Abstract
Inflammasomes are increasingly implicated in regulating immunity, but how their activation relates to function of human dendritic cells (DCs) is unknown. Here we show that DC maturation stimuli lead to rapid activation of caspase-1 in human monocyte-derived DCs. RNAi mediated inhibition of the inflammasome component ASC leads to marked inhibition of the capacity of lipopolysachharide (LPS)-matured DCs to stimulate antigen-specific T cells. RNAi mediated inhibition of Cathepsin B (CatB) also similarly inhibits the capacity of human DCs to stimulate immunity. The defective ability of ASC or CatB deficient DCs to stimulate T cells is independent of inflammasome-mediated processing of inflammatory cytokines and also includes DCs loaded with pre-processed peptide. Gene expression profiles of ASC or CatB deficient human DCs show marked overlap with downregulation of genes implicated in DC function. These data demonstrate an important role for ASC and CatB in regulating function of human DCs with overlapping effects on gene expression.
Keywords: human, dendritic cells, maturation, immunity, inflammasome
1.0. Introduction
Dendritic cells are potent antigen presenting cells (APCs) that provide a critical link between innate and adaptive immunity[1]. DCs can integrate diverse signals from pathogens or cellular damage leading to DC maturation, wherein they acquire capacity to serve as potent APCs[1].
Proteins of the NOD like receptor(NLR) family form a complex termed the inflammasome involved in the processing of inflammatory cytokines (particularly IL1β and IL18)[2]. Activation of inflammasomes is thought to involve an initial priming phase leading to the expression of inflammasome components and cytokine precursors, followed by activation phase, which leads to processing of mature cytokines[2]. Several models have been postulated to help explain inflammasome activation including potassium efflux, activation of reactive oxygen species (ROS), as well as release of cathepsinB (CatB) into the cytosol [2].
An important feature of DC maturation is the activation of lysosomes and enhanced lysosomal acidification, leading to enhanced activity of cysteine proteases, including cathepsins[3]. Maturation-associated lysosome activation likely activates several proteases [4, 5] but whether a single cathepsin plays a major role in regulating human DC function is not known.
Recent studies have implicated inflammasomes in the mechanism of action of chemical adjuvants, adaptive immunity to pathogens and tumors, as well as in autoimmunity [6–10]. In view of the central role of DCs in adaptive immunity, it is critical to understand how inflammasome activation relates to DC maturation in humans. Differentiation of DCs from monocytes serves as a useful model for inflammation associated DCs in both humans and mice [11]. Here we have examined the role of a key inflammasome component, apoptosis-associated speck-like protein containing CARD (ASC) and CatB (a protease implicated in inflammasome activation) in regulation of maturation and function of human monocyte-derived DCs.
2.0. Materials and Methods
2.1. Generation of DCs
Blood samples were obtained from healthy donors following informed consent approved by Yale IRB. DCs were generated from CD14+ monocytes cultured in the presence of GM-CSF and IL4 as described [12]. On day 5, immature DCs were used directly or matured overnight by either an inflammatory cytokine cocktail (IL-1β, IL-6, TNF-a and prostaglandin E2; Cyt-DCs) or 50ng/ml ultra-pure LPS (Sigma, St Louis, MO), as described [12]. Phenotype of DCs was assayed by flow cytometry after staining with antibodies against CD80, CD83 and CD86 (all from BD). Data were analyzed with Flowjo software (Tree Star, Ashland, OR).
2.2. Detection of activated Caspase-1
Activation of Caspase-1 was assayed with the FAM-YVAD-FMK Caspase-1 Detection Kit (Cell Technology Inc, Mountain View, CA). FAM-YVAD-FMK was added to the culture for 1 hr as per manufacturers protocol, washed twice with caspase-1 kit wash buffer, and detected using flow cytometry.
2.3. RNAi mediated inhibition of ASC and cathepsin B in dendritic cells
ON-TARGETplus SMARTpool SiRNAs (Dhamacon, Lafayette, CO) were used to inhibit human ASC (Human PYCARD, NM_145183) or CatB (HumanCTSB, NM_001908). CD14+ cells separated from magnetic column were resuspended at 105 cells per μl in phenol red free Opti-MEM® reduced-serum medium (Invitrogen, Carlsbad, CA). Cells were electroporated with 20 ng SiRNA in 0.4mm gap electroporation cuvettes using BTX ECM830 (Harvard Apparatus, Holliston, MA) at 500v, 2 pulses of 500ms. Inhibition of target genes was confirmed by western blot.
2.4. Detection of IL-1 β
For activation of the inflammasome, DCs were stimulated with 50 ng/ml LPS for 4 hours, then with 1 mM ATP for 15 minutes. Cell culture supernatant was assayed using the luminex system. For Luminex assay IL-1β antibody conjugated beads were used with the MILLIPLEX MAP Kit (Millipore, Billerica, MA) using LUMINEX100 System (Luminex Corporation, Austin, TX).
