Abstract
HNO binds with many different metals in organometallic and bioinorganic chemistry. To help understand experimentally observed metal centre effects, a quantum chemical investigation was performed, revealing clear general binding trends with respect to metal centre characteristics and the electronic origin for the first time.
HNO is an interesting molecule in chemistry and plays significant roles in many biological processes, such as vascular relaxation, enzyme activity regulation, and neurological function regulation.1–4 A number of the HNO biological interactions occur with metalloproteins, such as heme proteins,1–3, 5–8 Cu,Zn-SOD,9 and manganese quercetin dioxygenase.10 HNO has also been investigated in organometallic chemistry for over 40 years. A number of stable HNO metal complexes have been characterized with spectroscopic tools: OsCl2(CO)(HNO)(PPh3)2,11, 12 IrHCl2(HNO)(PPh3)2,11, 13 ReCl(CO)2(HNO)(PPh3)2,14 ReCl(CO)2(HNO)(PCy3)2,14 [Re(CO)3(HNO)(PPh3)2]+,15, 16 Ru(HNO)(‘pybuS4’),17 [OsBr(CO)2(HNO)(PPh3)2]+,16 RuHCl(CO)(HNO)(PiPr3)2,18, 19 OsHCl(CO)(HNO)(PiPr3)2,18, 19 Ru(TTP)(HNO)(1-MeIm),20 and [FeII(CN)5(HNO)]3−.21
Clearly, HNO can bind stably with many different metals. However, except for the most recent pentacyanoferrate HNO complex,21 all other HNO organometallic compounds are with late transition metals, suggesting that the metal centre may play a role in HNO binding. Given the broad tremendous interest in heme systems, it is interesting to note that while a Ru porphyrin complex with HNO was reported many years ago,20 only very recently the iron porphyrin complex of the conjugate base of HNO (i.e. NO−) was reported with the assistance of a specially designed porphyrin,22 and there is still no report of successful synthesis of iron porphyrin HNO complex. In contrast, most reported HNO metalloprotein systems are with Fe1–3, 5–8 and the protein environment was found to help stabilize the HNO binding in myoglobin (Mb).23 These results suggest that the early transition metal may bind with HNO weaker than the late transition metal, and thus need the aid of a special ligand or protein environment. Previous studies also indicate that, Mb can contain different metal centres, such as Mn,24 Co,25 Ru,26 and Zn,27 which can significantly modulate its electronic properties.
