Abstract
Objective
Although passive immunization with anti-HIV-1 Env IgG1 neutralizing monoclonal Abs (nmAbs) prevented simian-human immunodeficiency virus (SHIV) infection in rhesus monkeys (RMs), IgA nmAbs have not been tested. Here, we sought to determine whether human anti-HIV-1 dimeric (d)IgA1, dIgA2, and IgG1 differ in their ability to prevent mucosal R5 SHIV acquisition in RMs.
Design
DIgA1, dIgA2, and IgG1 versions of nmAb HGN194 were applied intrarectally (i.r.) in three RM groups 30 min before i.r. SHIV challenge.
Methods
After a control pharmacokinetic study showed that nmAb concentrations in rectal fluids over time were similar for all HGN194 isotypes, control and nmAb-treated animals were challenged i.r. with an R5 SHIV, and viral loads were monitored.
Results
Unexpectedly, dIgA1 provided the best protection in vivo – although all nmAbs showed similar neutralizing activity in vitro. Five out of the six dIgA1-treated RMs remained virus-free compared to only one out of six animals given dIgA2 (P=0.045 by log rank test) and two out of six RMs treated with IgG1 forms of the nmAb (P=0.12). Protection correlated significantly with virion capture activity by a given nmAb form, as well as inhibition of transcytosis of cell-free virus across an epithelial cell layer in vitro.
Conclusions
Our data imply that dIgA1-mediated capturing of virions in mucosal secretions and inhibition of transcytosis can provide significant prevention of lentiviral acquisition – over and above direct virus neutralization. Vaccine strategies that induce mucosal IgA, especially IgA1, should be developed as first-line of defense against HIV-1, a virus predominantly transmitted mucosally.
Keywords: R5 SHIV, immunoglobulin A isotypes, mucosal transmission, transcytosis, virion capture, HIV neutralization
Introduction
Although systemic passive immunization with IgG1 neutralizing monoclonal antibodies (nmAbs) targeting HIV-1 Env prevented simian-human immunodeficiency virus (SHIV) infection in rhesus macaques (RMs) [1-5], IgA nmAbs have not been tested in vivo. Human IgA consists of two subclasses, IgA1 and IgA2. While IgA1 predominates in blood, IgA2 is more prevalent in mucosal secretions, where both IgA isoforms are present as dimeric or polymeric secretory IgA (sIgA), having acquired J chain during synthesis and secretory component (SC) during epithelial transport. Dimeric IgA1 (dIgA1) has a longer hinge region, resulting in a 16 ± 3 nm distance between two Fab fragments [6] compared to 10 ± 2 nm in dIgA2 [7] (Fig. 1a,b). Anti-HIV-1 Env IgAs have been found in cervicovaginal washes of highly exposed persistently seronegative women (HEPS) [8]; recent data implied the presence of HIV-1 cervicovaginal neutralizing IgA1 in such individuals based upon the IgA isolation with jacalin, a lectin that preferentially binds to O-linked glycans present in the hinge region of IgA1 but not in IgA2 or IgG1 [9]. Moreover, it was shown that exposed, uninfected individuals developed anti-HIV-1 Env IgA1 after oral HIV-1 exposure [10]. Dimeric and monomeric IgA2 and IgG1 forms of b12 (an anti-CD4 binding site nmAb), were equally protective against epithelial adherence of HIV-1 in vitro [11], but dIgA1 and dIgA2 have not been directly compared, either in vitro or in vivo.
Fig. 1. HGN194 dIgA1, dIgA2, and IgG1 have relatively similar neutralization profiles and concentrations in rectal fluids after topical administration.