2.5. Allogeneic mixed lymphocyte reaction
CFSE labeled T cells were co-cultured with allogeneic DCs at T:DC ratio of 30:1 and the T cell proliferation was assayed 5–7 days later using flow cytometry.
2.6. Activation of influenza specific T cells
DCs from HLA-A2+ donors were infected with influenza virus (Influenza X-31, Charles River) or pulsed with HLA-A2-restricted flu-matrix peptide (Flu-MP) GILGFVFTL58–66 (5 μg/ml) prior to use as APCs for stimulation of influenza specific T cells at APC:T cell ratio of 1:30. IL2 (20 U/ml) was added on day 4 of culture. After 7–10 days of culture, the proliferation of flu virus specific T cells was analyzed by FACS using PE conjugated flu-matrix peptide GILGFVFTL bound HLA-A2 tetramer (Beckman Coulter, Brea, CA) and antibodies to CD8, CD3 and CD4.
2.7. SDS-PAGE and Western blotting
Cells were lysed in RIPA lysis buffer (Millipore, Temecula, CA), proteins were separated on NuPAGE 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to 0.45μm pore size Immobilon-P PVDF membrane (Millipore, Billerica, Massachusetts) using the Mini-PROTEAN Tetra System(Bio-Rad, Hercules, CA). The target proteins were then detected with HyGLO quick spray Chemiluminescent HRP antibody detection reagents (Denville scientific inc, Metuchen, NJ) and imaged with Bio-Rad ChemDoc XRS western blot detection system (Bio-Rad, Hercules, CA). The primary antibodies used were: rabbit polyclonal anti-human CatB and ASC (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit monoclonal anti-human cleaved IL-1β and β-Actin (Cell Signaling Technology, Danvers, MA), mouse monoclonal anti-human pro IL-1β (R&D, Minneapolis, MN). Secondary antibodies used include donkey anti-mouse IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA) and Anti-rabbit IgG HRP-linked Antibody (Cell Signaling Technology, Danvers, MA).
2.8. Gene expression profiling
CD14+ monocytes were electroporated with either non targeting (NT) control, ASC or CatB RNAi and then cultured with GMCSF and IL4 to generate immature DCs. Day 5 DCs were harvested and RNA was analyzed for gene expression profile using U133 plus 2 Affymetrix microarrays. Analysis of microarray data was carried out using Partek Genomics Suite. RNA normalization was used to normalize the raw expression data. We excluded the genes with fold change less than 1.5 fold and the expression less than 100 in one of the experimental conditions in each comparison to identify common differentially expressed genes to the comparisons between ASC vs. NT and CAT vs. NT. Genes with p values <0.05 for fold change compared to NT treated DCs were selected.
2.9. Statistical analysis
Data for treatment and control groups were compared using Student t test and p values lower than 0.05 were considered significant.
3.0. Results
3.1. Maturation induced activation of caspase-1
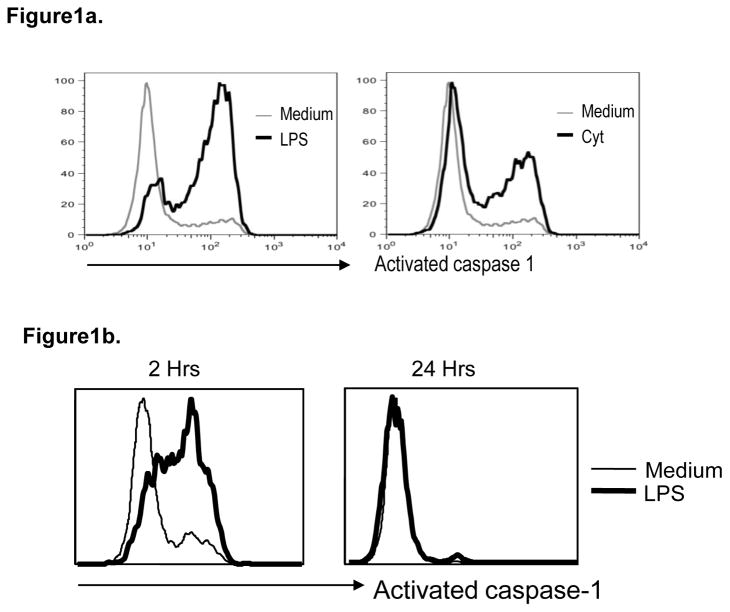
Exposure of monocyte-derived DCs (Mo-DCs) to both TLR ligands and inflammatory cytokines leads to DC maturation. Treatment of immature DCs (iDCs) with either LPS or cytokines led to a rapid but transient increase in activated caspase-1 detectable within 2 hours (Figure 1a), but returning to baseline by 24 hours (Figure 1b). Together these data show that transient activation of caspase-1 is an early feature of DC maturation.