However, there are no prior experimental or computational studies evaluating metal centre effects systematically to offer general HNO binding trends and the electronic origin of such trends, which can facilitate numerous organometallic and bioinorganic investigations, given the broad interest of HNO.1–4 Here, we present the first report to help understand the electronic origin of metal centre effects on HNO binding and their influences on structures, stabilities, electronic properties, and spectroscopic properties. To facilitate a systematic comparison of metals in different rows and periods in the periodic table, a common porphyrin platform was employed as M(Por)(5-MeIm)(HNO), where M, Por, and 5-MeIm stand for metal, porphyrin, and 5-methylimidazole (a Cβ truncated histidine residue, the heme axial ligand in Mb), respectively. Although HNO was found to react with metals of several different electronic configurations, the reported stable HNO metal complexes and heme proteins with spectroscopic characterizations are with the diamagnetic d6 configuration.11–21, 28, 29 So, the following d6 metals were investigated: Mn(I), Fe(II), Co(III), Tc(I), Ru(II), Rh(III), Re(I), Os(II), and Ir(III). Except for Tc (included for the systematic investigation), all other metals have been found experimentally to bind with HNO.10–21, 28, 29 For comparison, the diamagnetic d10 metal Zn(II) and s0 main group metal Mg(II) were also included in this investigation. The quantum chemical method used here was basically the same as used before for HNO/RNO/NO metal complexes.30
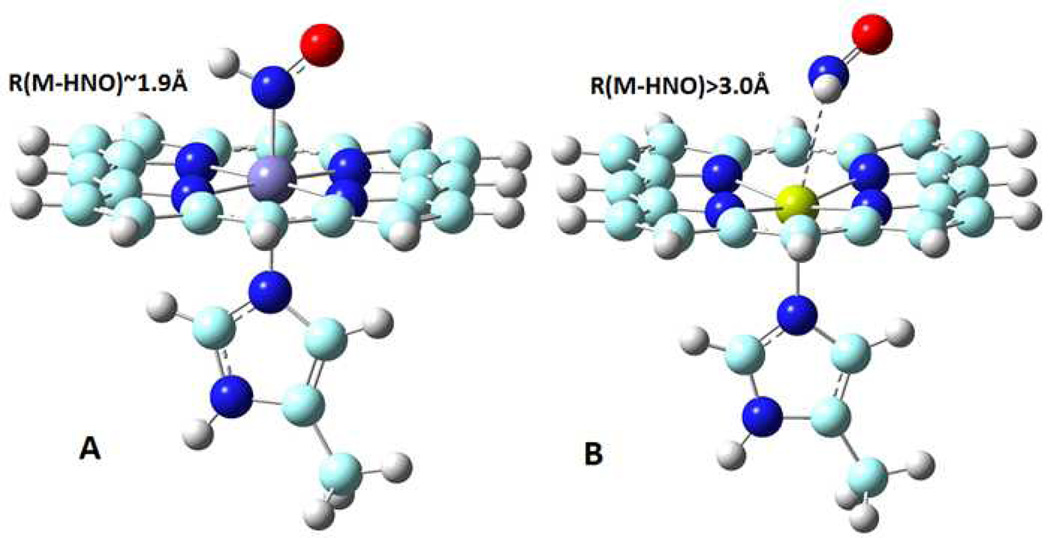
The first interesting thing to note is that all these calculated HNO metalloporphyrins fall into two clusters. One includes all d6 metals, and the other contains the d10 and s0 metals. Structures of HNO metal complexes in each cluster are similar, as illustrated in Fig.1A and 1B for the former and latter clusters, respectively. The former cluster for d6 metals involves stable binding of HNO via the metal centre, with ~1.9 Å metal-nitrogen binding distances and ca. −20 to −60 kcal/mol binding electronic energies. In contrast, the metal HNO (via N) distances are >3.0 Å for the d10 and s0 metals, indicating basically no binding. In fact, the overall binding electronic energies are only ca. −3 kcal/mol in such systems, which is consistent with the Van der Waals type of interaction between HNO and the porphyrin moiety shown in Fig.1B. Once the entropic effect is included, the total binding Gibbs free energies for the second cluster are actually positive (see Fig.2), suggesting that it is unfavourable to form such complexes. These results provide the first computational evidence that stable HNO metal complexes involve d6 metals, but not d10 or s0 metals, which is consistent with experimental findings.11–21, 28, 29
Fig. 1.
Typical molecular structures of HNO complexes with d6 metals (A) and d10/s0 metals (B). Atom colour scheme: N-blue, O-red, C-cyan, H-grey, metal centres using different colours.
