Abstract
A phase II non-randomized single arm trial of oral panobinostat (LBH589) was conducted in patients with low or intermediate-1 risk myelodysplastic syndrome (MDS). The objective of this study was to determine the clinical efficacy, safety, and tolerability of panobinostat in this patient population. The study would stop early if there was a chance >99% that the average overall response rate was <15%. Thirteen patients were treated: Median age was 70 years (range 47–84), 70% were transfusion dependent, 70% had intermediate-1 risk MDS. Most patients were diploid but one patient with del(5q), one with trisomy 8, one with complex cytogenetics, and two with deletion of 20q were included. Approximately 40% had previous therapy for MDS. Of the 13 patients, 1 (8%) achieved hematological improvement (HI-E and HI-P, duration 3 months) and 6 (46%) had stable disease for a median duration of 6 months (range 1.7–13.6). Treatment was withheld in one patient because of QTc prolongation but no other serious adverse effects were otherwise observed. Panobinostat did not consistently induce histone acetylation as measured with Western blot. This data indicates that panobinostat at the dose and schedule studied here has limited activity in lower risk MDS.
Treatment options for patients with lower risk (low and intermediate-1 by IPSS [1]) myelodysplastic syndrome (MDS) are limited [2]. Options include lenalidomide [3] for patients with alterations of chromosome 5 and the hypomethylating agents [4].The use of this last group of drugs is not well understood in this subset of patients. Allogeneic stem cell transplantation [5] is usually reserved for patients with more advanced disease and the impact of erythroid growth factors is controversial at this time [6]. A subset of patients may benefit from immune manipulation [7]. Therefore, newer agents are needed for patients with lower risk MDS. Panobinostat is a very potent panhistone deacetylase inhibitor (HDACi) currently being investigated in a number of human malignancies [8]. HDACis were initially developed as differentiation agents in human leukemia and have been shown to be safe and active in patients with myeloid malignancies [9]. Based on this, we hypothesized that single-agent panobinostat could be active in lower risk MDS. Oral route of administration and safety profile further increased interest in this approach.
In conclusion, panobinostat given as a single agent orally at a dose of 20 mg thrice a week followed by 1 week of rest has limited clinical activity in patients with lower risk MDS. Because of the favorable toxicity profile, it is possible that combination strategies or other doses and schedules maybe more effective and worth investigating.
Methods
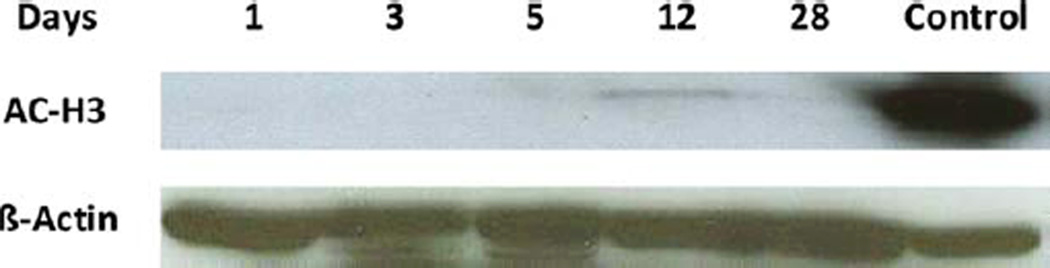
To test this concept, we designed a phase II open-label efficacy and toxicity trial of panobinostat for patients above 18 years of age with int-1 risk or transfusion dependent low-risk MDS. Patients could receive panobinostat as first treatment line for their hematological disease. Patients who had received prior therapy were allowed in the study. Appropriate renal, hepatic, and cardiac functions were required. Patients were excluded if they had previous HDACi treatment or if they were currently treated with valproic acid for other conditions. Patients with history of cardiac pathology such as rhythm alterations were excluded from the study. Use of drugs that could induce QT prolongation and CYP3A4 inhibitors were not allowed. Panobinostat was administered at a dose of 20 mg orally three times a week for three consecutive weeks with cycles repeated every 4 weeks for as long as possible unless toxicity of progression. The primary objective of the study was overall response rate defined by International Working Group [10]. A maximum of 40 patients could be enrolled. The study was to stop early if the expected response rate was <15%. Stopping rules were as follows: Stop if the number of patients with hematologic improvement/the number of patients evaluated is ≤0/15 or 1/32. The study also contained a stopping rule for non-hematological toxicity. Toxicity was graded using CTCAE version 3.0. Thirteen patients were enrolled between August 2009 and December 2010. Patient-related characteristics are shown in Table I. Median age was 70 years of age (range 47–84, 84% of patients older than 60), 70% were transfusion