Figure 1. Activation of caspase 1 with maturation of human Mo-DCs.
Monocyte derived immature DCs were cultured alone (Medium) or in the presence of lypopolysaccharide (LPS) or inflammatory cytokines (Cyt). Induction of activated caspase-1 was detected using flow cytometry.
a. Figure shows induction of activated caspase-1 in DCs at 2 hrs after treatment with both LPS as well as inflammatory cytokines.
b. Figure shows induction of activated caspase-1 in DCs at 2 and 24 hrs after treatment with LPS.
3.2. Deficient T cell stimulation by ASC deficient DCs
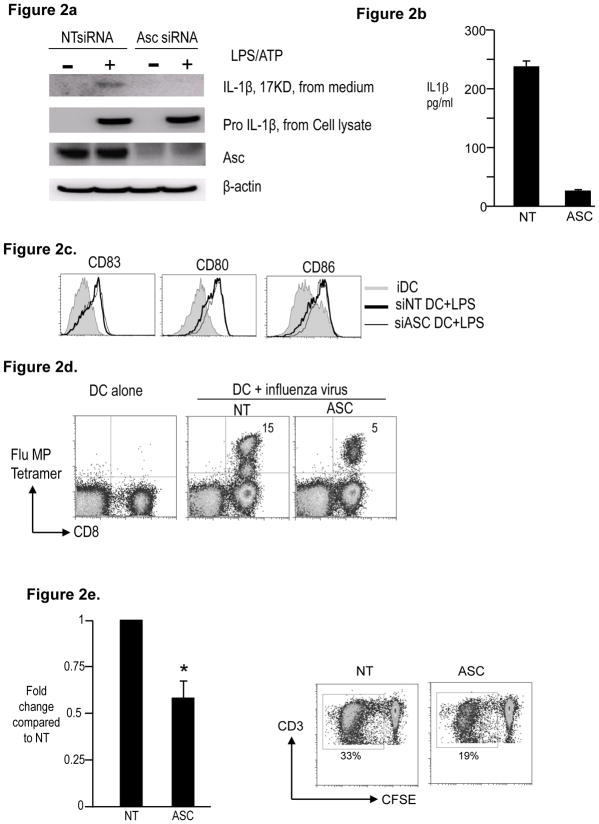
The temporal association between DC maturation and caspase-1 activation suggested that early activation of the inflammasome may play a role in DC function. Therefore we analyzed the effect of inhibition of ASC, an integral inflammasome component, on DC maturation. As expected, RNAi mediated knockdown of ASC inhibited IL1β (but not pro-IL1β) in response to LPS, indicating inhibition of the inflammasome (Figure 2a, 2b). Similar reduction in IL1β was observed in influenza infected DCs (Figure 2c). ASC deficiency did not alter LPS-induced upregulation of HLADR, CD80/86 and CD83 (Figure 2d), suggesting that ASC is not required for these phenotypic changes observed with DC maturation. However ASC inhibition led to clear decline in stimulation of influenza matrix peptide (MP)-specific CD8+ T cells by influenza-infected DCs (Figure 2e). ASC-deficient DCs are also less efficient at inducing stimulation of allogeneic T cells in a MLR (Fig 2f). Therefore these data show that ASC deficiency leads to inhibition of the capacity of human DCs to stimulate T cell immunity.
Figure 2. Effect of RNAi mediated inhibition of ASC on maturation and function of human Mo-DCs.
HLA A2.1+ CD14+ monocytes obtained from peripheral blood were electroporated using ASC siRNA or a control non-targeting (NT) siRNA. The cells were cultured in 1% plasma supplemented with IL4 and GMCSF. On day 5, DCs were matured as indicated.
a. NT or ASC siRNA treated DCs were cultured alone or in the presence of LPS and ATP. The cells were examined for the presence of ASC and pro- IL1β protein.β-actin was used as loading control. The cell supernatant was examined for the presence of 17KD active form of IL-1β
b. Some of the supernatant as in Figure 2a was also examined for the presence of IL1β by luminex.
c. Day 5 immature NT or ASC siRNA treated DCs were infected with influenza virus for one hour and matured overnight using LPS. Cell culture supernatants were harvested at 24 hrs and assayed for the presence of IL1β using the luminex assay.
d. NT or ASC siRNA treated DCs were cultured in medium alone (iDC) or in the presence of LPS (LPS). After 24 hours, the expression of CD80, CD83, CD86 and HLADR was monitored using flow cytometry. Figure is representative of 5 similar experiments.