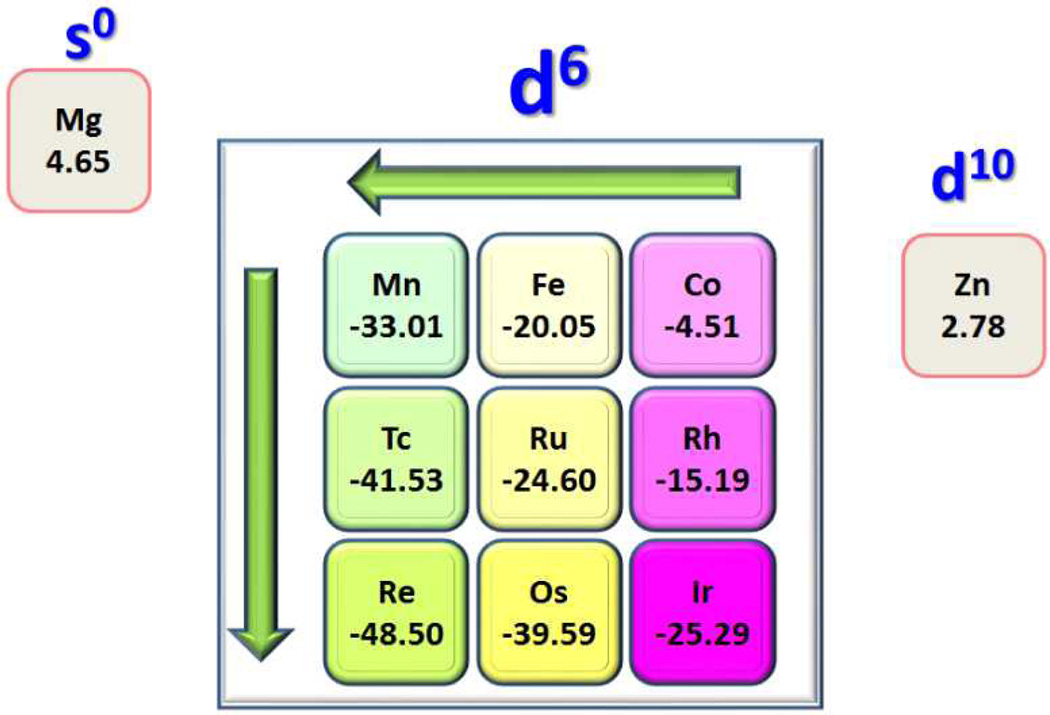
Fig. 2.
Binding Gibbs free energies (unit: kcal/mol).
To further investigate the metal centre effects and explore the electronic origin, we then focused on the first cluster of d6 metals to evaluate their HNO binding energies, electronic charges, and NO vibrational frequencies which were experimentally found to be excellent probes to characterize different HNO metal systems.11–21, 29 Because there is an excellent correlation between the binding electronic energies (ΔE’s) and the binding Gibbs free energies (ΔG’s) for these compounds, with a linear correlation coefficient R2 = 0.999, the following discussion is on the ΔG’s, which also include the contribution from entropic effect.
Interestingly, although they have the same d6 electronic configuration, there is a clear dependence of the binding Gibbs free energy on the location of the metal in the periodic table, as shown in Fig.2. There are two clear trends. The first trend is that in each of the three studied groups, the HNO binding stability always increases from early transition metal to late transition metal. For instance, for group 8 metals, ΔG decreases from −20.05 kcal/mol for Fe(II) to −24.60 kcal/mol for Ru(II) to −39.59 kcal/mol for Os(II). This is consistent with the overall feature observed in previous experimental systems.11–21 But these computational results provide a more direct evidence of this metal centre effect because the ligands are the same here. This systematic investigation also surveys more metals in a comparable way, which suggests that this metal centre effect regarding early vs. late transition metals may be a general feature of HNO metal binding. A second interesting trend is that for metals in the same period, such as Mn(I), Fe(II), and Co(III), ΔG increases from −33.01 kcal/mol for Mn(I) to −20.05 kcal/mol for Fe(II) to −4.51 kcal/mol for Co(III), suggesting that HNO binding are more stable with the low oxidation state metal than with the high oxidation state metal, given that the metals are in the same period and same electronic configuration. Again, this trend remains the same for all three studied periods.
