvl-R, 5′-GTCCTT TCACTTTAATTTCATGAGT-3′; lukF-Pvl-F, 5′-AGTCAAATC ATCAGTTGTTACATCA-3′; lukF-Pvl-R, 5′-TCTATCTGTTTA GCTCATAGGATTTTT-3′. We compared the amplified genes with the published PVL sequence (GenBank accession no. DQ993352).
To quantify the production of PVL in vitro, S. aureus strains were grown in Mueller-Hinton II broth. The protein LukF-PV was quantified by enzyme-linked immunosorbent assay, as described elsewhere [1].
To simulate SSTI in our mouse model, the hair on the back of 6–8-week-old female BALB/c mice (National Cancer Institute) was shaved, and the skin was disinfected with ethanol. Single-punch biopsies were performed on their backs, resulting in 5-mm-diameter full-thickness excision wounds. A suspension containing 107 colony-forming units (CFUs) of high-PVL-producing (strains 2 and 7) or low-PVL-producing (strains 1, 4, and 8) S. aureus strains in phosphate-buffered saline (PBS) was inoculated directly onto the wound. Untreated mice were used as a control. Photographs of the wounds were taken daily to follow gross visual wound healing, as assessed by the area of the wound uncovered by the migrating epithelia. The area of each wound was measured in a vertical and horizontal fashion on days 3 and 7, using a caliper. Then, these measurements were averaged to get a representative size of each lesion. On day 7, the mice were euthanized, and wound sections were obtained for histological and CFU analysis. Wound sections were homogenized in PBS and plated onto tryptic soy agar. Excised skin lesion tissues were embedded in paraffin and stained with either hematoxylin-eosin, to observe the morphology, or Gram stain, to observe the bacteria. Animal experiments were approved by the Animal Care and Use Committee.
Prism software (version 5.0; GraphPad) was used for statistical analysis. P values were calculated by analysis of variance and were adjusted by use of the Bonferroni correction; differences for which P<.05 were considered significant.
Results
Molecular epidemiologic and experimental studies have yielded conflicting results regarding the role of PVL in the pathogenesis of CA-MRSA infection. PVL production by CA-MRSA strains is highly variable, which could contribute to these conflicting results [1]. In a recent cohort study, we determined that 21 of 60 MSSA strains and 31 of 43 MRSA strains that cause SSTIs carry the PVL gene (unpublished data). Four PVL-positive MRSA strains from blood, collected at the same time, were also included for further characterization.
PFGE and multilocus sequence typing identified most (>90%) of the strains as clone USA300 (patterns I and III) or the closely related clone USA400 (pattern V). Two other PFGE patterns (II and IV) were identified. Pattern II is a CC8 clone of spa type t1171 with a USA300-like PFGE pattern. All MRSA strains had antibiotic resistance patterns that were consistent with CA-MRSA. Ten distinct PFGE patterns were identified among PVL-positive MSSA strains, and none were similar to USA300, as described elsewhere [5]. Polyclonality among PVL-positive MSSA strains has been reported, which stands in contrast to the consistently reported homogeneity among PVL-positive MRSA strains [13]. The diverse genetic background of PVL-positive MSSA strains supports the notion that PVL is a strain-independent virulence determinant among these isolates.
Next, the production of LukF-PV was determined in these distinct MRSA and MSSA clones. All 15 S. aureus strains produced PVL, but their levels of toxin production varied considerably (Figure 1B), irrespective of growth rates (data not shown). Eight of 15 strains produced 405–809 ng/mL PVL; the remaining strains produced PVL in excess of 1000 ng/mL, which was considerably higher than previously reported. Four of 5 strains that produced >2000 ng/mL PVL were MSSA strains, and the high-PVL-producing MRSA strain (strain 2) exhibited a spa type (t1171) (Figure 1C).
Figure 1.