e. NT or ASC siRNA treated DCs were infected with influenza virus, matured with LPS and then used to stimulate autologous T cells. Some T cells were cultured with uninfected autologus DCs (DC alone). The presence of Flu MP specific CD8+ T cells was examined using the HLA A2.1 specific Flu MP tetramer by flow cytometry. The numbers in the figure represent tetramer positive CD3+ T cells. The figure is representative of 3 different donors.
f. NT or ASC siRNA treated day 5 iDCs were co-cultured with CFSE labeled allogenic T cells at a ratio of 1:30. 5 days later proliferating CD3+ T cells were enumerated using flow cytometry analysis of the CFSE diluting CD3+ T cells. Figure shows the ability of ASC siRNA treated DCs to stimulate Allo-MLR as compared to those treated with control NT siRNA. Figure represents 4 different experiments. * p<0.05
3.3. Deficient T cell stimulation by CatB deficient DCs
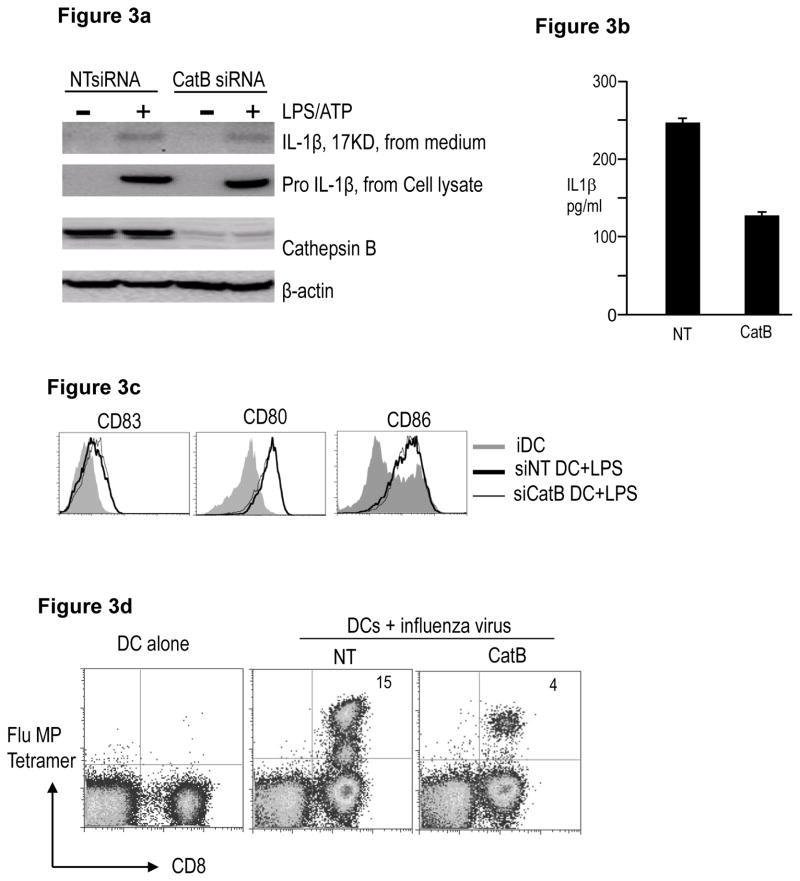
Prior studies have suggested a role for lysosomal injury and cytosolic release of cathepsin B (CatB) in some models of inflammasome activation [13]. As lysosomal acidification and protease activation are common features of DC maturation, we reasoned that CatB may be involved in this process. RNAi mediated inhibition of CatB led to partial reduction in IL1β production in response to LPS/ATP (Figure 3a, 3b), which is consistent with the finding in other studies that CatB deficient macrophages express IL1β in response to particulate NLRP3 agonists[14, 15]. CatB deficiency also led to partial reduction in IL1β production by influenza infected DCs (Figure 3c). As with ASC deficient DCs, inhibition of CatB did not inhibit LPS induced up regulation of HLADR, CD80/CD86 and CD83 (Figure 3d). However following influenza infection, CatB deficient DCs also had reduced capacity to stimulate influenza MP-specific CD8+ T cells (Figure 3e). Taken together, these data suggest that as with ASC, CatB deficient DCs are also defective in their capacity to stimulate antigen-specific T cells.
Figure 3. Effect of RNAi mediated inhibition of Cathepsin B on maturation and function of human Mo-DCs.
