Abstract
Acne vulgaris is the most common skin disorder affecting millions of people worldwide and inflammation resulting from the immune response targeting Propionibacterium acnes plays a significant role in its pathogenesis. In this study, we have demonstrated that P. acnes is a potent inducer of Th17 and Th1, but not Th2 responses in human PBMCs. P. acnes stimulated expression of key Th17-related genes, including IL-17A, RORα, RORc, IL-17RA and IL-17RC, and triggered IL-17 secretion from CD4+, but not CD8+ T cells. Supernatants from P. acnes-stimulated PBMCs were sufficient to promote the differentiation of naïve CD4+CD45RA T cells into Th17 cells. Furthermore, we found that the combination of IL-1β, IL-6 and TGF-β neutralizing antibodies completely inhibited P. acnes-induced IL-17 production. Importantly, we showed that IL-17-expressing cells were present in skin biopsies from acne patients but not from normal donors. Finally, vitamin A (all-trans retinoic acid) and vitamin D (1,25-dihydroxyvitamin D3) inhibited P. acnes-induced Th17 differentiation. Together, our data demonstrate that IL-17 is induced by P. acnes and expressed in acne lesions and that both vitamin A and vitamin D could be effective tools to modulate Th17-mediated diseases such as acne.
Introduction
Acne vulgaris is a multifactorial chronic disorder of the pilosebaceous follicles of human skin and its pathogenesis is not yet completely understood (Kim, 2005; Zouboulis, 2001; Zouboulis et al., 2005). The inflammatory nature of acne has been associated with the host immune response targeting Propionibacterium acnes, a commensal human skin bacterium found in the pilosebaceous follicles.
The innate immune system recognizes P. acnes via Toll-like receptor 2 (TLR2) (Kim et al., 2002), leading to the secretion of inflammatory cytokines, including IL-8 and IL-12. P. acnes has also been shown to stimulate production of inflammatory cytokines such as IL-8, TNF-α, IL-1β by both human monocytic cell lines and freshly isolated PBMCs from acne patients and normal controls (Vowels et al., 1995). The adaptive immune response system also plays a central role in the inflammation observed in acne, resulting from the recruitment of activated T helper 1 (Th1) lymphocytes to early acne lesions (Mouser et al., 2003). While the function of innate immune cells and Th1 cells in acne inflammation have been studied, the role of Th17 cells in acne is yet to be elucidated.
Th17 cells are characterized by the production of IL-17A and IL-17F belonging to the IL-17 family of cytokines. IL-17A and IL-17F target mostly nonlymphoid cells, including fibroblasts, keratinocytes, endothelial cells and macrophages and induce the production of cytokines such as TNF-α, IL-6, GM-CSF, and matrix metalloproteinases (Damsker et al., 2010; Kolls and Linden, 2004). In addition to IL-17A and F, Th17 cells produce IL-6, IL-21 and IL-22 and exhibit effector functions distinct from the Th1 and Th2 cells (Harrington et al., 2005; Langrish et al., 2005; Liang et al., 2006; Nurieva et al., 2007; Park et al., 2005; Zheng et al., 2007; Zhou et al., 2007). An important outcome of these effects is localized chronic tissue inflammation, which is often associated with extracellular matrix destruction (Miossec, 2003).
Th17 cells are potent inducers of tissue inflammation and have been associated with the pathogenesis of many autoimmune disorders such as psoriasis, rheumatoid arthritis, Crohn’s disease and multiple sclerosis (Amadi-Obi et al., 2007; Lock et al., 2002; Teunissen et al., 1998). Immune modulators such as ATRA (All-trans retinoic acid) and 1,25D3 (1,25-dihydroxyvitamin D3) share the retinoid X receptor (RXR) as a common receptor for retinoid signaling and have been tried as potential therapeutic agents in a variety of autoimmune and inflammatory diseases (Mucida et al., 2007).
Herein, we have evaluated whether Th17 cells are involved in the inflammatory response towards P. acnes and explored a possible role for vitamin A (ATRA) and vitamin D (1,25D3) in modulating Th17 differentiation.
Results
P. acnes lab strain and clinical isolates stimulate production of IL-17 and IL-22
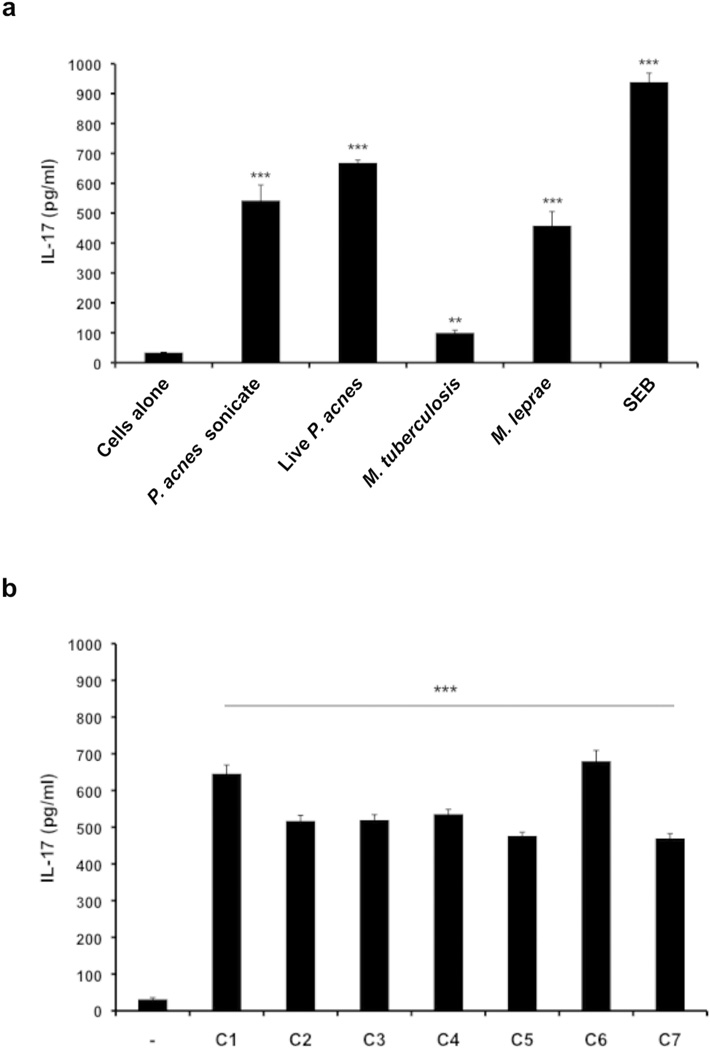
Increased IL-17 production is observed in response to pathogenic microbes and in inflammatory skin conditions such as psoriasis (Hino et al., 2011; Infante-Duarte et al., 2000; Tokura et al., 2010). We sought to determine if the causative pathogen of acne vulgaris, P. acnes, could stimulate the production of IL-17 in human peripheral blood mononuclear cells (PBMCs). We observed that both live P. acnes and P. acnes sonicate (ATCC strain 6919) stimulated the production of IL-17 (Fig. 1a), which was optimally induced seven days after activation. Other cutaneous pathogens, including Mycobacterium tuberculosis and Mycobacterium leprae, showed significantly lower IL-17 induction in comparison to P. acnes. Meanwhile, Staphylococcal enterotoxin B (SEB) was a potent inducer of IL-17, corroborating a previous study (Islander et al., 2010). In addition, we tested seven P. acnes clinical isolates obtained from acne patients and found that all clinical isolates tested induced IL-17 protein secretion ranging from approximately 500–700 pg/ml (Fig. 1b; p<0.001). Stimulation of PBMCs with both the P. acnes lab strain and clinical isolates also mediated robust IL-22 protein secretion (supplementary Fig. S1a and S1b; P<0.001).