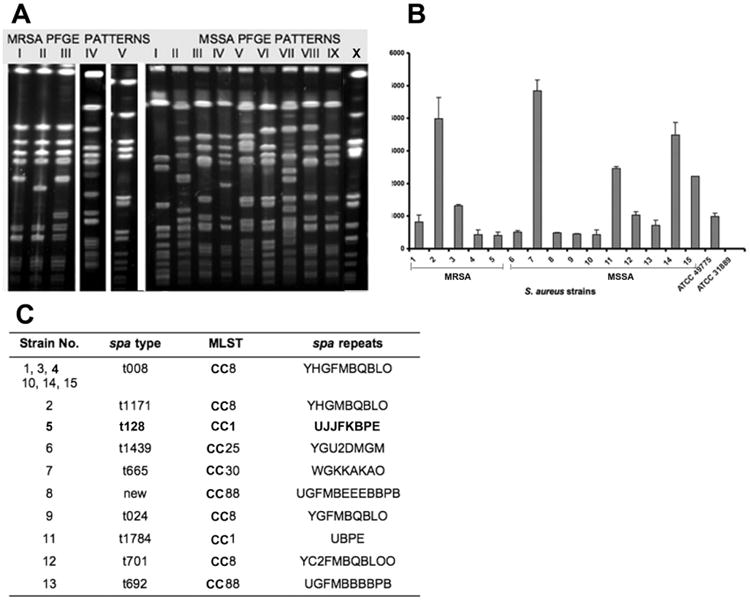
Characterization of Panton-Valentine leukocidin (PVL)–positive Staphylococcus aureus strains in the Bronx, New York. A, Pulsed-field gel electrophoresis patterns of PVL-positive methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) strains. Patterns I, III, and V represent USA300 and USA400 and constitute the majority of patterns found (>90%), whereas 10 different patterns were detected in PVL-positive MSSA strains. B, Varying levels of PVL toxin production by 5 MRSA clones and 10 MSSA clones. C, spa and multilocus sequence typing (MLST) of 5 MRSA and 10 MSSA clones. Only the high-PVL-producing MRSA clone (strain 2) has different spa and MLST types, but 8 different spa types were found in MSSA clones.
To determine whether differences in production were correlated with mutations in the PVL gene, the gene sequences of distinct MRSA and MSSA clones were compared with that of a standard MSSA strain (CP000255). In total, 4 mutations in lukS-PV and 2 in lukF-PV were detected, of which only 2 in lukS-PV (A at nucleotide positions 470 and 527) resulted in amino acid changes. In contrast to the results of PFGE pattern analysis, the sequences of lukS-PV and lukF-PV were identical in 4 MSSA clones and 4 MRSA clones, whereas variability was observed in the other MSSA and MRSA clones. MRSA and MSSA strains that produced high toxin levels did not exhibit specific mutations.
The effect of PVL production by S. aureus on virulence was investigated using a mouse model of skin infection (Figure 2). High-PVL-producing strains (2 and 7) significantly increased the mean size of the eschar (Figure 2B). On day 3, the mean size of the eschar in mice infected with high-PVL-producing strains reached ∼6.4 mm, whereas the mean size of the eschar in mice infected with low-PVL-producing strains was ∼5.25 mm for strain 8 (P < .05) and ∼4.6 mm for strains 1 and 4 (P < .001). For uninfected control mice, the mean size of the eschar on day 3 was ∼5.4 mm (P < .05). On day 7 (Figure 2B), the mean size of the eschar in mice infected with high-PVL-producing strains was ∼6 mm, whereas the mean size of the eschar in uninfected control mice and in mice infected with low-PVL-producing strains was ∼4.7 mm (P<.05) and ∼3.7 mm (P<.001), respectively.
Figure 2.
