SUMMARY
Background
Gut directed hypnotherapy can reduce IBS symptoms but the mechanisms underlying this therapeutic effect remain unknown.
Aim
We determined the effect of hypnotherapy and educational intervention on brain responses to cued rectal distensions in IBS patients.
Methods
44 women with moderate to severe IBS and 20 healthy controls (HCs) were included. Blood oxygen level dependent (BOLD) signals were measured by functional Magnetic Resonance Imaging (fMRI) during expectation and delivery of high (45 mmHg) and low (15 mmHg) intensity rectal distensions. Twenty-five patients were assigned to hypnotherapy (HYP) and 16 to educational intervention (EDU). 31 patients completed treatments and post treatment fMRI.
Results
Similar symptom reduction was achieved in both groups. Clinically successful treatment (all responders) was associated with significant BOLD attenuation during high intensity distension in the dorsal and ventral anterior insula (cluster size 142, p=0.006, and cluster size 101, p=0.005, respectively). Moreover HYP responders demonstrated a pre-post treatment BOLD attenuation in posterior insula (cluster sizes 59, p=0.05) while EDU responders had a BOLD attenuation in prefrontal cortex (cluster size 60, p=0.05). Pre-post differences for expectation conditions were almost exclusively seen in the HYP group. Following treatment, the brain response to distension was similar to that observed in HCs, suggesting that the treatment had a normalizing effect on the central processing abnormality of visceral signals in IBS.
Conclusions
The abnormal processing and enhanced perception of visceral stimuli in IBS can be normalized by psychological interventions. Symptom improvement in the treatment groups may be mediated by different brain mechanisms.
INTRODUCTION
Several studies have demonstrated a beneficial effect of hypnotherapy in the treatment of Irritable Bowel Syndrome (IBS), a common chronic gastrointestinal disorder characterized by abdominal pain, bloating and altered bowel habits, which is often associated with increased trait anxiety.1-5 In a recently published randomized controlled study, it was demonstrated that gut-directed hypnotherapy significantly improved IBS symptoms after 3 months when compared to supportive therapy or waiting list, and that this improvement was more pronounced for sensory symptoms, such as pain and bloating, than for bowel habit disturbances.2 This finding confirmed earlier reports that hypnotherapy can improve abdominal pain and overall symptoms in IBS.1, 6 Previous work has also demonstrated that hypnotherapy treatment may affect the perception of experimental visceral stimuli, both in hypersensitive and hyposensitive patients.7-10 However, changes in the perception of rectal distension were not correlated with symptom improvement in pediatric or in adult patients.9-11 Despite the successful use of hypnotherapy for the treatment of IBS symptoms, the neurobiological mechanisms underlying this mind based therapy remain largely unknown.
Rectal balloon distension and cued expectation of such high intensity distensions has traditionally been used to evoke abnormal brain responses in IBS.12 Evoked brain responses can be visualized by functional Magnetic Resonance Imaging (fMRI), where changes in the blood oxygen level dependent (BOLD) response to rectal stimuli cause measurable fMRI signal changes. In fMRI, a regional increase in the BOLD response is interpreted as increased neural activity in this region. According to a recent meta-analysis of brain responses to rectal distension in IBS patients and HCs, patients had more BOLD activity in brain regions associated with visceral afferent signal processing, emotional arousal and cognitive modulation.13 In addition, several studies have provided evidence for an altered engagement of endogenous cortico limbic pontine pain modulatory systems.14, 15 When viewed together, a model of IBS symptom generation has been proposed which includes abnormal signaling in visceral afferent pathways as well as central pain amplification. The latter includes increased emotional arousal, cognitive abnormalities (hypervigilance, catastrophizing) and related alterations in the balance of descending inhibitory and facilitatory systems.16
The primary aim of the current study was to further examine the pathophysiological mechanisms of IBS and to determine the effect of a clinically successful course of hypnotherapy and educational intervention on brain responses to expected and delivered low and high intensity rectal distensions in a sample of female IBS patients with moderately to severe symptoms. Specifically, we aimed to test the following hypotheses: 1) IBS patients who respond successfully to a course of hypnotherapy with significant GI symptom reduction demonstrate changes in brain responses to both expected and delivered rectal distensions. 2) Brain responses in hypnotherapy responders differ from those observed in patients improving in response to IBS related education. 3) Post treatment patterns of brain activation in treatment responders are similar to responses seen in HCs.
METHODS
Subjects
Forty-four right-handed female IBS patients (mean age 35.5 years, range 20-60) fulfilling Rome III criteria were recruited from the Gastroenterology Clinic at the University Hospital in Linköping, Sweden and by referral from general practitioners in the County of Östergötland, Sweden. They were refractory to conventional IBS treatment as administered or prescribed by their primary care physician. Twenty-nine patients had IBS-M, 10 had IBS-D and 5 had IBS-C.
Subjects were evaluated by trained gastroenterologists and standard clinical investigations were performed to exclude organic gastrointestinal disease. Twenty healthy right-handed women were recruited by advertisement to the HC group (mean age 32.2 years, range 21-54). For participating, HCs received 1000 SEK in compensation. Exclusion criteria for all subjects were organic gastrointestinal disease, metabolic, psychiatric or neurological disorders, abdominal surgery, centrally acting medication, use of nicotine 3 months before trial and use of alcohol less than 24 hours before the fMRI scan. Three patients were excluded immediately from the study due to claustrophobia during the first scanner procedure. Twenty-five patients were assigned to hypnotherapy (HYP) and 16 to educational therapy (EDU), in weekly periods depending on availability of the hypnotherapist.
Eighteen patients completed the hypnotherapy, reasons for discontinuation were: start of centrally acting medication (n=1), non-compliance to the study protocol (n=5), panic attacks during hypnotherapy (n=1). In the HYP group two fMRI data sets were excluded from analysis due to: exceeding predefined motion parameters (n=1) and major scanner artifact (n=1).
Twelve patients completed the educational intervention, reasons for discontinuation were: pregnancy (n=1), start of centrally acting medication (n=1), unrelated disease (n=1). In the EDU group three fMRI data sets were excluded from analysis due to: exceeding predefined motion parameters (n=2) and major scanner artifact (n=1). Nineteen HC completed the fMRI procedure. Due to vertigo during the experimental procedure one HC dropped out and one data set was excluded from analysis due to incomplete data collection.