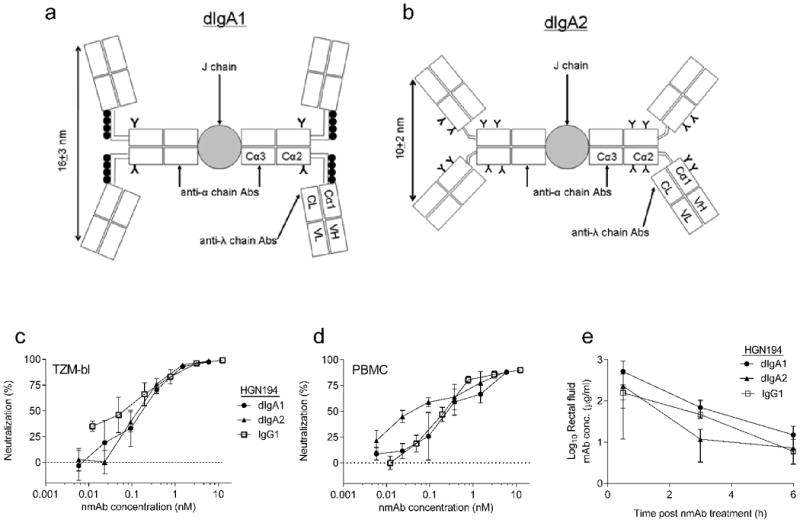
(a) Schematic representation of human dIgA1 and dIgA2 (b); (•) and (Y) indicate O-glycans and N-glycans, respectively. (c) Neutralization (TZM-bl assay) of SHIV-1157ipEL-p (challenge virus) by various HGN194 forms. Data represent results obtained from two independent experiments. (d) Neutralization (hPBMC assay) of SHIV-1157ipEL-p by various HGN194 forms. Data represent results obtained from two independent experiments. IgG1 VRC01 and Fm-6 (an anti-SARS IgG1 isotype control mAb), positive and negative controls for both assays, respectively (data not shown). (e) Pharmacokinetic study: average HGN194 concentrations in RM rectal fluids from each Ab-treated group (n = 4).
Only few vaccine efficacy studies have examined whether anti-HIV-1 Env IgA responses are correlated with protection. Bomsel et al. [12] described a link between mucosal IgA responses and protection as measured by IgA-mediated inhibition of transcytosis in vitro and protection from repeated intravaginal challenges of RMs vaccinated with HIV-1 gp41-bearing virosomes. In contrast, serum anti-HIV-1 Env IgA responses in the RV144 vaccine efficacy trial were associated with an increased risk of HIV-1 acquisition [13], whereas systemic IgG responses were significantly linked to a lower risk of HIV-1 acquisition. The RV144 results led to the hypothesis that anti-HIV-1 IgA responses may diminish the protective effects of IgG [13]. However, neither anti-HIV-1 IgA subclasses nor mucosal Ab responses were evaluated in RV144.
Here, we sought to test whether monoclonal anti-HIV-1 dIgA1, dIgA2, and IgG1 with the same epitope specificity differed in their ability to prevent mucosal R5 SHIV acquisition in RMs. Earlier, we showed that a high dose of intravenously administered IgG1 HGN194, which targets the conserved crown of the V3 loop [14], protected across clades against intrarectal (i.r.) R5 SHIV challenge [4]. We then generated different recombinant HGN194 isotypes - IgG1, dIgA1, and dIgA2 (Fig. 1a,b) - and evaluated their antiviral activity.
Methods
Cell lines, reagents, and virus
TZM-bl cells were purchased from the NIH AIDS Research and Reference Reagent Program (ARRRP). MAb Fm-6 and VRC01 were kindly provided by Drs. Wayne Marasco (Dana-Farber Cancer Institute) and John Mascola (Vaccine Research Center, NIH), respectively. gp1201157ip was prepared as previously described [15]. The SHIV-1157ipEL-p stock (grown in RM PBMC) had a p27 concentration of 792 ng/ml and 7.8 × 105 50% tissue culture infectious doses (TCID50)/ml as measured in TZM-bl cells.