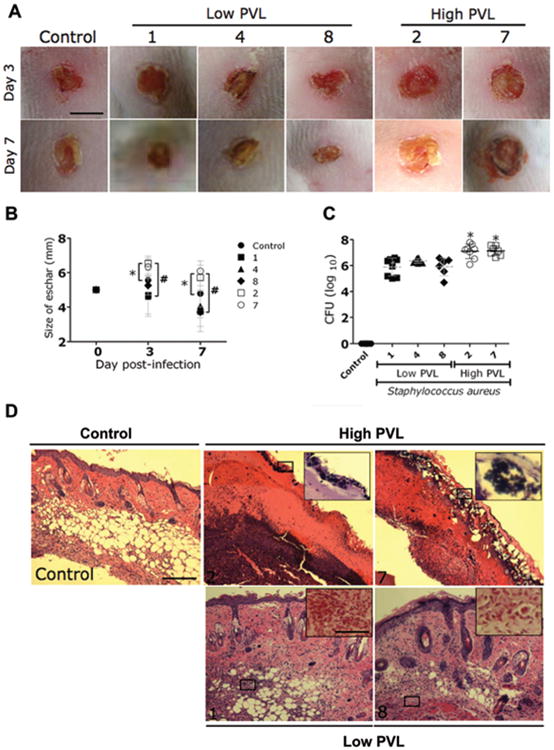
Comparison of virulence of Panton-Valentine leukocidin (PVL)–positive methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) strains with high and low PVL production in a murine skin and soft-tissue infection (SSTI) model. A, Wounds of BALB/ c mice uninfected (control) or infected with low- or high-PVL-producing strains of S. aureus on days 3 and 7. Scale bar, 5 mm. B, Analysis of wound size in the skin lesions of the mice. Data are the means of the results for 3 measurements at each time point, and error bars denote standard deviations. *P < .05; #P < .001. C, Wound bacterial burden in colony-forming units (CFUs). In mice infected intradermally with 107 CFUs of low-PVL-producing S. aureus strains, the wound bacterial burden was significantly lower than in mice infected with high-PVL-producing S. aureus strains. Uninfected mice were used as a control. There were 8 mice in the control group; 8 mice in each of the groups infected with strains 1, 2, and 4; 7 mice in the group infected with strain 7; and 6 mice in the group infected with strain 8. Dashed lines show the means of the results for 4 measurements, and error bars denote standard deviations. Asterisks denote statistical significance (P < .01), which was calculated by analysis of variance and adjusted by use of the Bonferroni correction. D, Histological analysis of uninfected mice (control) and mice infected with either high- or low-PVL-producing strains on day 7. Shown are representative hematoxylin-eosin–stained sections of the skin lesions, which demonstrate increased inflammation in SSTIs caused by high-PVL-producing S. aureus strains compared with low-PVL-producing strains. The insets show Gram staining of the sections shown in the black rectangles. Few or no S. aureus cells (purple) are seen in the SSTIs caused by low-PVL-producing S. aureus strains. Hematoxylineosin staining scale bar, 25 μm; Gram staining scale bar, 10 μm.
Mice infected with strains producing high levels of PVL had significantly higher microbial burdens than did uninfected control mice or mice infected with strains producing low levels of PVL (P< .01) (Figure 2C). In this assay, <30 CFUs were detected in the skin of uninfected mice, and S. aureus was not identified. Histological examinations revealed that the wounds of mice infected with high-PVL-producing strains had extensive neutrophil infiltration, along with extensive cell necrosis (Figure 2D). Gram staining of these tissue samples revealed large numbers of gram-positive cocci (Figure 2D). Tissue sections from the wounds of mice infected with low-PVL-producing strains showed minimal inflammation and no evidence of bacteria (Figure 2D). Notably, all strains had comparable doubling times (data not shown) at 37°C in vitro.
Discussion
Our results demonstrate significant variations in PVL production among clinical S. aureus strains from a small geographical area. Said-Salim [14] et al showed differential distribution and expression of PVL toxin in CA-MRSA strains by means of real-time PCR. Hamilton et al [1] also found marked variation in the toxin quantities produced by PVL-positive MRSA strains; however, that study did not include MSSA strains, and the MRSA strains, most of which were USA300, were collected in different institutions and were derived from a broad range of staphylococcal infections, including SSTIs, severe pneumonia, and necrotizing fasciitis. A correlation between severity of infection and PVL toxin production could not be established for those strains. In the study by Hamilton et al, the highest PVL production among the MRSA strains was 800 ng/mL, whereas among our collection 1 MRSA strain produced 2000 ng/mL and 1 MSSA strain produced 4800 ng/mL. Notably, in vitro studies with recombinant PVL have demonstrated a concentration-dependent effect leading to either apoptosis or necrosis in neutrophils exposed to different PVL concentrations [15]. Our findings contrast with those of Wardendurg et al [16], who found that wound size and bacterial burden were not increased in the presence of PVL genes in a clinical USA300 strain; however, the production of PVL by this strain was not quantified. Changes in PVL production may be the result of changes in promoter sequences, regulators, or other factors that affect protein levels. The expression of PVL may change during prolonged infection and in the setting of antibiotic treatment. However, our data suggest that there is a correlation between in vitro and in vivo expression. This would best be validated by experiments with mutants that have inducible PVL expression.