In total there were complete data from 16 patients in HYP, 9 patients in EDU and 18 HCs. Written and oral informed consent was obtained from all subjects. The study protocol was approved by the Regional Ethical Review Board in Linköping, Sweden, (DNR M71-09).
Study design
A study overview is presented in Figure 1.
Figure 1.
Flow chart overviewing the progress of patients and healthy controls during the course of the study.
Questionnaires
IBS Severity Scoring System (IBS SSS)
The IBS SSS is used for rating IBS symptoms.17 The scoring system incorporates 5 items: abdominal pain severity, pain frequency, bowel distension, bowel dysfunction and quality of life/global well-being. Total maximum score is 500. Mild, moderate and severe symptom burden are indicated by scores of 75 to 175, 175 to 300 and > 300, respectively. Responders were defined a priori as a pre-post treatment reduction of at least 50 points in IBS-SSS.17
Visceral Sensitivity Index (VSI)
The visceral sensitivity index (VSI) consists of a 15-item scale to measure gastrointestinal symptom-specific anxiety by assessing the cognitive, affective, and behavioural response to fear of GI sensations, symptoms, and the context in which these occurs.18
The Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HAD) is a self-assessment scale that was developed for detecting states of depression and anxiety in medical outpatient settings.19 The scale consists of 7 items each for anxiety and depression, graded on a 4-point scale. The sum score for anxiety and depression respectively, range from 0 to 21 with a higher score indicating more pronounced symptoms.
Gastrointestinal symptom diary
Subjects recorded their gastrointestinal symptoms on validated diary cards 29 during 2 weeks, before and after treatment. Along a 24 hour time axis they recorded episodes of abdominal pain and graded the pain intensity into light, moderate, or intense.
Ratings of present intensity and unpleasantness of gastrointestinal symptoms
A scale ranging from 0-10 was used to asses (a) the subjects current intensity of gastrointestinal symptoms and (b) abdominal unpleasantness during the fMRI session protocol. Zero indicates no intensity / no unpleasantness and 10 indicate very high intensity / very intolerable unpleasantness.
Hypnotherapy
The written gut-directed hypnotherapy script used for the intervention was developed by MSj (an experienced hypnotherapist) and has been used clinically for several years. All subjects were treated by MSj in a standard course of seven one hour long sessions of individual hypnotherapy (approximately one session per week). During the first session the therapist established a working alliance with the patient and informed the patient about the hypnotherapy treatment. The following six hypnotherapy sessions contained: induction of the hypnotic state, and hypnotic suggestions with the intention to reduce threat perception and gut symptoms and to increase overall physical relaxation. Subjects received a pre-recorded compact disc with the same content as in the clinical sessions. Subjects were instructed to practice at home on a daily basis.
Educational intervention
The subjects received seven individual 45 minute sessions with different tutorials covering gastrointestinal anatomy and physiology, IBS-symptoms, diet and the theory of different IBS treatments. The sessions began with 20 minutes when the subject studied material covering the session topic and then a 25 minute discussion followed. Tutors included gastroenterologists and experienced physiotherapists specialized in functional bowel and pelvic floor disorders.
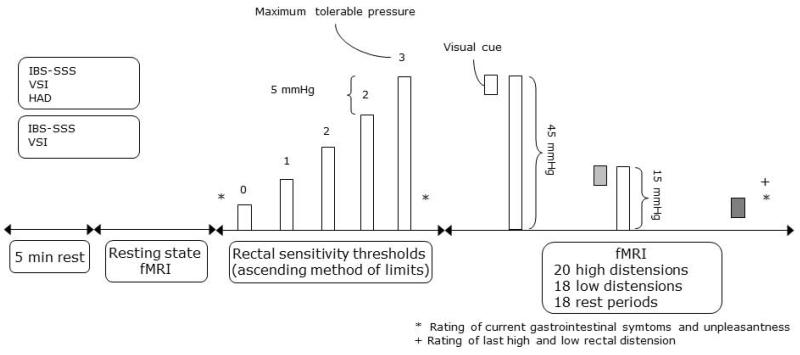
fMRI session protocol (Figure 2)
Figure 2. fMRI session protocol.
After 5 min rest and collection of resting state data (reported elsewhere) rectal sensitivity thresholds during rectal distension were determined using ascending method of limits: 0=no sensation, 1=sensation, 2=urgency and 3=maximum tolerable pressure. Twenty visually cued high and 18 low intensity rectal distensions were pseudorandomly delivered with 18 rest periods. Rating of last low and high rectal intensity distension indicated by +. Ratings of current gastrointestinal symptoms and unpleasantness indicated by *.
Dates of sessions did not coincide with menses. Subjects were instructed to fast for 4 hours before the experiment. A rectal balloon (maximum volume 520 ml, made of polyethylene, attached to a polyethylene tube) was installed and tested by a person with experience of this procedure. Subjects were placed in the MR scanner and fitted with headphones (allowing two-way communication) and high-resolution video goggles for visual stimulus presentation (Resonance Technology Inc., California, USA). The experimental paradigm was designed by using Superlab Pro 4 software (Cedrus Corp., San Pedro, USA) and presented via a standard PC in the goggles. During the initial 5 minutes of the experiment the subjects were instructed to rest and then a 10-minute fMRI sequence collecting resting-state data was performed (data reported elsewhere).
Pressure thresholds during rectal distension
An electronic barostat (Dual Drive Barostat, Distender series II; G&J Electronics Inc., Toronto, Canada) was used for rectal distensions. Distension protocols were performed using Protocol Plus Deluxe (v 6.7R). Protocol Plus Data Scanner (v 4.9) was used to analyze the recorded barostat data. Using ascending method of limits with phasic distensions the subjects’ sensory thresholds during rectal distension were evaluated in the scanner. The balloon was inflated for 30 seconds followed by a 30 seconds long period of rest with pressure of 2 mmHg. The increment of pressure was 5 mmHg. After each distension the subjects were instructed to verbally rate the distension according to a scale from 0-3 (0 = no sensation, 1= sensation, 2 = urgency to defecate and 3 = maximum tolerable pressure). When the subjects reported maximum tolerable pressure the distension protocol was ended. Before and after the thresholding procedure the subject was asked to assess their current intensity of gastrointestinal symptoms and unpleasantness.