In vitro neutralization assays
MAbs were incubated with virus for 1 h at 37°C and cells were added. The TZM-bl assay was performed as described [16]. The human PBMC assay was performed as reported [4].
Passive immunization and mucosal SHIV-1157ipEL-p challenge
All primate studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the U.S. (see supplemental digital content). First, four adult RMs per group were enrolled in an independent pharmacokinetic study with HGN194 dIgA1, dIgA2, and IgG1. For each Ab-treated group, 1.25 mg (in 2.1 ml phosphate-buffered saline (PBS)) of nmAb was applied intrarectally (i.r.), and rectal fluids were collected prior and 30 min, 3 h, 6 h, and 24 h after nmAb treatment (Table S1).
Next, six infant RMs per group were treated i.r. once with 1.25 mg (in 2.1 ml of PBS) of: HGN194 dIgA1 (Group 1); HGN194 dIgA2 (Group 2); and HGN194 IgG1 (Group 3). Eleven untreated animals served as controls (Group 4). All monkeys were challenged i.r. with 31.5 50% animal infectious doses (AID50) of SHIV-1157ipEL-p [17].
All infant RMs aged between 8 to 12 months at time of challenge and were Mamu B08 and B17 negative. Mamu A01-positive RMs were evenly distributed in each group, as were RMs with different FcγRIIIa genotypes (Table S2).
Binding of HGN194 isotypes to gp1201157ip, and HGN194 nmAb concentrations in RM rectal fluids
Costar 96-well plates were coated with gp1201157ip (1 μg/ml) ON at 4°C. After blocking, HGN194 nmAbs were applied for 1 h at room temperature, and binding was detected with goat polyclonal anti-human HRP-Abs directed either against the α chain (IgA-specific) (Rockland) or the λ chain (Millipore). Detection of HGN194 nmAbs in plasma and rectal fluids was performed with polyclonal goat anti-human IgG-specific or α chain-specific (IgA specific) HRP Abs (Rockland). HGN194 dIgA1 or dIgA2 or IgG1 were included as standard ranging from 7.8 to 0.2 ng/ml for IgG1, and 60 to 1 ng/ml for dIgA1 and dIgA2. TMB substrate (Invitrogen) was added and 5 min later, the reaction was stopped with 1 N sulfuric acid and OD values were measured at 450 nm with a microplate reader (Berthold).
Assessment of plasma viral RNA levels
Plasma viral RNA levels were measured as described [18, 19].
Virion capture assay
96-well plates (Costar) were coated with α chain-specific goat anti-human serum IgA or Fc-specific goat anti-human IgG Abs (Jackson Immunoresearch) overnight (ON) at 4°C. Following blocking, nmAbs were added at 5 μg/ml for 1 h at room temperature. SHIV-1157ipEL-p was added ON at 37°C. After incubation for 1 h with 0.5% Triton, the amount of virus captured by the nmAbs was measured by p27 assay (ABL).
Inhibition of transcytosis
HEC-1A cell (ATCC) monolayers were created on 0.4 μm polyethylene terephthalate (PET) membrane-hanging transwell inserts (Millipore). Electrical resistance of >400 mOhms/cm2 across the membrane confirmed monolayer integrity. Cell-free SHIV-1157ipEL-p (2 ng/ml of p27) was preincubated for 1 h at 37°C alone or with various concentrations of HGN194 dIgA1, dIgA2, or IgG1, or control IgG1 (anti-dengue virus 3, a kind gift from Dr. Dennis Burton, Scripps Research Institute). Next, virus or virus/nmAb mixtures were added to the apical surface of the cell monolayer in the upper chamber. After 12 h, fluid in the lower chamber (“subnatant fluid”) was collected and used to measure viral RNA copy numbers by RT-PCR [18, 19].
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5 for Windows (GraphPad Software Inc.). The Wilcoxon rank sum test was used to compare HGN194 nmAb concentrations in rectal fluids and the amounts of virus captured by HGN194 nmAb dIgA1 versus dIgA2.