We propose that some of the discrepancy regarding the contribution of PVL to virulence may be the result of differences in toxin expression between the individual strains. Increasing evidence suggests that the PVL gene and staphylococcal cassette chromosome mec type IV are transmitted independently by lysogenic phages [10], and it is not clear whether certain strains are more prone to infection. It is conceivable that infection with this phage has occurred for a longer time among MSSA strains and will eventually spread to more MRSA clones or that more MSSA strains will acquire resistance to methicillin. In our study, the PVL sequences were identical between the 4 USA300 and USA400 strains, as well as between some MSSA clones. Specific clones did not correlate with quantity of PVL production. Similar gene variations were found in recent studies [17, 18], but these variations have not been linked to PVL protein levels.
In summary, our data document significant differences in virulence between high- and low-PVL-producing MSSA and MRSA strains. This observation warrants closer monitoring and further investigation. Future studies should include analysis of PVL production in large numbers of S. aureus strains to determine whether high-PVL-producing strains constitute an independent risk factor, as is the case with Clostridium difficile. Given the emergence of CA-MRSA–related pneumonia in influenza virus–infected patients, these results could be highly relevant if new influenza virus strains emerge [11].
Acknowledgments
We acknowledge many other relevant publications that could not be cited because of the reference limit. We thank Dr Barry M. Kreiswirth and Jose R. Mediavilla for spa typing, and we thank Dr Xiabo Wang, Natalie Robiou, and Dr Mircea Radu Mihu for technical help. We also thank Dr Marilou Corpuz for assistance with obtaining permission from the Institutional Review Board.
Financial support: Northeast Biodefense Center (grant U54-AI057158-Lipkin); Office of Research and Development, Medical Research Service, US Department of Veterans Affairs.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Hamilton SM, Bryant AE, Carroll KC, et al. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin Infect Dis. 2007;45:1550–8. doi: 10.1086/523581. [DOI] [PubMed] [Google Scholar]
- 2.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 3.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 4.Lowy FD, Aiello AE, Bhat M, et al. Staphylococcus aureus colonization and infection in New York State prisons. J Infect Dis. 2007;196:911–8. doi: 10.1086/520933. [DOI] [PubMed] [Google Scholar]
- 5.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 6.Daskalaki M, Rojo P, Marin-Ferrer M, Barrios M, Otero JR, Chaves F. Panton-Valentine leukocidin-positive Staphylococcus aureus skin and soft tissue infections among children in an emergency department in Madrid, Spain. Clin Microbiol Infect. 2009 Jun 6; doi: 10.1111/j.1469-0691.2009.02830.x. electronically published ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Issartel B, Tristan A, Lechevallier S, et al. Frequent carriage of Panton-Valentine leucocidin genes by Staphylococcus aureus isolates from surgically drained abscesses. J Clin Microbiol. 2005;43:3203–7. doi: 10.1128/JCM.43.7.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–3. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 9.Brown EL, Dumitrescu O, Thomas D, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–64. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RK, George R, Nguyen-Van-Tam JS. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14:1187–92. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko J, Muramoto K, Kamio Y. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with lukM. Biosci Biotechnol Biochem. 1997;61:541–4. doi: 10.1271/bbb.61.541. [DOI] [PubMed] [Google Scholar]
- 13.Monecke S, Slickers P, Ellington MJ, Kearns AM, Ehricht R. High diversity of Panton-Valentine leukocidin-positive, methicillin-susceptible isolates of Staphylococcus aureus and implications for the evolution of community-associated methicillin-resistant S. aureus. Clin Microbiol Infect. 2007;13:1157–64. doi: 10.1111/j.1469-0691.2007.01833.x. [DOI] [PubMed] [Google Scholar]
- 14.Said-Salim B, Mathema B, Braughton K, et al. Differential distribution and expression of Panton-Valentine leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 2005;43:3373–9. doi: 10.1128/JCM.43.7.3373-3379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genestier AL, Michallet MC, Prevost G, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Baxindependent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–27. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Hara FP, Guex N, Word JM, et al. A geographic variant of the Staphylococcus aureus Panton-Valentine leukocidin toxin and the origin of community-associated methicillin-resistant S. aureus USA300. J Infect Dis. 2008;197:187–94. doi: 10.1086/524684. [DOI] [PubMed] [Google Scholar]
- 18.Wolter DJ, Tenover FC, Goering RV. Allelic variation in genes encoding Panton-Valentine leukocidin from community-associated Staphylococcus aureus. Clin Microbiol Infect. 2007;13:827–30. doi: 10.1111/j.1469-0691.2007.01763.x. [DOI] [PubMed] [Google Scholar]