Fig.1. P. acnes lab strain and clinical isolates stimulate production of IL-17 in human PBMCs.
a) PBMCs were cultured (2–5 × 106/ml) in the presence of P. acnes sonicate (2 µg/ml), live P. acnes (0.5 muliplicity of infection), M. tuberculosis (5 µg/ml), M. leprae (5 µg/ml), and Staphylococcus enterotoxin B (SEB 2 µg/ml) for seven days. b) PBMCs (2–5 × 106/ml) were cultured either in the presence or absence of seven P. acnes clinical isolates (C1–C7). Levels of IL-17 accumulated in culture supernatants were measured using ELISA. Experiments were performed at least five times using PBMCs from five different donors with similar results. (** p ≤ 0.05, ***p ≤ 0.001)
P. acnes triggers a Th17 and Th1 response
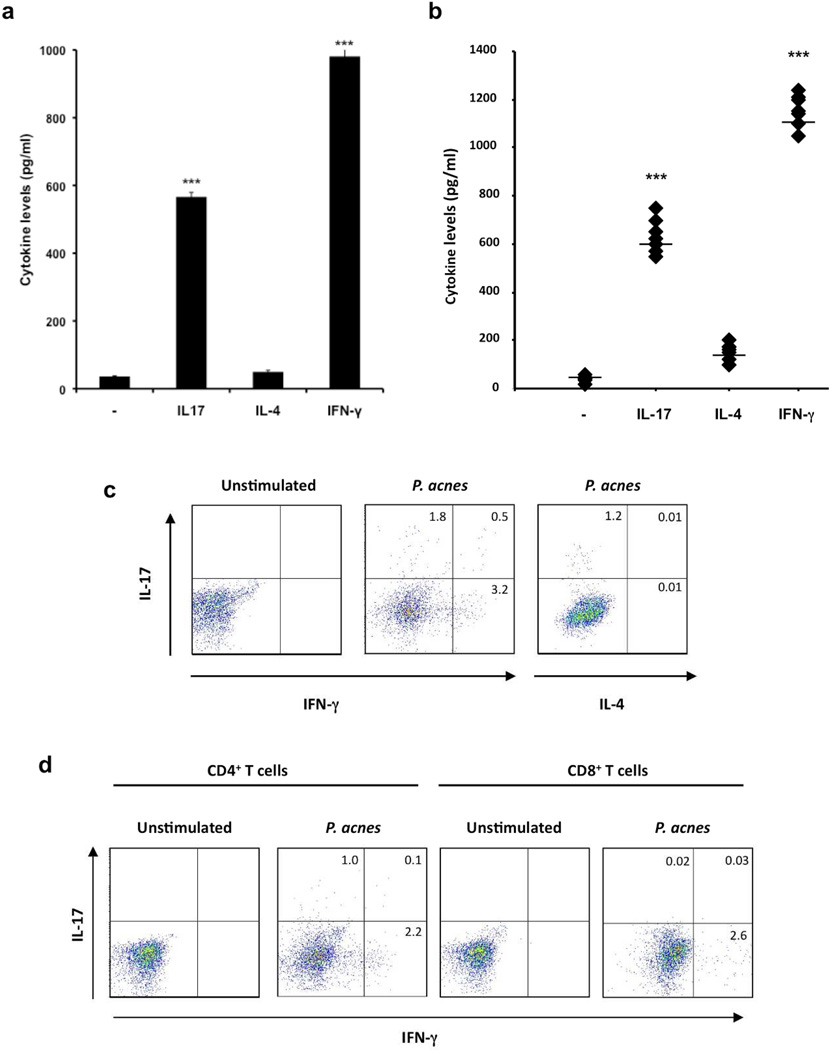
Infectious and inflammatory diseases are commonly characterized as Th1, Th2 or Th17, based on the subsets of T cells involved in host defense or disease pathogenesis. Therefore, we next wanted to characterize the T cells resulting from P. acnes stimulation based on their ability to produce IFN-γ(Th1), IL-4 (Th2), and/or IL-17 (Th17). We found that P. acnes induced IL-17 and IFN-γbut not IL-4 (Fig. 2a). In addition, we found that all seven P. acnes clinical isolates induced significant levels of IL-17 and IFN-γprotein expression, but minimal levels of IL-4, as measured by both ELISA (Fig. 2b; p<0.001) and flow cytometry (Fig. 2c). Our data suggest that P. acnes induces both Th17 and Th1 immune responses as measured by IL-17 and IFN-γ, respectively.