Results
HGN194 dIgA1, dIgA2, and IgG1: neutralization of SHIV-1157ipEL-p and pharmacokinetics after topical administration
First, we compared the neutralization potency of the different HGN194 forms (Fig. 1a,b) against the R5-tropic clade C SHIV-1157ipEL-p [17], the intended challenge virus. In TZM-bl cells, all isotypes exhibited similar neutralization profiles (IC50 values, 0.08-0.12 nM) and completely neutralized SHIV-1157ipEL-p (Fig. 1c). Similar neutralization results were obtained in a PBMC-based assay (Fig. 1d). Next, a pharmacokinetic study was performed with adult RMs to determine nmAb concentrations in rectal fluids of animals treated i.r. with 1.25 mg of HGN194 dIgA1, dIgA2, or IgG1 (Fig. 1e). Notably, RMs only have one IgA isotype that resembles human IgA2, with a hinge region shorter than that in human IgA1. To measure whether human dIgA1 and dIgA2 were equally stable after i.r. application, rectal fluids were collected before nmAb treatment, and 30 min, 3 h, 6 h and 24 h post-treatment (Table S1). At 30 min after nmAb delivery, we observed no significant differences in rectal fluid nmAb concentrations among the three groups (dIgA1/dIgA2, P=0.343, dIgA1/IgG1, P=0.486, dIgA2/IgG1, P=0.686). Because the IgA1 hinge region is known to be more susceptible to proteolysis by bacterial enzymes than that of IgA2, we assessed the intactness of dIgA1 and dIgA2 in rectal fluids collected 30 min after i.r. nmAb application. Notably, proteolysis of the two IgA isotypes did not differ (data not shown). Given these control data, we decided to perform the i.r. SHIV challenge 30 min after the i.r. passive immunization.
Passive immunization with HGN194 dIgA1, dIgA2, and IgG1 against R5 SHIV-1157ipEL-p
We then enrolled three groups of six RM infants and treated them i.r. with 1.25 mg of HGN194 dIgA1, dIgA2, or IgG1 at 30 min before i.r. challenge with SHIV-1157ipEL-p (31.5 AID50). Interestingly, five out of six dIgA1-treated RMs remained aviremic throughout (Fig. 2a), while only one out of six dIgA2-treated RMs (Fig. 2b) and two out of six IgG1-treated monkeys (Fig. 2c) remained virus-free. All controls were viremic by week 2 (Fig. 2d) (viral RNA loads, 4.5 × 105- 1.9 × 107 copies/ml). The time to viral load >50 copies/ml was compared using the log rank test (two-sided P-values). Clearly, HGN194 dIgA1 conferred better protection than dIgA2 against mucosal SHIV acquisition (P = 0.045); compared to IgG1, a trend towards better protection with dIgA1 was observed (P = 0.115) (Fig. 2e).
Fig. 2. HGN194 dIgA1 protects rhesus macaques significantly better than its dIgA2 form against SHIV acquisition as shown by passive intrarectal (i.r.) immunization.
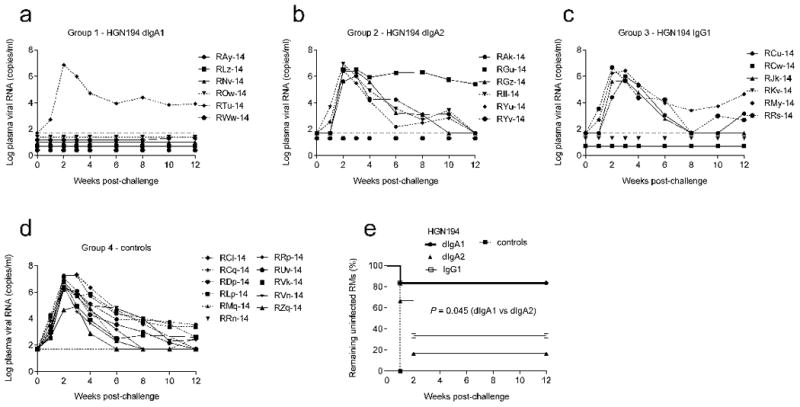
RM infants received topical treatment with the different HGN194 versions (1.25 mg in 2.1 ml) 30 min before challenge with high-dose SHIV-1157ipEL-p; (a) HGN194 dIgA1 (Group 1; n = 6), (b) HGN194 dIgA2 (Group 2; n = 6), (c) HGN194 IgG1 (Group 3; n = 6), and (d) none (control Group 4; n = 11). Viral RNA loads were measured by RT-PCR (detection limit: 50 copies/ml [18, 19]). (e) Percentage of RMs remaining uninfected over time by Kaplan-Meier plot.