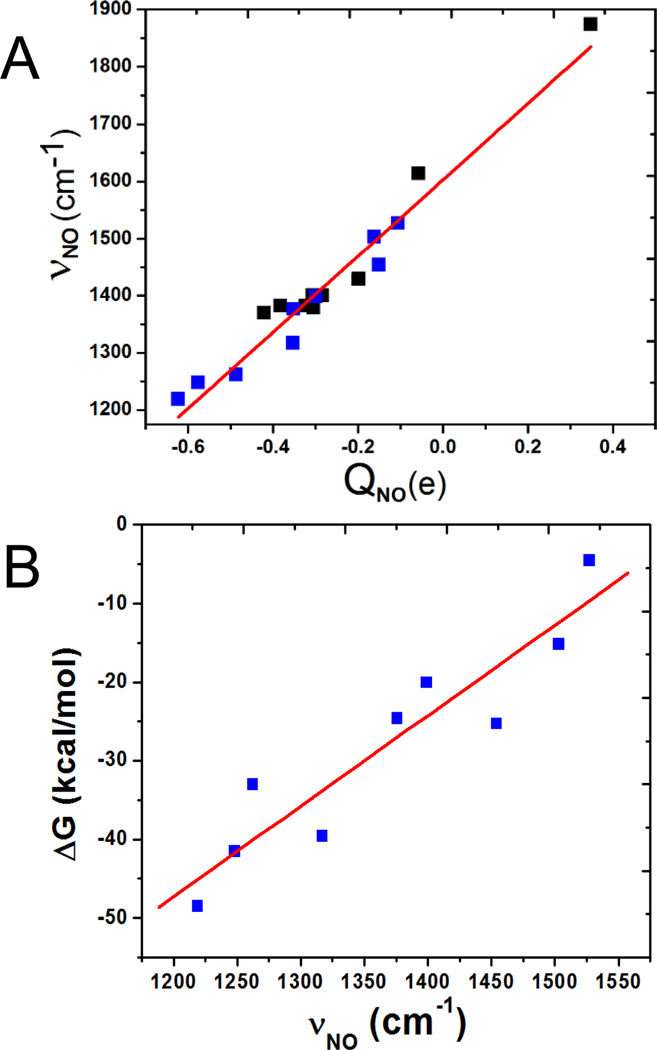
To explore the electronic origin of these metal centre effects on HNO binding, electronic charges were investigated. Our prior work on a few Ru and Fe HNO complexes show that stronger HNO binding entails more back-donation of metal electrons to the anti-bonding πNO* orbital in HNO, resulting in increased NO charge (QNO), which is associated with longer NO bond length (RNO) and smaller NO vibrational frequency (νNO).30 So, the NO geometric, electronic, and spectroscopic properties are all indicators of the metal back-donation effect. Compared to that investigation of HNO/RNO/NO compounds, here, a number of different HNO metal complexes were investigated. Some bond lengths, charges, and vibrational frequencies were reported in Table 1. It is interesting to note that the computed data basically have the same inter-relationships within these three characteristic properties, as with the previous HNO/RNO/NO systems. For instance, as shown in Fig.3A, there is an excellent overall correlation between νNO and QNO among all these NO-containing systems (black and blue data points are for previous and current systems) with R2 = 0.976. This suggests that the HNO bonding in all the studied metal complexes have basically the same mechanism, dominated by metal back-donation to πNO* orbital in HNO. There are also good correlations between binding ΔG and RNO/QNO/νNO, as illustrated in Fig.3B for ΔG vs. νNO with a linear correlation coefficient R = 0.931. These results indicate that the metal back-donation to the π-acid HNO is the principal driving force for HNO binding.
Table 1.
Bond lengths, charges and vibrational frequencies
| Compound | RNO (Å) |
RNH (Å) |
RMN (Å) |
QNO (e) |
νNO (cm−1) |
|---|---|---|---|---|---|
| Mn(Por)(5-MeIm)(HNO) | 1.278 | 1.039 | 1.829 | −0.487 | 1262 |
| Tc(Por)(5-MeIm)(HNO) | 1.282 | 1.035 | 1.953 | −0.576 | 1248 |
| Re(Por)(5-MeIm)(HNO) | 1.291 | 1.033 | 1.951 | −0.623 | 1219 |
| Fe(Por)(5-MeIm)(HNO) | 1.246 | 1.041 | 1.808 | −0.299 | 1399 |
| Ru(Por)(5-MeIm)(HNO) | 1.249 | 1.040 | 1.941 | −0.352 | 1376 |
| Os(Por)(5-MeIm)(HNO) | 1.261 | 1.038 | 1.930 | −0.353 | 1317 |
| Co(Por)(5-MeIm)(HNO) | 1.211 | 1.044 | 1.860 | −0.106 | 1527 |
| Rh(Por)(5-MeIm)(HNO) | 1.218 | 1.042 | 1.963 | −0.162 | 1503 |
| Ir(Por)(5-MeIm)(HNO) | 1.228 | 1.040 | 1.951 | −0.151 | 1454 |
Fig. 3.