Expectation and visceral stimulus fMRI paradigm
fMRI data were acquired while 18 low (15 mmHg) and 20 high (45 mmHg) intensity rectal distension (duration 15 s) were delivered in a pseudo randomized order. Each distension was preceded by a visual cue (duration 3 s) predicting the size of the distension (certain expectation). The high and low intensity distensions were signaled by an orange and blue cue respectively. The time between the cue and the beginning of the inflation was jittered by 2, 4 or 6 seconds. Between the distensions the subjects had 18 rest periods (safety baseline) of 14, 16 or 18 seconds duration, signaled by a grey cue (3 s) in pseudo randomized order. The total duration of the visceral stimulus paradigm was 24 minutes. After the protocol the subjects were again asked to assess their current gastrointestinal symptoms and unpleasantness and to rate the sensation of the last high and low intensity distension.
fMRI data acquisition
A 1.5 T MRI scanner (Philips Achieva; Philips, Best, The Netherlands) was used to acquire brain images. Functional brain images (axial and interleaved) were acquired by using a blood oxygen level dependent (BOLD) sensitive gradient echo sequence, employing the following acquisition parameters: Repetition time (TR) = 3 s; Echo time (TE) = 40 ms; flip angle = 90°; resolution 3 × 3 × 3 mm3, slice gap = 0.5 mm and number of slices = 35. The number of image volumes acquired during the fMRI session was 482.
Data analysis
Statistical parametric mapping 8 (SPM8) (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used for the preprocessing and statistical analysis of the BOLD fMRI data. The 482 images acquired during fMRI were realigned to the first image of the time series to correct for movement during scanning. The images were normalized to a standard brain atlas in Montreal Neurological Institute (MNI) space to allow for voxel-wise statistical testing between subjects. Finally, the images were smoothed using an 8 mm full width half maximum (FWHM) Gaussian kernel to reduce image noise and to ameliorate differences in intersubject localization. Subjects were excluded from further analysis if the BOLD fMRI images exceeded the predefined movement threshold of >3 mm or contained scanner artifacts when visually inspected.
To estimate the correlation between the time series of the measured BOLD response and the evoked rectal stimuli, we applied a general linear model (GLM) with 4 regressors representing the different conditions of the stimuli, 1 regressor representing the safety baseline, and 6 regressors representing movements during scanning. The 4 conditions during the fMRI experiment were as follows: 1) expectation of high intensity distension, 2) high intensity distension (45 mmHg), 3) expectation of low intensity distension, and 4) low intensity distension (15 mmHg). These conditions were compared to the safety baseline in a first level analysis of fixed effects in each subject.
In the second level analysis of within group and pre-post treatment effects, a region of interest (ROI) approach was applied. A priori defined ROIs included regions and sub regions associated with emotional arousal, processing of afferent signals and endogenous pain modulation: amygdala (AMY), hippocampus (HIPP), pregenual and subgenual anterior cingulate cortex (pACC and sgACC), anterior mid cingulate cortex (aMCC), periaqueductal grey (PAG), thalamus (THA), ventrolateral and dorsolateral prefrontal cortex (VLPFC and DLPFC) and ventral and dorsal anterior insula (aINS), mid INS (mINS) and posterior INS (pINS). One-sample t-tests were performed to evaluate brain activation caused by rectal stimuli in each group, HYP and EDU separately. To evaluate treatment effects within the two treatment groups paired t-tests were used. To compare treatment effects difference images of activity between before and after were created in SPM8 and entered in two group t-tests. Results were considered to be significant if the peak voxel p-value in ROIs was less than 0.05 corrected for multiple comparisons using family wise error (FWE) correction.
Correlation analysis was performed between significant pre-post treatment changes in symptoms of all therapy responders and correspondingly, significant pre-post treatment changes of BOLD response in ROIs. For the correlation analysis, changes in symptoms were entered as a covariate in SPM8 and inclusively masked by the significantly changed cluster as estimated by the analysis of treatment effects. Eigenvariates in peak voxels were extracted as measures of the correlation. The eigenvariates represents the β-values in the regression model. The correlation analysis between the eigenvariates and the pre-post treatment effects in symptoms (Pearsońs r) was performed in GraphPrism 4. The alpha level for significance was set at 0.05.
RESULTS
At baseline there were no significant differences between the two IBS groups in IBS-SSS, VSI, HAD and sensory rectal distension pressure thresholds.
Behavioral responses
Subjects who completed HYP treatment (n=18) reduced their IBS-SSS score from 342 (SD 65) to 233 (SD 89) (p<0.0001), and their VSI score from 48 (SD 18) to 34 (SD 18) (p<0.0001). Subjects who completed EDU therapy (n=13) reduced their IBS-SSS score from 340 (SD 77) to 256 (SD 94) (p=0.02) and their VSI score from 48 (SD 15) to 36 (SD 13) (p=0.005). There were no statistical difference in improvement in SSS or VSI between HYP and EDU. Figure 3 shows the effect of hypnotherapy on abdominal pain, one of the major symptoms of IBS. Thirteen subjects in the hypnotherapy and 7 subjects in the educational control group responded to therapy. Combined responders from both groups (n=20) demonstrated a significant decrease in VSI score from mean 47 (SD 17) to 33 (SD 17) (p<0.0001), GI-symptom intensity from mean 5.1 (SD 2.3) to 3.7 (SD 2.5) and unpleasantness 5.3 (SD 2.8) to 4 (SD 2.5) after the thresholding procedure (p=0.01 and p=0.02). The behavioral data of the responders of each subgroup are shown in Table 1. Pre-post treatment symptoms of all subjects who were included into the fMRI analysis are shown in the supporting information.
Figure 3. Frequency and intensity of abdominal pain (hours per 14 days) before and after hypnotherapy. Median and interquartile range are shown in the graph. After hypnotherapy the total numbers of pain hours decreased significantly from median 45.5 hours (range 5.5-225) to 25 hours (range 0-223 hours), paired sign test.
Table 1.