Capture of SHIV-1157ipEL-p by HGN194 dIgA1, dIgA2, and IgG1
Prompted by this surprise finding, we assessed the ability of the different HGN194 versions to retain cell-free virus by virion capture assay (Fig. 3a). Although all forms bound free virions, HGN194 dIgA1 captured significantly more virus than dIgA2 at both virus concentrations tested (P = 0.029; Wilcoxon rank sum test). We verified by ELISA that the anti-Cα Ab used to capture dIgAs on the plates bound equal amounts of either dIgA1 or dIgA2 (data not shown). HGN194 IgG1 could not be compared directly to the dIgA forms, as a different capture Ab was used.
Fig. 3. HGN194 dIgA1 captures significantly more virions than dIgA2 and blocks transcytosis.
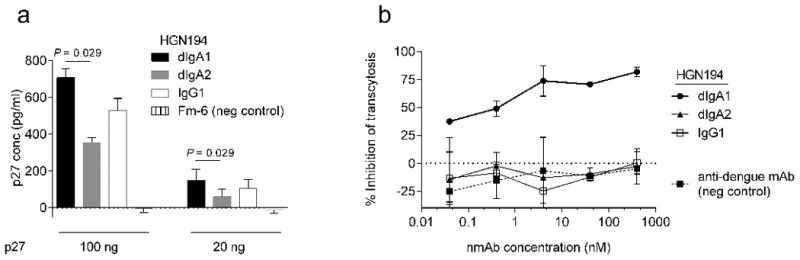
(a) Virion capture assay using SHIV-1157ipEL-p and the HGN194 forms listed; HGN194 IgG1 could not be compared to dIgA forms as a different capture Ab was used. (b) Inhibition of cell-free SHIV-1157ipEL-p transcytosis across a tight epithelial barrier; anti-SARS IgG1 Fm-6 and anti-dengue IgG1, negative controls.
Inhibition of transcytosis of cell-free SHIV-1157ipEL-p by HGN194 dIgA1, dIgA2, and IgG1
Inhibition of the transcytosis of cell-free virus across polarized epithelial cells was then tested with the three HGN194 versions, using the anti-dengue virus IgG1 mAb as negative control (Fig. 3b). Only HGN194 dIgA1 blocked transcytosis of cell-free SHIV-1157ipEL-p, the challenge virus, whereas dIgA2, and IgG1 showed the same levels as the unrelated anti-dengue control mAb. These data indicate that only HGN194 dIgA1 inhibited transcytosis of cell-free virus across an epithelial cell layer in vitro. Our findings regarding IgG1 are in line with a previous study that used cell-free virus, in which none of the IgG1 nmAbs tested inhibited transcytosis, including b12 and 2F5 [20].