Fig.2. P. acnes stimulate production of IL-17A and IFN-γbut not IL-4 in PBMCs.
PBMCs were cultured (2–5 × 106/ml) in the presence of P. acnes sonicate (2 µg/ml) or P. acnes clinical isolates for 7 days. a) Levels of IL-17, IL-4 and IFN-γaccumulated in culture supernatants were measured using ELISA. Experiments were performed at least five times using PBMCs from five different donors with similar results. b) PBMCs (2–5 × 106/ml) were cultured either in the presence or absence of seven P. acnes clinical isolates (C1–C7). Levels of IL-17, IL-4, and IFN-γaccumulated in culture supernatants were measured using ELISA. Experiments were performed at least three times using PBMCs from three different donors with similar results. The overall group effect was statistically significant (p<0.001). c) Flow cytometry of PBMCs stimulated with P. acnes sonicate for 7 days. Intracellular cytokine staining for IFN-γ, IL-4 and IL-17 was performed on day 7. Plots are gated on CD4+ T cells. Each panel is representative of four independent experiments. d) Flow cytometry of peripheral blood stimulated with P. acnes sonicates for seven days. Intracellular cytokine staining for IFN-γand IL-17 was performed on day seven. Plots are gated on CD4+ and CD8+ T cells. Each panel is representative of three independent experiments. Data represent mean ± SD (** p ≤ 0.05, ***p ≤ 0.001)
We next characterized the IL-17 and IFN-γ-expressing T cells based on CD4 and CD8 expression. P. acnes induced both IL-17 and IFN-γin CD4+ T cells (Fig. 2d). On the other hand, CD8+ T cells produced only IFN-γbut not IL-17, suggesting that the production of IL-17 was restricted to CD4+ T cells in response to P. acnes.
P. acnes induces Th17-related genes
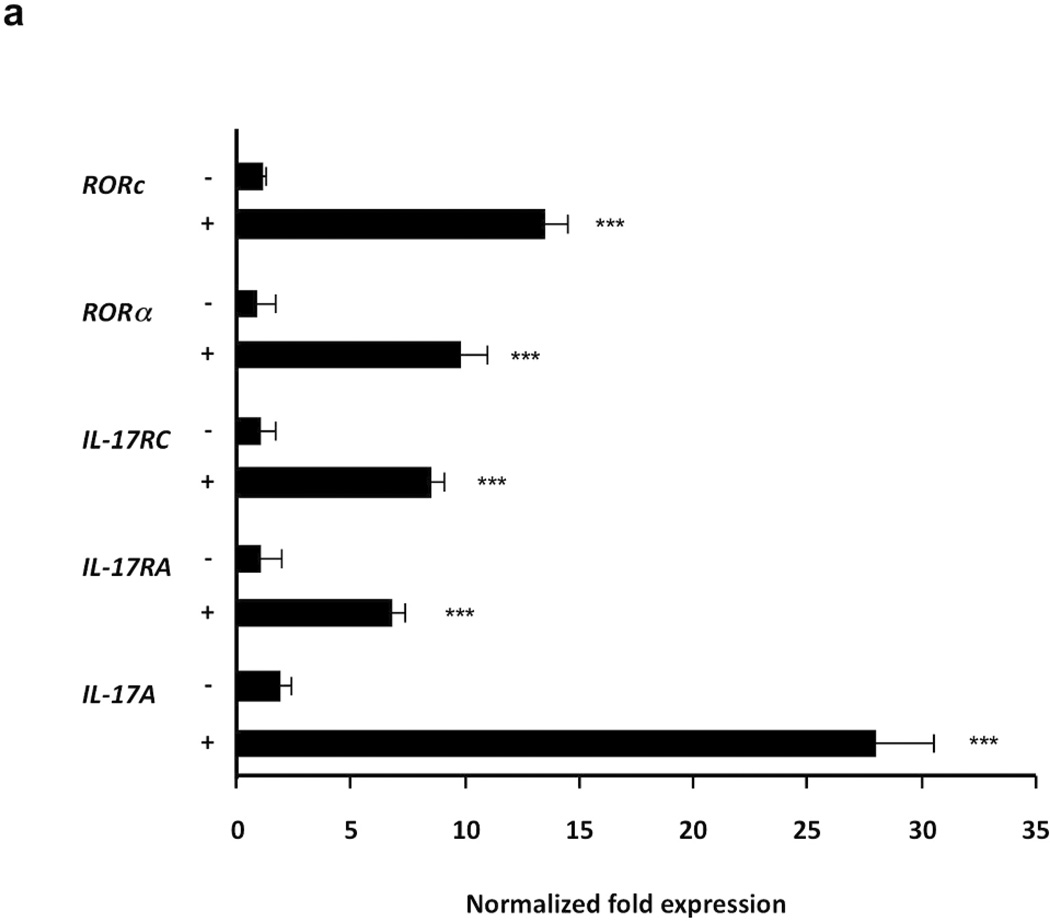
The hallmark of human Th17 cells is the expression of IL-17 and key differentiation markers including RORα and RORc. P. acnes induced IL-17 gene expression (28-fold), as well as expression of retinoic orphan receptors Rora (10-fold) and Rorc (14-fold). PBMCs stimulated with P. acnes also were induced to express IL-17RA (7-fold) and IL-17RC (8-fold), two receptor genes that partner together to mediate responses to IL-17A and IL-17F (Toy et al., 2006) (Fig. 3; p<0.001). Our data suggest that P. acnes triggers the expression of key genes involved in Th17 differentiation, including IL-17, IL-17 receptor genes and the transcriptional factors Rorc and Rora.
Fig.3. Induction of IL-17, IL-17RA, IL-17RC, RORc and RORa mRNAs expression in PBMCs stimulated with P. acnes.