Behavioural data before and after intervention for HYP and EDU responders. Mean, standard deviation and p-value for paired t-tests are shown.
| HYP responders (n=13) |
EDU responders (n=7) |
|||||
|---|---|---|---|---|---|---|
| Before | After | P- value |
Before | After | p- value |
|
| VSI (score) | 45.9 (19.6) | 31.5 (18.2) | 0.0005 | 49.8 (14.7) | 36.4 (14.4) | 0.05 |
|
Rectal distension, sensation (mmHg) |
13.8 (5.7) | 14.2 (4.5) | NS | 10.7 (4.5) | 16.2 (4.8) | NS |
|
Rectal distension, urgency (mmHg) |
22.1 (6.2) | 23.1 (5.6) | NS | 22.1 (4.9) | 25.0 (6.1) | NS |
|
Rectal distension, maximum tolerable pressure (mmHg) |
42.3 (12.7) | 40.8 (13.1) | NS | 34.3 (5.3) | 44.2 (8.9) | 0.03 |
|
GI symptom intensity, before thresholding (score) |
2.3 (1.6) | 2.9 (2.6) | NS | 3.0 (2.5) | 2.3 (2.4) | NS |
|
GI symptom intensity, after thresholding (score) |
5.4 (2.1) | 4.2 (2.6) | 0.04 | 4.3 (2.9) | 2.9 (2.3) | NS |
|
GI symptom intensity, after fMRI protocol (score) |
6.7 (2.8) | 5.4 (2.9) | 0.06 | 5.4 (3.9) | 4.9 (2.9) | NS |
|
GI unpleasantness, before thresholding (score) |
3.5 (2.3) | 3.1 (2.9) | NS | 2.3 (2.4) | 2.4 (2.3) | NS |
|
GI unpleasantness, after
thresholding (score) |
5.4 (2.1) | 4.4 (2.5) | 0.05 | 4.7 (4.0) | 3.4 (2.5) | NS |
|
GI unpleasantness, after
fMRI protocol (score) |
7.2 (2.8) | 6.0 (3.0) | NS | 5.4 (3.7) | 5.4 (2.3) | NS |
|
Rating of last mild
distension (score) |
1.3 (0.9) | 1.3 (0.9) | NS | 1.3 (0.9) | 1.3 (1.1) | NS |
|
Rating of last aversive
distension (score) |
2.9 (0.3) | 2.9 (0.3) | NS | 2.7 (0.5) | 3.0 (0) | NS |
Brain responses
Treatment effects on brain responses were assessed in three different analyses. 1) Pre-post treatment data in all responders (n=20). 2) Comparison between pre- and post-treatment brain response of IBS patients with the brain response of HCs. 3) Differences in brain responses between IBS subjects who responded to hypnosis (HYP responders) versus those who responded to education (EDU responders). The present study may be limited by the fact that the data of several subjects had to be excluded which reduced the number of complete data sets. One unexpected problem was that several subjects exceeded motion thresholds during data collection in the scanner dependent on the very intense rectal stimulus preventing them to lie still.
All IBS treatment responders
BOLD responses during both the high intensity distension and expectation of such distension were significantly reduced post treatment in aINS and vlPFC, while AMYG, HIPP, and pINS showed reductions only during the distension. There was no pre-post treatment increase of BOLD during the rectal distensions. Pre-post treatment BOLD responses during all conditions are shown in Table 2.
Table 2.
Within group pre-post treatment BOLD response of all therapy responders (n=20). Results calculated using paired t-tests and thresholded at p ≤ 0.05 corrected for multiple comparisons (FWE) at peak level. MNI-coordinates (x, y, z) indicated. L = left, R = right. NS = no significant findings.
| Region of interest (ROI) |
Cluster size |
Peak p FWE |
x | y | z | ||
|---|---|---|---|---|---|---|---|
| Aversive rectal distension (45 mmHg) | |||||||
|
Reduction of
BOLD response |
AMY | L | 6 | 0.05 | −30 | −2 | −14 |
| HIPP | L | 18 | 0.01 | −34 | −14 | −14 | |
| aINS (dors) | L | 142 | 0.006 | −28 | 22 | −6 | |
| aINS (vent) | L | 101 | 0.005 | −28 | 22 | −8 | |
| pINS (dors) | L | 65 | 0.03 | −32 | −16 | 16 | |
| VLPFC | L | 96 | 0.03 | −28 | 22 | −8 | |
| Increase of BOLD response | NS | ||||||
| Mild rectal distension (15 mmHg) | |||||||
| Increase of BOLD response | NS | ||||||
| Expectation of aversive rectal distension | |||||||
|
Reduction of
BOLD response |
aINS (dors) | L | 30 | 0.03 | −28 | 22 | −6 |
| aINS (vent) | L | 49 | 0.03 | −28 | 22 | −6 | |
| VLPFC | L | 112 | 0.02 | −44 | 50 | −10 | |
| Increase of BOLD response | NS | ||||||
| Expectation of mild rectal distension | |||||||
| Increase of BOLD response | HIPP | R | 24 | 0.05 | 32 | −20 | −16 |
| THAL | L | 41 | 0.04 | −14 | −12 | 6 | |
Significant correlations between pre-post symptom improvement in VSI score and BOLD attenuation in the aINS (Figure 4 a), and between GI symptom improvement and BOLD decrease in HIPP (Figure 4b) were observed for the high Intensity distension. In contrast, no significant correlations were observed during the expectation condition.
Figure 4 a-b.
Correlations between pre-post treatment improvement in symptoms versus reduction in blood oxygen level dependent response (increasing blood oxygen level dependent activity on the y-axis of the figure is interpreted as a pre-post treatment blood oxygen level dependent response reduction) during high intensity distension in all treatment responders.
Treatment responders vs healthy controls
Before therapy, treatment responders (n=20) showed significantly more BOLD responses than HCs (n=18) during the high intensity distension in several brain regions including vlPFC, aMCC, pACC and sgACC. After treatment, these differences were no longer observed, except in vlPFC. BOLD responses of all treatment responders before and after therapy, compared with HCs are shown in Table 3.
Table 3.