Binding of HGN194 dIgA1, dIgA2, and IgG1 for monomeric gp1201157ip
Finally, we analyzed the binding of each isotype to gp1201157ip, the HIV-1 clade C Env carried by the challenge virus, SHIV-1157ipEL-p. By surface plasmon resonance, HGN194 IgG1 showed better binding to monomeric gp1201157ip immobilized on the CM5 chip than either dIgA1 or dIgA2 (Fig. 4a). Steric hindrance may decrease access to the epitope for dimeric Abs in comparison to IgG1. When dIgA1 and dIgA2 were directly immobilized on the CM5 chip, binding of monomeric gp1201157ip as analyte to each Ab was comparable as judged by the response units (Fig. 4b). Moreover, there were no differences in avidity of the three HGN194 mAbs to gp1201157ip (Fig. S1). We also analyzed HGN194 isotype binding to gp1201157ip immobilized on ELISA plates with Abs directed either against a constant region on the human IgA α chain (IgA specific; Figs. 1a, b, arrows) or the human λ chain (recognizing all isotypes; Figs. 1a, b, arrows). When detection was performed with polyclonal anti-human λ-chain Abs, dIgA1 seemingly bound better to gp120 than did IgG1 and dIgA2 (Fig. 4c). However, consistent with the Biacore data, detection with polyclonal anti-human IgA α-chain Abs showed similar binding of dIgA1 and dIgA2 to gp1201157ip (Fig. 4d), implying that equal numbers of dIgA1 and dIgA2 molecules were bound to gp120 immobilized on the plates. This finding suggests that the secondary anti-λ Abs may have better access to the cognate λ constant region in dIgA1 compared to dIgA2. The latter has a substantially shorter hinge region that results in tighter stacking of the Fabs and Fc part, thereby compromising access of the secondary anti-λ Abs to the λ constant region. Furthermore, the IgA2 Cα1 region has two potential N-linked glycosylation sites versus none in the dIgA1 isotype. Consequently, sugar moieties could also hinder access of the secondary anti-λ Abs in dIgA2 (Fig. 1b) – and possibly also access of antigen to the antigen-binding sites. Together, these data indicate that the HGN194 IgA isotypes had similar binding properties for monomeric gp1201157ip.
Fig. 4. HGN194 dIgA1 and dIgA2 have similar affinities for monomeric gp1201157ip.
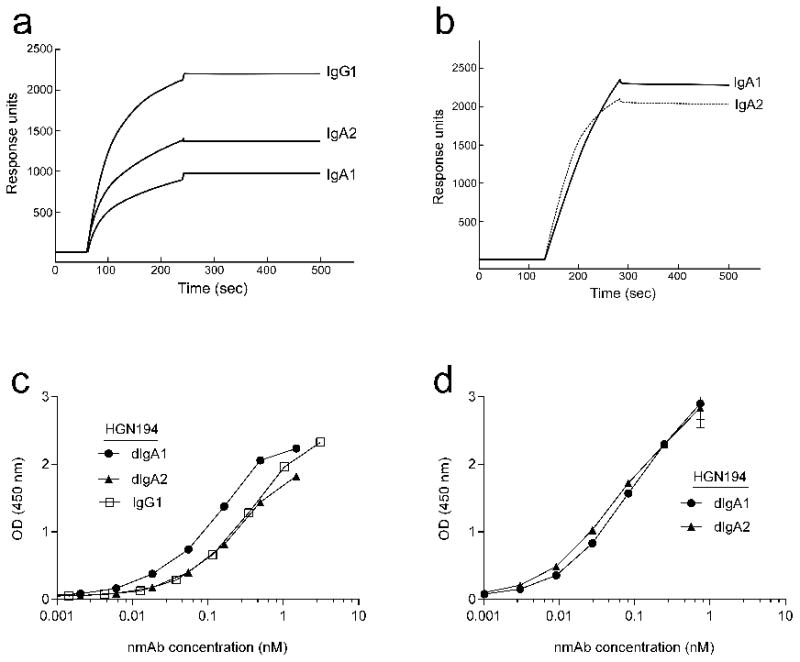
(a) Surface plasmon resonance (SPR) analysis: HGN194 dIgA1, dIgA2, and IgG1 binding to monomeric gp1201157ip. Gp1201157ip was immobilized on a CM5-chip and Abs flowed over as analytes, with association and dissociation phases of up to 3 min (flow rate, 30 μl/min). Qualitative SPR responses are shown for IgG1, dIgA1 and dIgA2 analytes (60 nM) interacting with chip-bound, soluble monomeric gp1201157ip. (b) SPR analysis of binding of soluble monomeric gp1201157ip to chip-immobilized HGN194 dIgA1 and dIgA2. Abs were coupled to a CM5-chip to a surface density of 5,600 response units (RU) for dIgA1 and 4,800 RU for dIgA2, respectively. Different concentrations of gp1201157ip were injected as analyte in randomized duplicate runs for 3 min (flow rate, 30 μl/min) and representative sensograms of gp1201157ip binding (20 nM) to dIgA1 and dIgA2 are shown. (c) ELISA binding of HGN194 isotypes to gp1201157ip: detection with goat anti-human λ chain Abs, and (d) goat anti-human α-chain specific. The data shown are representative of two independent experiments.