HLA A2.1+ CD14+ monocytes were electroporated with either Cathepsin B (CatB) or control NT siRNA. The cells were cultured in 1% plasma supplemented with IL4 and GMCSF. On day 5, DCs were matured as indicated.
a. NT or Cathepsin B (CatB) siRNA treated DCs were cultured alone or in the presence of LPS and ATP. The cells were examined for the presence of cathepsin B and pro- IL1β protein. β-actin was used as loading control. The cell supernatant was examined for the presence of 17KD active form of IL-1β
b. Some of the supernatant as in Figure 3a was also examined for the presence of IL1β by luminex.
c. Day 5 immature NT or CatB siRNA treated DCs were infected with influenza virus for one hour and matured overnight using LPS. Cell culture supernatants were harvested at 24 hrs and assayed for the presence of IL1β using the luminex assay.
d. NT or CatB siRNA treated DCs were cultured in medium alone (iDC) or in the presence of LPS (LPS). After 24 hours, the expression of CD80, CD83, CD86 and HLADR was monitored using flow cytometry. Figure is representative of 4 similar experiments.
e. NT or CatB siRNA treated DCs were infected with influenza virus, matured with LPS and then used to stimulate autologous T cells. The presence of Flu MP specific CD8+ T cells was examined using the HLA A2.1 specific Flu MP tetramer using flow cytometry. The numbers in the figure represent tetramer positive CD3+ T cells. The figure is representative of 3 different donors.
3.4. Mechanism of altered DC function in ASC and CatB deficient DCs
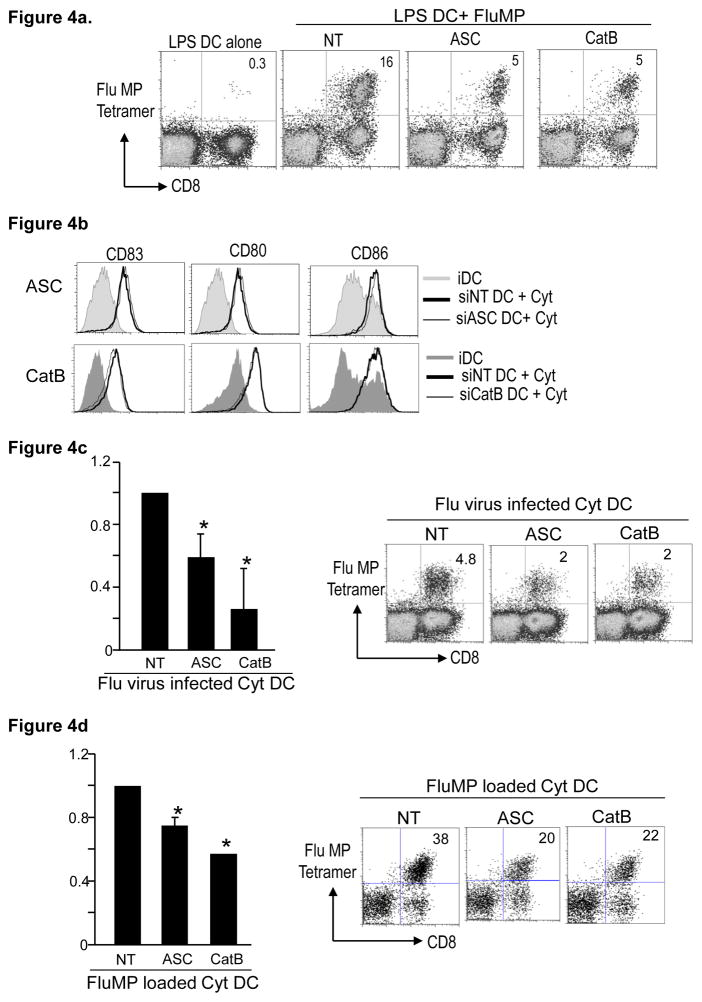
In order to test whether the reduced capacity of ASC and CatB-deficient DCs to stimulate T cell responses was related to altered processing of antigen, we tested the capacity of these DCs to stimulate Flu-MP specific T cells after pulsing with exogenously loaded MP peptide. However, both ASC and CatB deficient DCs were still defective in their ability to expand MP-specific T cells (Fig 4a), suggesting that this defect is not related to alteration of antigen-processing. The reduced capacity of ASC or CatB deficient DCs to stimulate T cell immunity can also not be ascribed to alteration in maturation-associated phenotypic remodeling, as there were no differences in LPS-induced expression of MHC and costimulatory molecules (HLA-DR, CD80, CD86) in these DCs (Figure 2c and 3c). Defective function of ASC or CatB-deficient DCs was also not explained by altered viability of these DCs (not shown). One of the best studied roles for ASC is as a component of the inflammasome, which is required for processing of inflammatory cytokines. ASC and CatB-deficient DCs upregulated HLADR, CD80/CD86 and CD83 in response to exogenous inflammatory cytokines (Fig 4b). However these DCs still exhibited reduced capacity for stimulation of MP-specific T cells following influenza infection (Fig 4c). ASC or CatB-deficient peptide pulsed Cyt-DCs were also deficient at stimulating T cells (Fig 4d), indicating that the reduced capacity of ASC and CatB deficient DCs is at least in part independent of phenotypic remodeling induced by inflammatory cytokines. Taken together, these data point to deficient antigen presentation in CatB or ASC-deficient DCs as opposed to defects in antigen processing, or maturation-associated phenotypic remodeling.