Brain activation of all therapy responders (n=20) compared with brain activity of HCs (n=18) before and after treatment. Results calculated using unpaired t-tests and thresholded at p ≤ 0.05 corrected for multiple comparisons (FWE) at peak level. MNI-coordinates (x, y, z) indicated. L = left, R = right. NS = no significant findings. Complete results are shown in the supporting information.
| Region of interest (ROI) |
Cluster size |
Peak p FWE |
x | y | z | |||
|---|---|---|---|---|---|---|---|---|
| IBS responders before treatment compared with HCs | ||||||||
|
Aversive
distension (45 mmHg) |
IBS> HCs | VLPFC | L | 180 | 0.01 | −40 | 16 | −18 |
| aMCC | L | 49 | 0.04 | −4 | 38 | 34 | ||
| pACC | L | 411 | 0.01 | −8 | 50 | 4 | ||
| sgACC | L | 20 | 0.02 | −2 | 16 | −10 | ||
| sgACC | R | 26 | 0.05 | 0 | 18 | −10 | ||
| IBS< HCs | HIPP | L | 26 | 0.05 | 34 | −26 | −12 | |
|
Mild
distension (15 mmHg) |
IBS>HCs | pACC | L | 85 | 0.04 | −36 | −8 | −12 |
| pACC | R | 104 | 0.03 | 2 | 40 | 10 | ||
| IBS<HCs | NS | |||||||
|
Expectation
aversive distension |
IBS> HCs | aINS (vent) | R | 14 | 0.05 | 32 | 22 | −14 |
| NS | ||||||||
|
Expectation
mild distension |
IBS>HCs | pINS (dors) | R | 12 | 0.006 | 32 | −26 | 20 |
| IBS< HCs | NS | |||||||
| IBS responders after treatment compared with HCs | ||||||||
|
Aversive
distension (45 mmHg) |
IBS>HCs | VLPFC | R | 87 | 0.05 | 32 | 18 | −22 |
| IBS<HCs | pINS (dors) | L | 16 | 0.05 | −32 | −10 | 18 | |
|
Mild
distension (15 mmHg) |
IBS>HCs | NS | ||||||
| IBS<HCs | NS | |||||||
|
Expectation
aversive distension |
IBS>HCs | AMY | L | 37 | 0.004 | 28 | 4 | −18 |
| IBS<HCs | NS | |||||||
|
Expectation
mild distension |
IBS>HCs | AMY | L | 45 | 0.001 | 26 | 2 | −20 |
| IBS<HCs | NS | |||||||
Hypnosis vs Education group responders
The 13 HYP responders had a significant pre-post treatment BOLD attenuation in the aINS both during the high intensity distension (which also showed reduction in pINS) and during the expectation condition, while aINS reduction in the EDU responders was only seen during the distension (in addition to reductions in vlPFC) Data shown in Table 4.
Table 4.
Pre-post treatment BOLD response of hypnotherapy responders (n=13) and educational responders (n=7). Results calculated using paired t-tests and thresholded at p ≤ 0.05 corrected for multiple comparisons (FWE) at peak level. MNI-coordinates indicated. L = left, R = right. NS = no significant findings. Complete results are shown in the supporting information.
| Region of interest (ROI) |
Cluster size |
Peak p FWE |
x | y | z | |||
|---|---|---|---|---|---|---|---|---|
| Aversive rectal distension (45 mmHg) | ||||||||
|
Reduction of
BOLD response |
HYP
responders |
aINS (vent) | L | 40 | 0.04 | −30 | 22 | −8 |
| pINS | L | 59 | 0.05 | −30 | −18 | 16 | ||
|
EDU
Responders |
aINS (dors) | L | 79 | 0.02 | −30 | 20 | 0 | |
| aINS (vent) | L | 27 | 0.05 | −28 | 22 | −2 | ||
| VLPFC | L | 60 | 0.05 | −40 | 38 | −2 | ||
|
Increase of
BOLD response |
HYP
responders |
NS | ||||||
|
EDU
responders |
NS | |||||||
| Mild rectal distension (15 mmHg) | ||||||||
|
Reduction of
BOLD response |
HYP
responders |
pINS | L | 86 | 0.03 | −32 | −16 | 12 |
|
EDU
responders |
NS | |||||||
|
Increase of
BOLD response |
HYP
responders |
NS | ||||||
|
EDU
responders |
NS | |||||||
| Expectation of aversive rectal distension | ||||||||
|
Reduction of
BOLD response |
HYP
responders |
aINS (dors) | L | 39 | 0.02 | −32 | 16 | 8 |
| aINS (dors) | L | 43 | 0.05 | −28 | 20 | −6 | ||
| aINS (vent) | L | 57 | 0.04 | −28 | 20 | −6 | ||
|
EDU
responders |
NS | |||||||
|
Increase of
BOLD response |
HYP
responders |
NS | ||||||
|
EDU
responders |
NS | |||||||
| Expectation of mild rectal distension | ||||||||
|
Reduction of
BOLD response |
HYP
responders |
HIPP | L | 17 | 0.05 | −36 | −26 | −10 |
| pINS | L | 11 | 0.03 | −38 | −18 | 22 | ||
| THAL | R | 41 | 0.04 | 12 | −26 | 16 | ||
|
EDU
responders |
NS | |||||||
|
Increase of
BOLD response |
HYP
responders |
AMY | R | 4 | 0.05 | 20 | −8 | −22 |
| HIPP | R | 9 | 0.04 | 22 | −10 | −24 | ||
| PAG | L | 2 | 0.05 | 0 | −30 | −6 | ||
|
EDU
responders |
HIPP | R | 26 | 0.009 | 32 | −20 | −14 | |
Significantly more pronounced pre-post reductions in BOLD response in the HYP responders compared to the EDU responders were seen during the low intensity distension, and included the right aINS (cluster size 17, p=0.03, [28 30 4]), the right mid INS (cluster size 16, p=0.04, [36 6 −6]) and the right aMCC (cluster size 70, p=0.03, [8 32 38]).
When adding treatment non-responders to the analysis there were no pre-post treatment differences in HYP group (n=16) during the high intensity distension and a reduction of BOLD in sgACC in the EDU group (n=9). Data for all conditions are shown in Table 5.
Table 5.