PBMCs were cultured (2–5 × 106/ml) with P. acnes sonicate (2 µg/ml). Real time PCR of IL-17, IL-17RA, IL-17RC, RORc and RORa mRNA expression was analyzed 24 hours following P. acnes stimulation. Gene expression was normalized to the housekeeping genes GAPDH and quantified by the comparative method 2−ΔΔCT. Data are representative of four independent experiments. Data represent mean ± SD (***p ≤ 0.001)
Supernatants from PBMCs treated with P. acnes differentiate naïve CD4+CD45RA T cells to IL-17 producing Th17 cells
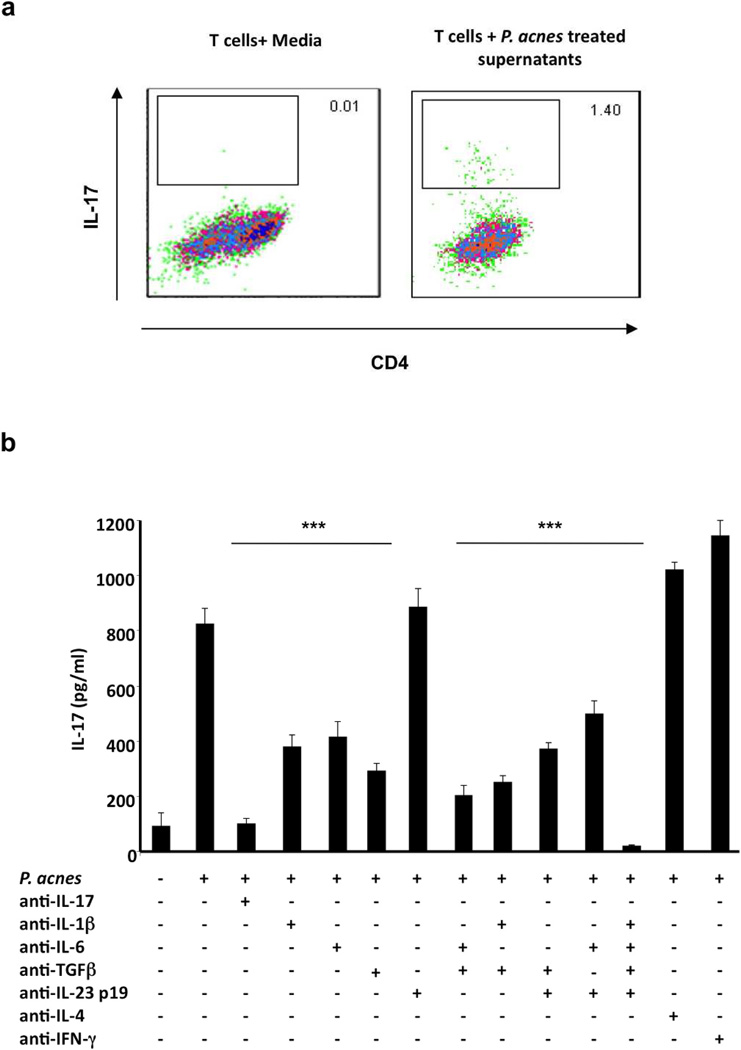
The differentiation of Th17 cells occurs in the presence of specific cytokines including IL-1β, IL-6 and TGF-β. We hypothesized that culture supernatants from P. acnes-stimulated PBMCs were sufficient to induce naïve CD4+CD45RA T cells to differentiate into Th17 cells. We isolated naïve CD4+CD45RA T cells from the peripheral blood of healthy donors and stimulated them with plate-bound anti-CD3 and soluble anti-CD28 monoclonal antibodies for six days in the presence or absence of culture supernatants derived from P. acnes-stimulated PBMC. Those naïve T cells activated in the presence of supernatants from P. acnes-stimulated PBMCs produced IL-17, with the frequency of IL-17 secreting cells increasing from 0.01 to 1.4% (Fig. 4a). We also observed differentiation of naïve T cells into Th1 cells as measured by IFN-γ(4.25 %), but not Th2 cells, as measured by IL-4 (Supp Fig S2). Treatment with anti-CD3 alone did not facilitate differentiation into IL-17 producing cells (Supplementary Fig S2). These data indicate that activation of PBMCs with P. acnes triggers the secretion of cytokines that promote the differentiation of naïve T cells into Th1 and Th17 cells.
Fig.4. Supernatants from PBMCs treated with P. acnes differentiate naïve CD4+T cells to IL-17 producing T cells.
a) PBMCs (2–5 × 106/ml) were stimulated overnight with P. acnes sonicate (2 µg/ml). Culture supernatants were then collected and used to stimulate naive CD4+CD45RA T cells for seven days in 96 well plates with plate bound anti-CD3 and soluble CD28. Cells were harvested and intracellular cytokine staining for IL-17 was performed. Each panel is representative of three independent experiments. b) PBMCs (2–5 × 106/ml) were cultured with IL-17, IL-1β, IL-6, TGF-β, IL-23p19, IL-4 and IFN-γneutralizing antibodies for one hour followed by seven days of stimulation with P. acnes. IL-17 production was then measured using ELISA. Data are representative of four independent experiments. Data represent mean ± SD (** p ≤ 0.05, ***p ≤ 0.001)
To determine the key cytokines involved in the development and differentiation of P. acnes-induced IL-17-expressing T cells, we incubated PBMCs for one hour with neutralizing antibodies to IL-17, IL-1β, IL-6, TGF-β, IL-23p19, IL-4 and IFN-γprior to treatment with P. acnes. The cells were cultured and evaluated for cytokine expression by ELISA on day seven. P. acnes induced the production of IL-17 which could be inhibited in the presence of neutralizing antibody to IL-17. The addition of IL-1β, IL-6, IL-23p19 or TGF-β neutralizing antibody alone led to the reduction of IL-17 protein expression by approximately 40–50% (p<0.001) (Fig. 4b). In addition, the combination of all four antibodies completely abrogated P. acnes-induced IL-17 production (p<0.001). In contrast, neutralization of IL-23p19, IL-4 or IFN-γalone had no effect on IL-17 production. No significant change in IL-17 production was found in the presence of isotype control antibodies (data not shown). Finally, differentiation of naïve T cell into Th1 cells as measured by IFN-γ production was blocked by neutralizing IL-12p40; in contrast, the presence of the Th17 associated neutralizing antibodies had no affect (Supplementary Fig S2b). Therefore, IL-1β, IL-6 and TGF-β appear to regulate P. acnes-induced IL-17 responses and corroborate previous studies demonstrating that these cytokines are important for the generation and maintenance of Th17 T cells.