Figure 4. Effect of exogenous IL1B on the maturation and function of ASC and CatB RNAi treated DCs.
HLA A2.1 positive CD14+ monocytes were electroporated with either ASC, CathepsinB (CatB) or non targeting control (NT) siRNA. The cells were cultured in 1% plasma supplemented with IL4 and GMCSF and used for the following experiments on day 5 of culture.
a. NT, ASC or CatB electroporated day 5 immature DCs were matured using LPS. After 24 hrs in culture, the DCs were loaded with the HLA A2.1 specific influenza matrix peptide and used to stimulate autologous T cells at a 1:30 ratio. After 10 days in culture in the presence of IL2, the expansion of the Flu MP specific T cells was examined using the Flu MP tetramer using flowcytometry. The numbers in the figure represent tetramer positive CD3+ T cells. Figure represents 3 different donors *p<0.05 compared to NT treated DCs.
b. NT, ASC or CatB siRNA treated immature DCs were cultured alone or in the presence of inflammatory cytokine cocktail (IL1B, TNFα, PGE2 and IL6). After 24 hours the DCs were examined for their expression of CD83, CD80, CD86 and HLADR using flowcytometry. The figure is representative of 4 similar experiments.
c. NT, ASC or CatB siRNA treated DCs were infected with influenza virus, matured with inflammatory cytokines and then used to stimulate autologous T cells. The presence of Flu MP specific CD8+ T cells was examined using a HLA A2.1 Flu MP specific tetramer by flow cytometry. The figure is representative of 3 different donors. *p<0.05 compared to NT treated DCs.
d. NT, ASC or CatB siRNA treated Day5 immature DCs were matured using inflammatory cytokines. After overnight culture, the DCs were loaded with the A2.1 fluMP peptide and then cocultured with autologus T cells. The presence of Flu specific T cells was examined using the FluMP tetramer using flowcytometry. The figure is representative of 3 different donors.*p<0.05 compared to NT treated DCs.
3.5. Changes in gene expression in ASC and CatB-deficient DCs
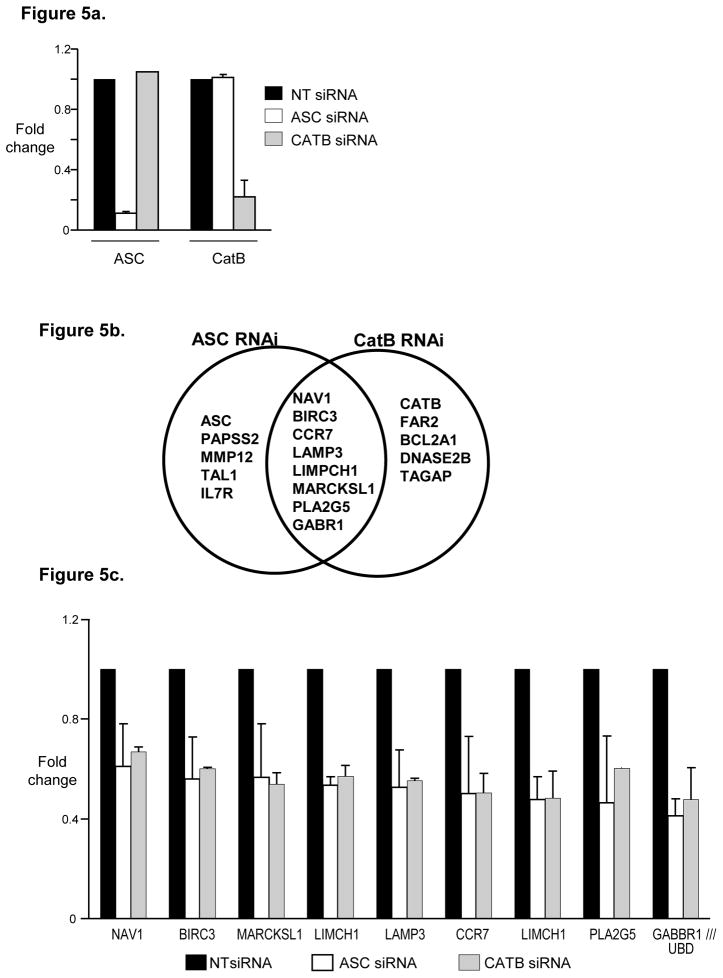
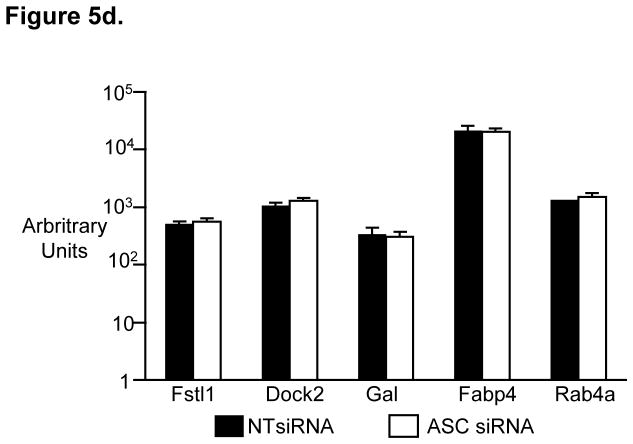
As the effect of ASC and CatB deficiency on DC function appeared to be independent of best known roles of these proteins in the cytosol, we explored an alternate possibility that roles of ASC as well as CatB in the nucleus and changes in gene expression may be important [16–18]. RNAi-mediated inhibition of both ASC and CatB was specific for that gene without cross inhibition (Fig 5a). Knockdown of both ASC as well as CatB led to inhibition of only a small number of genes. Interestingly, there was a marked overlap between genes altered in the setting of ASC and CatB-deficiency. 8 of 13 genes (61%) significantly altered in ASC-deficient DCs were also decreased in CatB-deficient DCs (Fig 5b). Furthermore, the degree of change in these shared genes is very similar between ASC and CatB deficient DCs (Fig 5c). This list of shared genes included genes such as CCR7 and LAMP3 which have been previously shown to be important for DC function and homing [19, 20]. Ippagunta et al recently described similar reduction of only a limited set of genes in ASC-deficient murine DCs to a similar degree as in our