Pre-post treatment BOLD response of all patients who received hypnotherapy (n=16) and educational therapy (n=9). Results calculated using paired t-tests and thresholded at p ≤ 0.05 corrected for multiple comparisons (FWE) at peak level. MNI-coordinates (x, y, z) indicated. L = left, R = right. NS = no significant findings. Complete results are shown in the supporting information.
| Region of interest (ROI) |
Cluster size |
Peak p FWE |
x | y | z | |||
|---|---|---|---|---|---|---|---|---|
| Aversive rectal distension (45 mmHg) | ||||||||
|
Reduction of
BOLD response |
HYP | NS | ||||||
| NS | ||||||||
| EDU | sgACC | L | 50 | 0.03 | −4 | 24 | −8 | |
|
Increase of BOLD
BOLD response |
HYP | NS | ||||||
| EDU | NS | |||||||
| Mild rectal distension (15 mmHg) | ||||||||
|
REDUction of
BOLD response |
HYP | aINS | L | 39 | 0.04 | −34 | −12 | 2 |
| EDU | NS | |||||||
|
Increase of
BOLD response |
HYP | NS | ||||||
| EDU | AMY | L | 32 | 0.01 | −24 | −8 | −14 | |
| Expectation of aversive rectal distension | ||||||||
|
Reduction of
BOLD response |
HYP | aINS (dors) | L | 64 | 0.005 | −34 | 14 | 6 |
| aINS (dors) | L | 67 | 0.03 | −28 | 20 | −6 | ||
| aINS (vent) | L | 89 | 0.03 | −28 | 20 | −6 | ||
| mINS | L | 8 | 0.05 | −34 | 10 | 8 | ||
| VLPFC | L | 211 | 0.05 | −44 | 42 | −2 | ||
| EDU | NS | |||||||
|
Increase of
BOLD response |
HYP | NS | ||||||
| EDU | NS | |||||||
| Expectation of mild rectal distension | ||||||||
|
Reduction of
BOLD response |
HYP | THAL | R | 30 | 0.05 | 12 | −26 | 16 |
| pINS | L | 16 | 0.007 | −38 | −18 | 22 | ||
| pACC | L | 189 | 0.04 | −12 | 40 | 8 | ||
| EDU | NS | |||||||
|
Increase of
BOLD response |
HYP | AMY | R | 9 | 0.02 | 20 | −8 | −22 |
| HIPP | R | 19 | 0.02 | 22 | −10 | −24 | ||
| EDU | AMY | R | 7 | 0.03 | 28 | −4 | −14 | |
| HIPP | R | 14 | 0.03 | 30 | −18 | −18 | ||
Hypnosis responders vs healthy controls (Figure 5 a and b)
Figure 5a.
Blood oxygen level dependent response during high intensity rectal distension before (top panel), after (middle panel) a course of successful hypnotherapy in IBS patients. Blood oxygen level dependent response to the same stimuli in healthy controls is shown in the bottom panel. Images thresholded at p < 0.01 uncorrected. Red color represents increased and blue color decreased blood oxygen level dependent response. Numbers indicates slice level.
Figure 5b.
Blood oxygen level dependent response during expectation of high intensity rectal distension before (top panel), after (middle panel) a course of successful hypnotherapy in IBS patients. Blood oxygen level dependent response to the same stimuli in healthy controls is shown in the bottom panel. Images thresholded at p < 0.01 uncorrected. Red color represents increased and blue color decreased blood oxygen level dependent repsonse. Numbers indicates slice level.
Before hypnotherapy, during high intensity distension, HYP responders had significantly higher BOLD response than HCs in left mINS (cluster size 94, p=0.02, [−44 2 −6]), left pINS (cluster size 59, p=0.03, [−42 2 −8]), right pINS (cluster size 96, p=0.05, [44 −2 −10]) and left VLPFC (cluster size 61, p=0.01, [−40 18 −20]). These differences were no longer seen after therapy.
DISCUSSION
Hypnotherapy aims to induce a state of deep relaxation with focused attention, teaching patients to control symptoms and physiological functions that are not usually easily accessible to conscious manipulation.20 Education can also reduce symptom related anxieties and worries, but has no specific effect on attention and relaxation. Using both of these treatment strategies in IBS patients, the main findings of the present study were: 1) Clinically successful treatment (all responders) was associated with significant BOLD signal reductions during both high intensity distension and expectation of such distension in the aINS, a brain region which receives interoceptive, cognitive and affective modulatory input, and which is essential for the conscious awareness of visceral sensations. 21-23 2) In the aINS, a significant correlation between treatment induced reduction of GI related anxiety (VSI) with BOLD decrease was observed. 3) Brain responses associated with symptom reduction during HYP and EDU showed similarities both during distension and expectation in the form of reductions in aINS activity, but differed in other responses, suggesting that somewhat different brain mechanisms may mediate symptom improvement to the two different treatments. 4) Following treatment, the brain response to high intensity distension was similar to that observed in HCs, suggesting that psychological/cognitive treatment has a normalizing effect on the central processing of visceral stimuli in IBS.
Treatment responders
Patients who responded to treatment (all responders) demonstrated a reduced activation in the aINS during the high intensity distension, consistent with a reduced conscious perception of signals from the viscera. Given the role of the aINS in integrating interoceptive, cognitive and affective inputs, and its role in a salience network24 one could speculate that treatment responders have enhanced their cognitive ability to attenuate interoceptive signals from the gut, and/or reduced the role of affective input in sensory amplification. Even though the present sample was not large enough to allow for the isolated evaluation of non-responders, when non-responders were included into the analysis pre-post treatment, the observed changes in aINS were less pronounced in both treatment groups, suggesting that these changes were related to symptom improvement. This interpretation was further supported by showing a significant correlation of BOLD signal changes in the aINS with symptom improvement.
Hypnotherapy versus educational therapy
HYP responders demonstrated a reduction of BOLD activity in both aINS and pINS, during the high intensity rectal distension. Decreased activation in pINS, which is the primary interoceptive cortex, is consistent with a reduced spinal afferent input to the brain.15 Since the balloon distension stimuli during the first and second fMRI were identical, the decrease in pINS could not be explained by primary changes in the visceral input to the CNS but rather had to be a consequence of primary central processes. Such central processes could include autonomic outflow to the gut resulting in changes in motility and activity of mechanosensitive primary afferents. Alternatively, alterations in the balance of facilitatory and inhibitory descending influences on dorsal horn neurons could change the excitability of these neurons and result in increased ascending interoceptive input to the brain. However this central inhibition could also be at the level of the pINS itself, not only at the dorsal horn.