Plots of νNO vs. QNO (A) and ΔG vs. νNO (B).
With this common HNO bonding and binding mechanism in the studied metal systems, the trends observed in this work can then be understood. First, because d10 and s0 metals basically cannot donate electrons, while the d6 metals can, only d6 metals were found to bind with HNO stably, but not the d10 and s0 metals, consistent with experimental observations.11–21, 28, 29 Second, in the same group, the late transition metal is softer than the early transition metal, possessing higher capability of back-donation to HNO, so it is relatively easier to synthesize HNO complexes with late transition metal centres, consistent with more of such complexes were reported in the area of organometallic chemistry.11–21, 28, 29 Third, in the same period and with the same electronic configuration, the metal with a low oxidation state can provide more back-donation than that with a high oxidation state, therefore, it binds with HNO stronger.
In summary, this work not only explained the current experimental observations of metal centre effects, but also provided the first straightforward evidence of general trends of HNO binding regarding metal’s electronic configuration, position in the periodic table, and oxidation state. It also revealed for the first time the common bonding mechanism that dominates the HNO binding in various metal complexes, which is metal back-donation to the strong π-acid HNO. These results shall facilitate future organometallic and bioinorganic studies of HNO metal systems.
Acknowledgments
This work was supported by the NIH grant GM-085774 to YZ.
Footnotes
All the molecules investigated in this work were subject to full geometry optimizations and subsequent frequency calculations to verify that they are the minimum energy states in their potential energy surfaces. Atomic charges are from the natural population analysis. The binding energy was calculated as a difference of the energy of M(Por)(HNO)(5-MeIm) and the sum of the energies of HNO and M(Por)(5-MeIm). The Gibbs free energy was calculated for room temperature. The computational method is basically the same as used previously,30 i.e. mPWVWN with CEP-121G for metal center, 6–311G++(2d,2p) for atoms directly bonded with the metal center and HNO, and 6–31G* for other atoms. Calculations were carried out with Gaussian 03 program, by M. J. Frisch et al., Gaussian Inc., Wallingford, CT, 2004.
Contributor Information
Weihai Fang, Email: fangwh@bnu.edu.cn.
Yong Zhang, Email: yong.zhang@stevens.edu.
Notes and references
- 1.Miranda KM. Coord. Chem. Rev. 2005;249:433–455. [Google Scholar]
- 2.Averill BA. Chem. Rev. 1996;96:2951–2964. doi: 10.1021/cr950056p. [DOI] [PubMed] [Google Scholar]
- 3.Farmer PJ, Sulc F. J. Inorg. Biochem. 2005;99:166–184. doi: 10.1016/j.jinorgbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Roncaroli F, Videla M, Slep LD, Olabe JA. Coord. Chem. Rev. 2007;251:1903–1930. [Google Scholar]
- 5.Rusche KM, Spiering MM, Marletta MA. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- 6.Huang JM, Sommers EM, Kim-Shapiro DB, King SB. J. Am. Chem. Soc. 2002;124:3473–3480. doi: 10.1021/ja012271v. [DOI] [PubMed] [Google Scholar]