IL-17-expressing cells are found in acne skin lesions
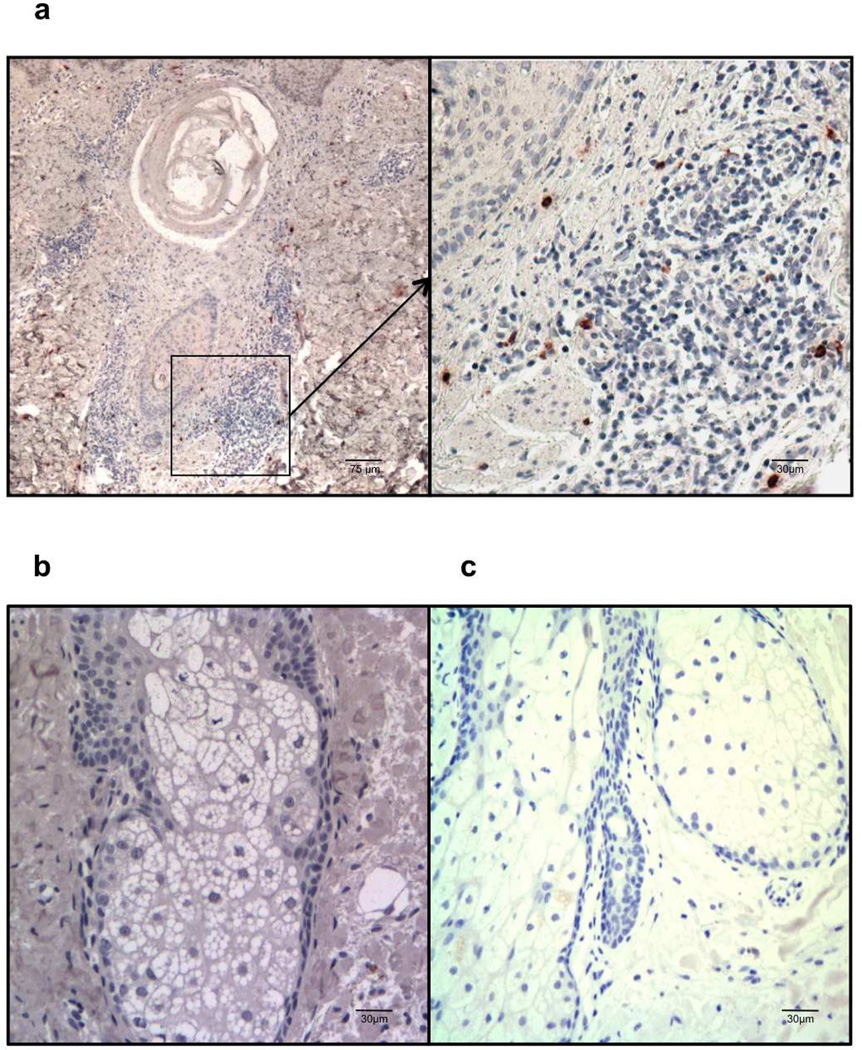
To date there are no studies clearly linking IL-17 to the pathogenesis of acne. Given our data that P. acnes induces a Th17 response in vitro, we sought to correlate these findings with in situ studies of IL-17 expression in skin biopsies from acne patients and healthy individuals. We found that IL-17-expressing cells were present in skin biopsies of acne patients and scattered around the dermis near the pilosebaceous unit (Fig. 5). On the other hand, IL-17 expression was not detectable in skin biopsies from healthy donors and isotype controls. Taken together, our immunohistochemistry results demonstrate that cells expressing IL-17 are detected in acne skin biopsies.
Fig.5. Immunohistochemistry of IL-17 in acne lesion.
Frozen skin sections were obtained from patients with acne lesion (a) and (b) and nomal control skin (c). Immunohistochemical staining was carried out using anti-human IL-17 antibody (a) and (c) and an isotype control (b). IL-17 expressing lymphoid cells (brown) can be seen scattered around the inflammation surrounding the pilosebaceous follicle (a). Data is representative of three independent experiments from three different lesions. Scale bars represent 75 Zm and 30 Zm respectively.
ATRA and 1,25D3 inhibit the development of Th17 cells
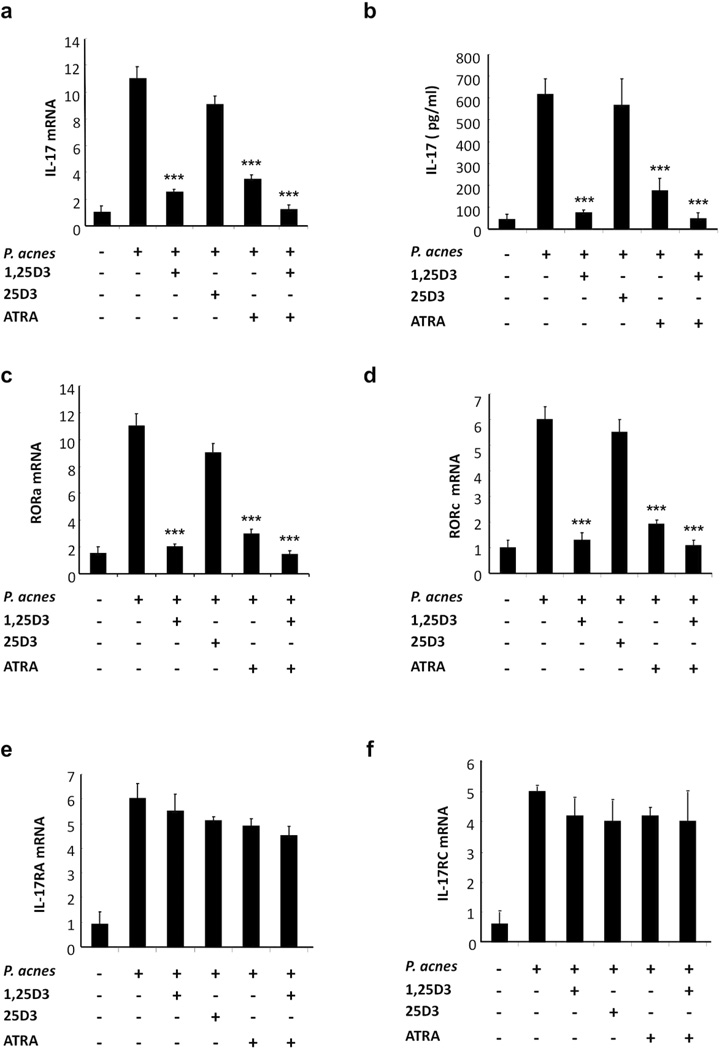
Vitamin A (ATRA) and vitamin D (1,25D3) share retinoid X receptor (RXR) as a common receptor for signaling, and their functions on inhibiting Th17 development are thought to be similar (Iwata et al., 2003; Sigmundsdottir et al., 2007). ATRA and 1,25D3 are commonly used dermatologic therapeutics as they have the ability to modulate the immune response. We therefore compared the role of ATRA and 1,25D3 on P. acnes-induced Th17 generation. P. acnes stimulation in the absence of ATRA and 1,25D3 (10−7 M) induced expression of IL-17 mRNA and protein expression (Fig. 6a; p<0.001, 6b; p<0.001). The addition of either ATRA, 1,25D3, or a combination of the two prior to activation with P. acnes downregulated the induction of both IL-17 mRNA and protein expression (Fig. 6a, 6b); the inactive form of vitamin D3 (25D3) had no effect. Similar results were obtained for the expression of RORa and RORc genes (Fig. 6c and 6d; p<0.001); both ATRA and 1,25D3 downregulated the induction of RORa (74 and 83%) and RORc (73 and 80%) expression, respectively. The combination of ATRA and 1,25D3 strongly inhibited RORa and RORc expression by 83% and 93%, respectively. In contrast, neither ATRA nor 1,25D3 had any significant inhibitory effect on IL-17RA and IL-17RC receptor gene expression (Fig. 6e, 6f; p<0.001). Finally, we determined that ATRA and 1,25D3 did not have a significant affect on cell viability (Supplementary Fig. S3). Our findings strongly suggest that both ATRA and 1,25D3 effectively inhibit the expression of genes required for the development and differentiation of P. acnes-related Th17 cells.
Fig.6. Effects of 1,25D3, 25D3 and ATRA on Th17 differentiation.
Human PBMCs were stimulated with P. acnes sonicate (2 µg/ml) in the presence of 1, 25D3 (10−7 M), 25D3 (10−7 M) and ATRA (10−7 M). IL-17 (a), RORa (c), RORc (d), IL-17RA (e) and IL-17RC (f) mRNA expression was analyzed 24 hours following P. acnes stimulation. Gene expression was normalized to the housekeeping genes GAPDH and quantified by the comparative method 2−ΔΔCT. Each panel is representative of three independent donors. b) PBMCs were cultured (2–5 × 106/ml) with 1,25D3, 25D3 and ATRA for one hour followed by seven days of stimulation with P. acnes (sonicate or live), IL-17 production was then measured using ELISA. Data are representative of four independent experiments. Data represent mean ± SD (***p ≤ 0.001)