It has been shown in several studies that hypnotic modulation can increase and decrease pain perception in both HCs and patients with chronic pain.25-28 Recently Abrahamsen demonstrated that hypnotic hypoalgesia induced a decrease of brain activity in the pINS.29 In the present study, we did not assess to what extent subjects managed to use self-hypnosis strategies during the scanning procedure. However, pre-post differences for the low intensity distension and for the two expectation conditions were almost exclusively seen in the HYP group and not in the EDU group, despite similar reductions in symptom ratings. This suggests that somewhat different brain regions may be involved in the symptom reduction by the two types of interventions.
In this study, the rectal distension was predicted by a visual cue, preparing the subjects for the imminent stimulus. During the expectation of the high intensity distension, HYP responders reduced their BOLD response in the left aINS. During expectation of the low intensity distension, HYP responders reduced their BOLD response in left HIPP, left pINS and right THAL, but showed increased BOLD response in right AMY and in the left PAG. In the subsequent distension there was a reduced activation in the left pINS. PAG is a part of the endogenous pain modulating system and allows regulation of nociceptive input both in facilitation and inhibition, mainly at the dorsal horn.28, 30 Earlier it has been demonstrated in both humans 31, 32 and animal models 33 that stimulation of PAG can produce a subsequent analgesic effect, and that AMYG input to the PAG is part of this endogenous pain modulation circuitry.34 Even though PAG activation in expectation of pain has been shown to be predictive of pain-related activation in the pINS,35 the current findings are most consistent with hypnosis induced engagement of the AMYG - PAG circuit in the attenuation of visceral sensation.
The educational intervention in the present study was effective in the treatment of IBS symptoms in a similar way than in earlier studies.36, 37 The EDU responders demonstrated a pre-post treatment decrease in aINS, but not in pINS suggesting that they, similarly to HYP responders, experienced a reduced perception of visceral stimuli after treatment. However, EDU but not HYP responders demonstrated a pre-post treatment BOLD decrease in vlPFC, during the high intensity distension. During the expectation of the high intensity stimulus there was a trend into the same direction. These findings suggest that educational therapy exerts its effect due to mechanisms involving the prefrontal cortex.
IBS treatment responders versus HCs
Before treatment IBS treatment responders had greater BOLD responses during high intensity distension in left vlPFC, left aMCC, left pACC and bilat sgACC and trends towards greater BOLD activation in right vlPFC, left aINS, left mINS and left pINS, when compared with HCs, Table 5. After treatment all these differences decreased or disappeared, indicating that BOLD decrease in these regions is in consonant with a normalization. Correspondingly, pre-post treatment BOLD reductions in the responders were demonstrated in left vlPFC, left aINS and left pINS and for EDU responders also left sgACC.
The high intensity distensions were associated with a decreased BOLD response in left vlPFC and ACC regions in the HCs, and after treatment also in IBS patients (Figure 4a). The right vlPFC has been involved in a variety of inhibitory processes,38 and activation of this region is involved in the cognitive modulation of pain,28 and during placebo analgesia.38, 39 Interestingly in the present study the pre-post reduction in left vlPFC were most pronounced in the EDU group, while HCs did not demonstrate a BOLD decrease in left vlPFC. Provided that negative BOLD response can be interpreted as a neural inhibition 40, 41 the present results suggest that education is associated with reduced engagement of left vlPFC and ACC during unpleasant visceral stimulation. As right vlPFC has clearly been implicated in many studies in pain, behavior and emotion inhibition, one could speculate that the education induced reduction in left vlPFC results in relative dominance of right vlPFC, e.g. increased inhibition.
Clinical implications
Gut directed hypotherapy as well as disease related education resulted in a clinically significant level of symptom improvement and decreased GI related anxiety. These subjective changes were correlated with a reduction in BOLD responses in the aINS to a high intensity visceral stimulus. This finding clearly demonstrates that the processing and perception of visceral stimuli can be modulated by psychological and cognitive treatment. Given that there is a subgroup of IBS patients with predominant disturbances in central processing and modulation of visceral afferent signals (“central pain amplification”), the present findings establish psychological therapy as an important strategy to treat an important aspect of IBS pathophysiology. Long-term treatment follow-up studies with brain imaging evaluation are needed to evaluate if brain response alterations persist, and if this therapy may be curative for selected patients.
Supplementary Material
Acknowledgments
This study was funded by County Council of Östergötland, Lions forskningsfond för folksjukdomar, Svenska Läkaresällskapets forskningsfond.and NIDDK grant DK048351 (EAM).
Footnotes
STATEMENT OF INTERESTS
1. Declaration of personal interests: The authors declare no competing financial interests
2. Declaration of funding interests:
STATEMENT OF AUTHORSHIP
Guarantor of the article:
Susanna Walter
Specific author contributions
1. MBO Lowén: Study design; acquisition of data; analysis and interpretation of data; drafting of manuscript
2. EA Mayer: Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
3. M Sjöberg: Study design; hypnotherapy
4. K Tillisch: Study design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
5. B Naliboff: Study design
6. J Labus: Study design; statistical analysis
7. P Lundberg: Study concept; technical support, revision of the manuscript
8. M Ström: Study concept and design; Administrative support; supervision; revision of the manuscript
9. M Engström:Study concept and design; acquisition of data; analysis and interpretation of data; study supervision
10. SA Walter: Study concept and design; acquisition of data; analysis and interpretation of data; drafting and final revision of the manuscript; obtained funding
All authors have approved the final version of the article and the authorship list.
Contributor Information
Mats B.O. Lowén, Department of Clinical and Experimental Medicine/Gastroenterology, Center for Medical Image Science and Visualization (CMIV), Linköping University Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
Emeran A. Mayer, The Gail and Gerald Oppenheimer Family Center for Neurobiology of Stress, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, USA.
Martha Sjöberg, Ersta Hospital, Karolinska Institute, Stockholm, Sweden.
Kirsten Tillisch, The Gail and Gerald Oppenheimer Family Center for Neurobiology of Stress, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, USA.
Bruce Naliboff, The Gail and Gerald Oppenheimer Family Center for Neurobiology of Stress, Department of Psychiatry, David Geffen School of Medicine at UCLA, Los Angeles, USA.
Jennifer Labus, The Gail and Gerald Oppenheimer Family Center for Neurobiology of Stress, Department of Psychiatry, David Geffen School of Medicine at UCLA, Los Angeles, USA.