Discussion
Our data showed that anti-HIV-1 dIgA1 provided significantly better protection in vivo than dIgA2 and IgG1 (Fig. 2), although the different HGN194 forms did not differ in their ability to neutralize the challenge virus (Fig. 1c, d). Notably, HGN194 dIgA1 was able to capture significantly more virions than HGN194 dIgA2 in vitro (Fig. 3a), making less cell-free virus available for transcytosis. Only HGN194 dIgA1 inhibited transcytosis of cell-free virus (Fig. 3b). In our assay, pre-incubation of nmAbs with virus allows the capture and possible aggregation of virus, mimicking the first step of virus encounter that occurs in the mucus. The better ability of HGN194 dIgA1 to capture virus particles in vitro translated in vivo to better protection against SHIV transmission.
We propose that due to its longer hinge and the more open conformation of the latter, dIgA1 may be able to bind one virion per Fab – maximally four per dIgA1. Our data hinted that dIgA2 could only capture approximately half of the amount of virus compared to dIgA1 (Fig. 3a), which may be due to only two virions being able to bind to dIgA2. Interestingly, the inter-Fab distance in dIgA1 of 16 ± 3 nm (Fig. 1a) is compatible with the peak distance between different spikes on a given HIV-1 virion (˜15 nm) [21], whereas the 10 ± 2 nm distance in dIgA2 is not (Fig. 1b). We postulate that the inter-spike distance is the minimal distance needed that will allow one virion to bind to each Fab of the four Fabs in dIgA1 when fully occupied. Although differences in inter-Fab distances may also result in different epitope specificities among Ab isotypes [22], this was not the case with our HGN194 isotypes as demonstrated by peptide competition in TZM-bl neutralization assays (Fig. S2). The differences in protection of the experimental RMs could also not be ascribed to differences in the interaction of the HGN194 dIgA isotypes with the RM CD89 IgA Fcα receptor; dIgA1 and dIgA2 bound similarly to CD89-expressing cells by flow cytometry (Fig. S3).
To conclude, this is the first demonstration of significant biological differences between the two human IgA isotypes with regard to their ability to provide protection against mucosal R5 SHIV challenge in vivo despite identical epitope specificity and in vitro virus neutralization. We linked better prevention of virus acquisition in the primates to a differential ability of the two dIgA isotypes to capture cell-free virions, therefore blocking transcytosis of cell-free virus across an intact epithelial cell layer in vitro. These data are of importance to HIV-1 vaccine development as they suggest that mucosal IgA isotypes should be examined in future vaccine trials and that vaccine strategies should be devised to preferentially induce the more protective antiviral IgA1 responses.
Supplementary Material
Acknowledgments
We thank Drs. Marian Neutra and Richard Blumberg for critical reading of this manuscript and Wayne Marasco (Boston) for mAb Fm-6. We thank Stephanie Ehnert, Chris Souder, and Kalpana Patel (Yerkes National Primate Research Center, (YNPRC)) for conducting the passive immunization and the Resource for Nonhuman Primate Immune Reagents for performing PK studies.