Discussion
IL-17 is a cytokine secreted by activated T cells and has been implicated in the pathogenesis of both infectious and inflammatory skin diseases such as Staphylococcus infection, psoriasis, contact hypersensitivity and atopic dermatitis (Cho et al., 2010; Guttman-Yassky et al., 2008; Hino et al., 2011; Pennino et al., 2010; Tokura et al., 2010). While inflammation induced by both innate and adaptive immune responses in acne has been studied, the role of IL-17 producing Th17 cells in acne pathogenesis has not been investigated. Here, we demonstrate the involvement of IL-17 in acne pathogenesis to our knowledge previously unreported. P. acnes stimulation of PBMCs induced production of IL-17 and IL-22, as well as the expression of key Th17 differentiation markers including RORα, RORc, and IL-17 receptors, IL-17RA and IL-17RC. Antibody neutralizing experiments demonstrated that IL-1β, IL-6 and TGF-β were key inducers of Th17 cell activation. Importantly, we described IL-17-expressing cells surrounding the inflamed pilosebaceous unit in skin biopsies from acne patients. Finally, ATRA and 1,25D3, two immunomodulators used for treatment of various skin diseases, inhibited the development of Th17 cells and IL-17 production. Together our data suggest that Th17 cells may play a role in the pathogenesis of acne.
Infectious and inflammatory diseases have been categorized based on the T cells and cytokines that characterize the diseased tissue. For example, a Th1 response predominates in psoriasis, while a Th2 response predominates in atopic dermatitis. In leprosy, a disease caused by Mycobacterium leprae, the protected tuberculoid patients mount predominantly a Th1 response while the susceptible individuals mount a Th2 response (Modlin, 1994). We found that P. acnes induced IFN-γbut not IL-4, similar to a previous study (Mouser et al 2004). However, we also showed that IL-17 was induced indicating that both Th1 and Th17 responses may be involved in the host response to P. acnes. Several infectious and inflammatory diseases involve both Th1 and Th17 response such as psoriasis, which initially was shown to be Th1-mediated, but recent work demonstrates the involvement of IL-17 (Kagami et al., 2010). Therefore, similar to psoriasis, treatments targeting both Th1 and Th17 cytokines in acne may be therapeutic. Interestingly, we found IL-17-expressing cells surrounding the pilosebaceous unit in acne lesion suggesting that our in vitro data is clinically relevant. It would be useful to investigate whether the levels of response of Th1 and Th17 and even Th2 might vary in various acne lesions, similar to the immune spectrum seen in leprosy patients, particularly given that Mycobacterium and P. acnes share similar genetic features (Barksdale and Kim, 1984).
We determined that distinct subsets of T cells were responsible for the secretion of IL-17 and IFN-γin response to P. acnes. P. acnes induced both IL-17 and IFN-γin CD4+ T cells, but not CD8+ T cells, which produced only IFN-γ. Our data support previous studies that suggest that inflammation in acne can be initiated by a P. acnes-specific CD4+ T cell response (Farrar and Ingham, 2004; Lodes et al., 2006; Teunissen et al., 1998). Indeed, it has been postulated that immunogenic P. acnes proteins released into the follicle could be processed by Langerhans cells, which could, in turn present these antigens to CD4+ T cells in local lymph nodes (Farrar and Ingham, 2004). Whether the Th17 cells found in the periphery migrate back to skin to induce subsequent inflammation in acne resulting in inflammation is not clear.