studies. One of these genes (Dock2) was then implicated in defective actin polymerization [21]. However none of the genes found to be altered in ASC-deficient murine DCs, including Dock2 were altered in ASC or CatB-deficient human DCs (Fig 5d). We were also unable to document differences in actin polymerization by phalloidin staining following DC maturation or differences in phagocytic uptake of fluorescently labeled ovalbumin or E.coli in these DCs (data not shown). Together, these data demonstrate that the changes in gene expression surprisingly overlap between ASC and CatB-deficient human DCs. While ASC-deficient DCs are functionally defective in both mouse and humans, the specific genes or mechanisms involved may differ between the two species.
Figure 5. SiRNA inhibition of ASC as well as Cathepsin B lead to changes in the gene expression of the treated DCs.
CD14+ monocytes were treated with either ASC, Cathepsin B or non targeting control (NT) siRNA. The CD14+ cells were cultured in 1% plasma supplemented with IL4 and GMCSF. On day 5 the DCs were harvested, RNA extracted and analyzed using the affymetrix U133 plus 2 chips. The experiments shown in figure 5 were done using two different donors.
a. Expression of ASC and CatB in DCs treated with NT, ASC or CatB siRNA.
b. Figure shows overlap between genes affected by treatment with ASC siRNA and those affected by treatment with CatB siRNA.
c. Expression of genes that are affected by both ASC as well as CatB siRNA treatment.
d. Expression in our data set of genes previously shown to be affected in ASC knockout mice in a study by Ippagunta et al[21].
4.0. Discussion
These data provide the first direct evidence that ASC and CatB play an important role in the regulation of APC function of human Mo-DCs. These findings therefore demonstrate an overlap between pathways that regulate DC function and activation of the inflammasome, and have implications for regulation of adaptive immunity in humans.
Several recent studies in mice have implicated a role for inflammasome activation in adaptive immunity to pathogens, mechanism of action of adjuvants, as well as models of autoimmunity [2, 10]. Some of the recent studies relating to the role of inflammasome in cell-mediated immunity however have yielded conflicting results, due in part to specific experimental conditions or mouse models used[22]. For example, Ichinohe et al. demonstrated impaired T cell responses to influenza in ASC and caspase-1 deficient but not NLRP3-deficient mice[7]. However other studies did not observe a difference in influenza specific adaptive immunity in NLRP3 or caspase-1 deficient mice [23, 24]. Differences in adaptive immunity observed by Ichinohe et al were attributed to effects of IL1β on T cell function[7]. However our data, suggest that primary defects in DC function may also underlie the impaired capacity of ASC deficient mice to induce protective T cell responses. Deficient function of ASC-deficient DCs is not restored by exogenous cytokines suggesting an inflammasome-independent effect. Inflammasome independent effects of ASC were recently described in murine models of arthritis and autoimmune encephalomyelitis[25–27].