Peter Lundberg, Center for Medical Image Science and Visualization, CMIV; Radiation Physics, Department of Medical and Health Sciences, Faculty of Health Sciences, Linköping University; Department of Radiation Physics UHL, County Council of Östergötland, Linköping, Sweden Center for Medical Image Science and Visualization, CMIV; Radiology, Department of Medical and Health Sciences, Faculty of Health Sciences Linköping University; Department of Radiology UHL, County Council of Östergötland, Linköping, Sweden.
Magnus Ström, Department of Clinical and Experimental Medicine/Gastroenterology, Linköping University, Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
Maria Engström, Department of Medical and Health Sciences (IMH)/Radiology, Linköping University, Sweden, Center for Medical Image Science and Visualization (CMIV) Linköping University, Sweden.
Susanna A. Walter, Department of Clinical and Experimental Medicine/Gastroenterology, Center for Medical Image Science and Visualization (CMIV), Linköping University Department of Gastroenterology, UHL, County Council of Östergötland, Linköping, Sweden.
REFERENCES
- 1.Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet. 1984;2(8414):1232–4. doi: 10.1016/s0140-6736(84)92793-4. [DOI] [PubMed] [Google Scholar]
- 2.Lindfors P, Unge P, Arvidsson P, et al. Effects of Gut-Directed Hypnotherapy on IBS in Different Clinical Settings-Results From Two Randomized, Controlled Trials. The American journal of gastroenterology. 2011 doi: 10.1038/ajg.2011.340. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Webb AN, Kukuruzovic RH, Catto-Smith AG, Sawyer SM. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2007;(4):CD005110. doi: 10.1002/14651858.CD005110.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Galovski TE, Blanchard EB. The treatment of irritable bowel syndrome with hypnotherapy. Applied psychophysiology and biofeedback. 1998;23(4):219–32. doi: 10.1023/a:1022209631047. [DOI] [PubMed] [Google Scholar]
- 6.Roberts L, Wilson S, Singh S, Roalfe A, Greenfield S. Gut-directed hypnotherapy for irritable bowel syndrome: piloting a primary care-based randomised controlled trial. The British journal of general practice : the journal of the Royal College of General Practitioners. 2006;56(523):115–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut. 1990;31(8):896–8. doi: 10.1136/gut.31.8.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lea R, Houghton LA, Calvert EL, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(5):635–42. doi: 10.1046/j.1365-2036.2003.01486.x. [DOI] [PubMed] [Google Scholar]
- 9.Simren M, Ringstrom G, Bjornsson ES, Abrahamsson H. Treatment with hypnotherapy reduces the sensory and motor component of the gastrocolonic response in irritable bowel syndrome. Psychosom Med. 2004;66(2):233–8. doi: 10.1097/01.psy.0000116964.76529.6e. [DOI] [PubMed] [Google Scholar]
- 10.Palsson OS, Turner MJ, Johnson DA, Burnelt CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig Dis Sci. 2002;47(11):2605–14. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- 11.Vlieger AM, van den Berg MM, Menko-Frankenhuis C, Bongers ME, Tromp E, Benninga MA. No change in rectal sensitivity after gut-directed hypnotherapy in children with functional abdominal pain or irritable bowel syndrome. The American journal of gastroenterology. 2010;105(1):213–8. doi: 10.1038/ajg.2009.613. [DOI] [PubMed] [Google Scholar]
- 12.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28(2):349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillisch K, Mayer EA, Labus JS. Quantitative Meta-Analysis Identifies Brain Regions Activated During Rectal Distension in Irritable Bowel Syndrome. Gastroenterology. 2011 doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53(11):1595–601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–96. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 18.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Whorwell PJ. IBS: Hypnotherapy-a wasted resource? Nature reviews. 2011;9(1):12–3. doi: 10.1038/nrgastro.2011.235. [DOI] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews. Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32(4):1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Cauda F, Costa T, Torta DM, et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62(1):343–55. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faymonville ME, Boly M, Laureys S. Functional neuroanatomy of the hypnotic state. J Physiol Paris. 2006;99(4-6):463–9. doi: 10.1016/j.jphysparis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Vanhaudenhuyse A, Boly M, Balteau E, et al. Pain and non-pain processing during hypnosis: a thulium-YAG event-related fMRI study. Neuroimage. 2009;47(3):1047–54. doi: 10.1016/j.neuroimage.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Schulz-Stubner S, Krings T, Meister IG, Rex S, Thron A, Rossaint R. Clinical hypnosis modulates functional magnetic resonance imaging signal intensities and pain perception in a thermal stimulation paradigm. Reg Anesth Pain Med. 2004;29(6):549–56. doi: 10.1016/j.rapm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamsen R, Dietz M, Lodahl S, et al. Effect of hypnotic pain modulation on brain activity in patients with temporomandibular disorder pain. Pain. 151(3):825–33. doi: 10.1016/j.pain.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neuroscience letters. 1999;262(1):61–4. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 31.Young RF, Brechner T. Electrical stimulation of the brain for relief of intractable pain due to cancer. Cancer. 1986;57(6):1266–72. doi: 10.1002/1097-0142(19860315)57:6<1266::aid-cncr2820570634>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Mayer DJ. Analgesia produced by electrical stimulation of the brain. Progress in neuro-psychopharmacology & biological psychiatry. 1984;8(4-6):557–64. doi: 10.1016/0278-5846(84)90015-0. [DOI] [PubMed] [Google Scholar]
- 33.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25(6):319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi S. Organization of neural systems for aversive information processing: pain, error, and punishment. Front Neurosci. 2012;6:136. doi: 10.3389/fnins.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1-2):101–10. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Ringstrom G, Storsrud S, Lundqvist S, Westman B, Simren M. Development of an educational intervention for patients with Irritable Bowel Syndrome (IBS): a pilot study. BMC gastroenterology. 2009;9:10. doi: 10.1186/1471-230X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringstrom G, Storsrud S, Posserud I, Lundqvist S, Westman B, Simren M. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22(4):420–8. doi: 10.1097/MEG.0b013e3283333b61. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman MD, Jarcho JM, Berman S, et al. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22(1):447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150(1):59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62(2):1040–50. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 41.Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–7. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.