Our data identified three cytokines, IL-1β, IL-6 and TGF-β to be critical for triggering the differentiation programs of naïve CD4+ T cells to IL-17 secreting Th17 cells in response to P. acnes. In contrast, neither IL-23, IFN-γor IL-4 was required for Th17 differentiation in vitro. This finding corroborate other studies showing the combinatorial role of IL-1β, IL-6 and TGF-β in the initiation of Th17 cell development (Acosta-Rodriguez et al., 2007; Korn et al., 2007; Li et al., 2007; Mangan et al., 2006; Sutton et al., 2006; Wilson et al., 2007; Zhou et al., 2007). The fact that we detected IL-17 differentiation in the absence of IL-23 suggests that IL-23 may be dispensable in the induction of P. acnes-specific IL-17 response. However, we do not exclude the possibility that IL-23 and other cytokines such as IL-21 and IL-22 may support the later stages of Th17 differentiation.
Finally, we examined the role of vitamin A and vitamin D in regulating the generation of Th17 cells. Both ATRA and 1,25D3 have has been shown to suppress Th17 generation via downregulation of RARα and the transcriptional factor RORc, which orchestrates Th17 differentiation (Chang et al., 2010; Ikeda et al., 2010; Mucida et al., 2007). We found that ATRA and 1,25D3 inhibited IL-17 mRNA and protein expression in response to stimulation with P. acnes. In addition, induction of RORa and RORc was also inhibited in the presence of ATRA or 1,25D3. On the other hand, ATRA and 1,25D3 had no significant effect on IL-17 receptor gene expression. These findings suggest a role for retinoids and vitamin D in regulating the expression of genes required for Th17 differentiation.
In summary, we demonstrate that, P. acnes is a potent inducer of IL-17, IL-22 and IL-17-associated genes in human PBMCs and that IL-17 producing cells are found in acne lesions. Our in vivo finding that IL-17+ lymphocytes are present in abundance near the pilosebaceous follicles suggests that Th17 cells may play a role in acne pathogenesis. In addition, we demonstrate the inhibitory effect of ATRA and 1,25D3 in P. acnes induction of IL-17, providing a therapeutic application of ATRA and 1,25D3 for the treatment of acne and other Th17 mediated skin disease.
Materials and Methods
PBMC isolation, stimulation and cytokine ELISAs
PBMCs were obtained from healthy donors after signed written informed consent as approved by the Institutional Review Board at UCLA in accordance with the Helsinki Guidelines. PBMCs were then isolated using Ficoll–Paque gradients (GE Healthcare) and plated onto 24 well tissue culture plates (2 × 106 to 5 × 106/well) in RPMI 1640 media containing 10% FBS (HyClone). Cells were cultured with media, P. acnes sonicate (2µg/ml), live P. acnes 0.5 multiplicity of infection (0.5 MOI), Mycobacterium tuberculosis sonicate (2 µg/ml), Staphylococcus enterotoxin B (2 µg/ml) or Mycobacterium leprae sonicate (2 µg/ml) for 7 days. IL-17 and IL-22 levels in culture supernatants were measured by ELISA following the manufacturer’s recommendations (R&D ELISA Development System, DY317 and DY782). Samples were assayed in triplicates.
P. acnes and Clinical Isolates
P. acnes strain ATCC 6919 was obtained from American Type Culture Collections and prepared by probe sonication. The level of endotoxin contaminating the P. acnes was quantified with a Limulus Amoebocyte Lysate assay (BioWhittaker) and found to be < 0.1 ng/ml. P. acnes cultures were also grown in the miniMACS anaerobic workstation (Don Whitley Scientific) as described before (Marinelli et al., 2012).
P. acnes clinical isolates were obtained from nasal skin microcomedones from patients attending the Division of Dermatology outpatient clinic at UCLA, or from donors recruited to the laboratory as previously described (Marinelli et al., 2012) and after signed written informed consent as approved by the Institutional Review Board at UCLA in accordance with the Helsinki Guidelines.
Surface and Intracellular staining
Cells were collected and left unstimulated or were stimulated with P. acnes in the presence of GolgiStop (BD) for the last five hours of incubation prior to staining. As a positive control for IL-17 cytokine secretion, cells were incubated for five hours with 50 ng/ml of Phorbol 12-Myristate 13-acetate, 500 ng/ml Ionomycin (Sigma), and Golgiplug (IFN-γand IL-17) or GolgiStop (IL-17 and IL-4) at the recommended concentrations (BD Pharmingen). Cells were first surface stained with fluorescein isothiocyanateconjugated anti-CD4 or anti-CD8 antibodies (BD) for 20 minutes on ice. Intracellular staining was performed using the Cytofix-Cytosperm buffer set following manufacturer’s protocol (BD). Cells were fixed with 2% paraformaldehyde, acquired on BD Biosciences FacsScan, and analyzed using CellQuest-Pro software (BD).
Purification and stimulation of naïve CD4+ T cells
Naïve CD4+ T cells were isolated by negative selection using the EasySep Human Naïve CD4+ T Cell Enrichment Kit (Stem Cell Technologies) following manufacturer’s recommendations. PBMCs were isolated and stimulated with media, live P. acnes (0.5 MOI) or P. acnes sonicate at 2 µg/ml for 24 hours. Supernatants were harvested after 24 hours and added to cultures containing naïve CD4+ T cells that had been stimulated with plate bound anti-human CD3 and anti-human CD28 (eBiosciences) antibodies. Intracellular cytokine staining was performed on day seven.
RNA isolation, cDNA synthesis and real-time PCR
PBMCs were stimulated with media or P. acnes sonicate. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following manufacturer’s protocol and treated with RNase-free DNase. RNA samples were reverse-transcribed to cDNA using iScript cDNA synthesis kit (BIO-RAD). Reactions were done at 25°C for 5 min, 42°C for 30 min and 85°C for 5 min. Real-time PCR was applied using iQ SYBR Green supermix (BIO-RAD). 40 cycles were carried out at 95°C for 5 min, then 95°C for 10 sec, 55°C for 20 sec, 72°C for 20 sec. GAPDH was used as a control. Gene expression level was quantified by the comparative method 2−ΔΔCT. The list of primers used in the study are summarized in supplementary table S1.