These data also suggest an important role for CatB in APC function of human DCs. One of the challenges in understanding the role of specific cathepsins in human cells is that peptide inhibitors often used to inhibit them can have significant off target effects[14, 15]. For example, proteome analysis of DCs treated with the commonly utilized CatB inhibitor CA074Me shows alteration in several proteins (unpublished data). Therefore we have explored RNAi mediated inhibition, which lacks this caveat. It is notable that the effects of CatB-deficiency were not solely due to defects in lysosomal antigen degradation, as even peptide-pulsed DCs were deficient at T cell stimulation. The role of CatB in activation of inflammasomes was initially proposed primarily in the context of particulate antigens such as silica or alum, leading to lysosomal destabilization and activation of NLRP3 inflammasome[2]. However, more recent studies have now implicated CatB in the activation of the inflammasome in response to several pathogens including bacteria[28–30], suggesting that the link between CatB and inflammasomes might not be restricted to particulate antigens or NLRP3.
Further studies are needed to better understand the mechanisms underlying the dysfunction induced by ASC or CatB deficiency in human DCs. The roles of these proteins in the cytosol (inflammasome activation and lysosomal proteolysis, respectively) have been extensively studied. Both cathepsins and ASC have recently also been localized to the nucleus, wherein they may regulate gene expression by altering mRNA stability [21], or proteolysis of nuclear transcription factors [17, 18]. Ippagunta et al recently described a inflammasome-independent effect of ASC on murine DCs by inhibiting Dock2 expression and actin polymerization [21]. We were able to detect ASC in the DC nuclei (data not shown), but were unable to detect changes in Dock-2 expression or actin polymerization. Both studies suggest that the effects of ASC on DC function may at least in part be inflammasome-independent. It is notable that some of the genes such as CCR7 altered in ASC-deficient human DCs may also play a role in homing [20], which can only be tested in vivo. Another novel finding in our study is that the effects of ASC and CatB-inhibition on gene expression in DCs are highly overlapping, suggesting that these proteins may share targets or substrates in the nucleus, which may contribute to the effects of these proteins.
In summary, we have shown for the first time that CatB and ASC have a major impact on the capacity of human DCs to stimulate antigen-specific T cell responses in culture. Both of these proteins may therefore be important targets of DC-mediated regulation of adaptive immunity in humans.
Acknowledgments
KMD is supported in part by funds from the National Institutes of Health, Doris Duke Foundation and the Dana Foundation.
Abbreviations
- ASC
apoptosis-associated speck-like protein containing a CARD
- NLR
NOD-like receptor
- LPS
lipopolysaccharide
- APC
antigen presenting cell
- DC
dendritic cells
- RNAi
RNA interference
- GMCSF
Granulocyte-macrophage colony stimulating factor
- MLR
mixed lymphocyte reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299(5611):1400. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 4.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21(2):155. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184(1):9. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 7.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand PK, Malireddi RK, Kanneganti TD. Role of the nlrp3 inflammasome in microbial infection. Front Microbiol. 2011;2:12. doi: 10.3389/fmicb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17(2):57. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, Steinman RM, Ravetch JV, Dhodapkar MV. Selective blockade of inhibitory Fc gamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A. 2005;102:2910. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4(8):e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77(10):4327. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009;182(5):3173. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceru S, Konjar S, Maher K, Repnik U, Krizaj I, Bencina M, Renko M, Nepveu A, Zerovnik E, Turk B, Kopitar-Jerala N. Stefin B interacts with histones and cathepsin L in the nucleus. J Biol Chem. 2010;285(13):10078. doi: 10.1074/jbc.M109.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedelind S, Poliakova K, Valeta A, Hunegnaw R, Yemanaberhan EL, Heldin NE, Kurebayashi J, Weber E, Kopitar-Jerala N, Turk B, Bogyo M, Brix K. Nuclear cysteine cathepsin variants in thyroid carcinoma cells. Biol Chem. 2010;391(8):923. doi: 10.1515/BC.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9(3):325. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 20.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 21.Ippagunta SK, Malireddi RK, Shaw PJ, Neale GA, Walle LV, Green DR, Fukui Y, Lamkanfi M, Kanneganti TD. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nat Immunol. 2011;12(10):1010. doi: 10.1038/ni.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38(8):2085. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti TD, Webby RJ. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci U S A. 2011;108(7):2927. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R, Van de Veerdonk FL, Netea MG, Joosten LA, Lamkanfi M, Kanneganti TD. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem. 2010;285(16):12454. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 184(9):4610. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdallah AM, Bestebroer J, Savage ND, de Punder K, van Zon M, Wilson L, Korbee CJ, van der Sar AM, Ottenhoff TH, van der Wel NN, Bitter W, Peters PJ. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J Immunol. 2011;187(9):4744. doi: 10.4049/jimmunol.1101457. [DOI] [PubMed] [Google Scholar]
- 29.Atianand MK, Duffy EB, Shah A, Kar S, Malik M, Harton JA. Francisella tularensis reveals a disparity between human and mouse NLRP3 inflammasome activation. J Biol Chem. 2011;286(45):39033. doi: 10.1074/jbc.M111.244079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2011;184(10):5743. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]