Funding support: This work was supported by the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) UCL-VDC grant 38637 (R.A.W.) and CAVD 50314 (E.L.R.), by NIH grants R37 AI34266 and R01 DE023049 (R.M.R.), P01 AI48240 (R.M.R. and S.-L.H.) and by ORIP P51 000165 awarded to the YNPRC and R24OD010947 (F.V.).
Footnotes
Conflicts of Interest: A. Lanzavecchia is the scientific founder of Humabs LLC, a company that develops human antibodies for treatment of infectious diseases. D. Corti, and G. Agatic are currently employees of Humabs. A. Lanzavecchia hold shares in Humabs.
Contributors: All authors participated in the critical review of the report. JDW, DC, AL, RAW, JLH, FV, QS RMR designed the study. JDW, AMS, MMM, NBS, SKL, VS, MK, SG, KR, DC, GH, BCB, KK, and GA performed experiments and analyzed data; ELR, DNF and DK analyzed data; SL performed statistical analysis; SLH contributed key reagents; JDW, AMS and RMR wrote the report.
References
- 1.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 3.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 4.Watkins JD, Siddappa NB, Lakhashe SK, Humbert M, Sholukh A, Hemashettar G, et al. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS One. 2011;6:e18207. doi: 10.1371/journal.pone.0018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer VG, Siddappa NB, Ruprecht RM. Passive immunization as tool to identify protective HIV-1 Env epitopes. Curr HIV Res. 2007;5:642–655. doi: 10.2174/157016207782418506. [DOI] [PubMed] [Google Scholar]
- 6.Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol. 2008;180:1008–1018. doi: 10.4049/jimmunol.180.2.1008. [DOI] [PubMed] [Google Scholar]
- 7.Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J Biol Chem. 2009;284:5077–5087. doi: 10.1074/jbc.M807529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo JJ, Plummer F, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14:1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 9.Choi RY, Levinson P, Guthrie BL, Payne B, Bosire R, Liu AY, et al. Cervicovaginal HIV-1 neutralizing IgA detected among HIV-1-exposed seronegative female partners in HIV-1-discordant kenyan couples. AIDS. 2012 doi: 10.1097/QAD.0b013e328359b99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselrot K, Saberg P, Hirbod T, Soderlund J, Ehnlund M, Bratt G, et al. Oral HIV-exposure elicits mucosal HIV-neutralizing antibodies in uninfected men who have sex with men. AIDS. 2009;23:329–333. doi: 10.1097/QAD.0b013e32831f924c. [DOI] [PubMed] [Google Scholar]
- 11.Mantis NJ, Palaia J, Hessell AJ, Mehta S, Zhu Z, Corthesy B, et al. Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region. J Immunol. 2007;179:3144–3152. doi: 10.4049/jimmunol.179.5.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen RA, Ong H, Kittel C, Ruprecht CR, Ferrantelli F, Hu SL, et al. DNA prime/protein boost immunization against HIV clade C: safety and immunogenicity in mice. Vaccine. 2006;24:2324–2332. doi: 10.1016/j.vaccine.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 16.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Unit. 2005;Chapter 12(Unit 12):11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 17.Siddappa NB, Watkins JD, Wassermann KJ, Song R, Wang W, Kramer VG, et al. R5 clade C SHIV strains with tier 1 or 2 neutralization sensitivity: tools to dissect env evolution and to develop AIDS vaccines in primate models. PLoS One. 2010;5:e11689. doi: 10.1371/journal.pone.0011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, et al. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000;16:1247–1257. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- 19.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Chomont N, Hocini H, Gody JC, Bouhlal H, Becquart P, Krief-Bouillet C, et al. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 22.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, et al. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A. 2012;109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