Cytokine blocking/Neutralization experiments
PBMCs were incubated with neutralizing antibodies for one hour prior to activation with P. acnes sonicate. The following antibodies were used: anti-IL-1β (1 µg/ml; R&D systems), anti-IL-17 (2 µg/ml; R&D systems), anti-IL-23 p19 (0.4 µg/ml; R&D systems), anti-TGF-β (1 µg/ml; R&D systems), anti-IL-4 (100 ng/ml; BD Pharmingen), anti-IL-6 (300 ng/ml; BD Pharmingen), and anti-IFN-γ(300 ng/ml; BD Pharmingen), anti IL-12p40/70 (300 ng/ml; BD Pharmingen). Samples were assayed in triplicates.
Immunohistochemistry
De-identified normal skin biopsy specimens obtained from the UCLA Translational Pathology Core Laboratory and typical closed comedone-type acne lesions were obtained from Dankook University Hospital after signed written informed consent of patients as approved by the Institutional Review Board in accordance with the Helsinki Guidelines for protection of human subjects. Immunohistochemical analysis was performed using the standard streptavidin–biotin technique, using the commercial kit HRP-AEC system following manufacturer’s recommendations (R&D Systems).
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay
Cell proliferation experiments were performed in 96-well plates (5 replicates) and 1,25D3 (10−7 M) and ATRA (10−7 M) treatments initiated at 24 hours post-seeding for 7 days. Stocks and dilutions of PLX4032/vemurafenib (Plexxikon) and AZD6244/selumetenib (Selleck Chemicals) were made in dimethyl sulfoxide (DMSO). Cells were quantified using CellTiter-GLO Luminescence (Promega) following the manufacturer’s recommendations
Statistical analysis
Experimental values were expressed as the means ± standard deviation (SD) for the number of separate experiments indicated in each case. To assess differences in expression level for all groups a generalized estimating equation (GEE) model was run because donors were repeatedly measured over time. Overall group F tests were performed and if significant, post hoc pairwise comparisons were run between the groups using Tukey’s multiple comparison criteria to control the familywise error rates. Both Tukey intervals and Tukey adjusted p-values were used for this analysis. Significant differences were considered for those probabilities < 5% (p < 0.05).
Supplementary Material
Acknowledgements
We thank Ping Fu (Tissue procurement and Histology Core Laboratory, UCLA) for her assistance with histological preparations. Flow cytometry was performed in the UCLA Jonnson Comprehensive Cancer Center (JCCC) and Centers for AIDS Research Flow Cytometry Core Facility supported by NIH Awards CA-16042 and AI-28697, by the JCCC, the UCLA AIDS Institute and the UCLA School of Medicine. This work was supported by NIH Grant R01-AR-053542 (J.K.). Statistical analysis was funded by the UCLA Clinical and Translational Science Institute Grant UL1TR00124
Abbreviations
- ATRA
All-trans retinoic acid
- IL-17
Interleukin 17
- PBMCs
Peripheral blood mononuclear cells
- Th17
T helper 17
- TGF-β
Transforming growth factor-β
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- RORC
Retinoic orphan receptor C
- RORA
Retinoic orphan receptor A
- 1,25D3
1,25-dihydroxyvitamin D3
Footnotes
Conflicts of Interest
The authors state no conflict of interest.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nature immunology. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Barksdale L, Kim KS. Propionibacterium, Corynebacterium, Mycobacterium and Lepra bacilli. Acta Leprol. 1984;2:153–174. [PubMed] [Google Scholar]
- Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PloS one. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar MD, Ingham E. Acne: inflammation. Clin Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. Journal of immunology. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hino R, Kabashima R, Kawakami C, Sugita K, Nakamura M, Tokura Y. Circulating Th17 cell fluctuation in psoriatic patients treated with topical calcipotriol and betamethasone butyrate propionate. Journal of the European Academy of Dermatology and Venereology : JEADV. 2011;25:242–244. doi: 10.1111/j.1468-3083.2010.03714.x. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, et al. 1alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. Journal of immunology. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus stimulates production of interleukin-17 from memory but not naive T cells. Infect Immun. 2010;78:381–386. doi: 10.1128/IAI.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. International immunology. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. The Journal of investigative dermatology. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–198. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Journal of immunology. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1-and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature medicine. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Secrist H, Benson DR, Jen S, Shanebeck KD, Guderian J, et al. Variable expression of immunoreactive surface proteins of Propionibacterium acnes. Microbiology. 2006;152:3667–3681. doi: 10.1099/mic.0.29219-0. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Marinelli LJ, Fitz-Gibbon S, Hayes C, Bowman C, Inkeles M, Loncaric A, et al. Propionibacterium acnes Bacteriophages Display Limited Genetic Diversity and Broad Killing Activity against Bacterial Skin Isolates. MBio. 2012;3 doi: 10.1128/mBio.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- Modlin RL. Th1-Th2 paradigm: insights from leprosy. The Journal of investigative dermatology. 1994;102:828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- Mouser PE, Baker BS, Seaton ED, Chu AC. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J Invest Dermatol. 2003;121:1226–1228. doi: 10.1046/j.1523-1747.2003.12550_6.x. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, et al. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. Journal of immunology. 2010;184:4880–4888. doi: 10.4049/jimmunol.0901767. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nature immunology. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. The Journal of experimental medicine. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. The Journal of investigative dermatology. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Tokura Y, Mori T, Hino R. Psoriasis and other Th17-mediated skin diseases. J Uoeh. 2010;32:317–328. doi: 10.7888/juoeh.32.317. [DOI] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. Journal of immunology. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infection and immunity. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC. Is acne vulgaris a genuine inflammatory disease? Dermatology. 2001;203:277–279. doi: 10.1159/000051771. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, et al. What is the pathogenesis of acne? Experimental dermatology. 2005;14:143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.