- 7.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. Proc. Natl. Acad. Sci. USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller TW, Cherney MM, Lee AJ, Francoleon NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. J. Biol. Chem. 2009;284:21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt H, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar MR, Zapata A, Ramirez AJ, Bowen SK, Francisco WA, Farmer PJ. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18926–18931. doi: 10.1073/pnas.1111488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy KR, Reed CA, Roper WR. J. Chem. Soc. D-Chem. Commun. 1970:1501–1502. [Google Scholar]
- 12.Wilson RD, Ibers JA. Inorg. Chem. 1979;18:336–343. [Google Scholar]
- 13.Melenkivitz R, Hillhouse GL. Chem. Commun. 2002:660–661. doi: 10.1039/b111645b. [DOI] [PubMed] [Google Scholar]
- 14.Southern JS, Green MT, Hillhouse GL, Guzei IA, Rheingold AL. Inorg. Chem. 2001;40:6039–6046. doi: 10.1021/ic010669m. [DOI] [PubMed] [Google Scholar]
- 15.Southern JS, Hillhouse GL, Rheingold AL. J. Am. Chem. Soc. 1997;119:12406–12407. [Google Scholar]
- 16.Melenkivitz R, Southern JS, Hillhouse GL, Concolino TE, Liable-Sands LM, Rheingold AL. J. Am. Chem. Soc. 2002;124:12068–12069. doi: 10.1021/ja0277475. [DOI] [PubMed] [Google Scholar]
- 17.Sellmann D, Gottschalk-Gaudig T, Haussinger D, Heinemann FW, Hess BA. Chem. Eur. J. 2001;7:2099–2103. doi: 10.1002/1521-3765(20010518)7:10<2099::aid-chem2099>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Marchenko AV, Vedernikov AN, Dye DF, Pink M, Zaleski JM, Caulton KG. Inorg. Chem. 2002;41:4087–4089. doi: 10.1021/ic025699j. [DOI] [PubMed] [Google Scholar]
- 19.Marchenko AV, Vedernikov AN, Dye DF, Pink M, Zaleski JM, Caulton KG. Inorg. Chem. 2004;43:351–360. doi: 10.1021/ic0349407. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Richter-Addo GB. J. Inorg. Biochem. 2004;98:1247–1250. doi: 10.1016/j.jinorgbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Montenegro AC, Amorebieta VT, Slep LD, Martin DF, Roncaroli F, Murgida DH, Bari SE, Olabe JA. Angew. Chem.-Int. Edit. 2009;48:4213–4216. doi: 10.1002/anie.200806229. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino J, Bari SE, Bikiel DE, Doctorovich F. J. Am. Chem. Soc. 2010;132:989–995. doi: 10.1021/ja905062w. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Ling Y, Zhang Y. J. Am. Chem. Soc. 2011;133:13814–13817. doi: 10.1021/ja204072j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi I, Li C-z, Ishida M, Yao Q. J. Electroanal. Chem. 1999;460:245–250. [Google Scholar]
- 25.Li Cz, Nishiyama K, Taniguchi I. Electrochim. Acta. 2000;45:2883–2888. [Google Scholar]
- 26.Li C.-z, Taniguchi I, Mulchandani A. Bioelectrochemistry. 2009;75:182–188. doi: 10.1016/j.bioelechem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Takashima H, Kawahara H, Kitano M, Shibata S, Murakami H, Tsukahara K. J. Phys. Chem. B. 2008;112:15493–15502. doi: 10.1021/jp807692w. [DOI] [PubMed] [Google Scholar]
- 28.Kumar MR, Pervitsky D, Chen L, Poulos T, Kundu S, Hargrove MS, Rivera EJ, Diaz A, Colon JL, Farmer PJ. Biochemistry. 2009;48:5018–5025. doi: 10.1021/bi900122r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Immoos CE, Sulc F, Farmer PJ, Czarnecki K, Bocian DF, Levina A, Aitken JB, Armstrong RS, Lay PA. J. Am. Chem. Soc. 2005;127:814–815. doi: 10.1021/ja0433727. [DOI] [PubMed] [Google Scholar]
- 30.Ling Y, Mills C, Weber R, Yang L, Zhang Y. J. Am. Chem. Soc. 2010;132:1583–1591. doi: 10.1021/ja907342s. [DOI] [PMC free article] [PubMed] [Google Scholar]