dependent, 70% had intermediate-1 risk MDS, most patients were diploid but one patient with del(5q), one with trisomy 8, one with complex cytogenetics, and two with deletion of 20q were included. Median percent of marrow blasts was 1% (range 1–6%). At start of therapy, median hemoglobin was 9.5 (range 7.5–11.2 G/dL), median platelet count was 56 (range 6–431 k/uL), and median white blood cell count was 4.6 (range 0.8–20.3 k/UL). Approximately 40% had previous therapy for MDS including hypomethylating agents, lenalidomide, and investigational agent. Median number of prior therapies in treated patients was two (range 1–4). Median duration of disease at time of enrollment was 10 months (range 1–50). Patients received a median of four cycles of panobinostat (range 1–9). Of 13 patients, one (8%) achieved a hematological improvement. This included both HI-E and HI-P for duration of 3 months. This patient was a 65-year-old woman with refractory cytopenia with multilineage dysplasia, no prior therapy, and diploid cytogenetics. No complete remissions or partial responses were documented. Six patients (46%) had stable disease for a median duration of 6 months (range 2–13.6). Median overall survival was 15 months (1–31 months). Two patients died because progression to acute myelogenous leukemia (AML). Therapy was well tolerated (Table II): no major adverse events were documented except for one patient that developed significant QTc prolongation. QTc interval increased from a baseline of 409 ms to 556 ms. The patient was asymptomatic and the event did not result in cardiac arrhythmia. At the time of event, patient also had severe anemia (hemoglobin 6 g/dL). QTc normalized with drug cessation and correction of anemia. Patient was discontinued from study drug. As a biomarker of molecular activity, histone H3 acetylation was measured in five patients with variable results. Induction of acetylation was documented in two (Fig. 1). Stopping rules for response or toxicity were not officially met. Despite this and due to the very modest clinical activity observed, the study was closed to new patient entry at MD Anderson Cancer Center on 3/2011.
TABLE I.
Patient Characteristics
| Patient characteristics (N = 13) | N (%) |
|---|---|
| Median age years (range) | 70 (47–84) |
| Male sex | 8 (62) |
| RA/RCMD/RARS/MDS-U | 11 (84) |
| RAEB | 1 (8) |
| MPN | 1 (8) |
| Cytogenetic | |
| Diploid | 8 (61) |
| Del(5q) | 1 (8) |
| +8 | 1 (8) |
| 20q− | 2 (15) |
| Complex | 1 (8) |
| Bone marrow blast % | |
| 0–4 | 11 (85) |
| 5–10 | 2 (15) |
| Prior transfusion | 9 (69) |
| Prior treatment | 5 (38) |
| Prior malignancy | 2 (15) |
| PS > 1 | 6 (46) |
| IPSS Int-1 | 11 (85) |
RA/RCMD/RARS/MDS-U: refractory anemia, refractory anemia with multilineage dysplasia, refractory anemia with ring sideroblasts, MDS-unclassifiable; RAEB, refractory anemia with excess blasts; MPN, myeloproliferative neoplasm; PS, performance status.
TABLE II.
Toxicity of Profile
| Toxicity | N (%) | Grade |
|---|---|---|
| Cardiac | 1 (8) | QTc prolongation |
| Fatigue | 2 (15) | 2 |
| Decreased appetite | 1 (8) | 2 |
| Rash | 1 (8) | 1 |
| Dizziness | 1 (8) | 1 |
| Nausea | 1 (8) | 1 |
| Dyspea | 1 (8) | 1 |
| Diarrhea | 1 (8) | 1 |
| Hyperbilirubinemia | 2 (15) | 1 |
Figure 1.
Induction of histone H3 acetylation by LBH589. Histone acetylation was measured as previously reported using Western blots [11]. AC-H3, acetylated histone H3. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Greenberg P, Cox C, LeBeau MM, Fenaux P, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 2.Garcia-Manero G. Myelodysplastic syndromes: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:490–498. doi: 10.1002/ajh.22047. [DOI] [PubMed] [Google Scholar]
- 3.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol. 2011;29:516–523. doi: 10.1200/JCO.2010.31.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 6.Jadersten M, Montgomery SM, Dybedal I, et al. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106:803–811. doi: 10.1182/blood-2004-10-3872. [DOI] [PubMed] [Google Scholar]
- 7.Zou JX, Rollison DE, Boulware D, et al. Altered naive and memory CD4+ T-cell homeostasis and immunosenescence characterize younger patients with myelodysplastic syndrome. Leukemia. 2009;23:1288–1296. doi: 10.1038/leu.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Manero G, Issa JP. Histone deacetylase inhibitors: A review of their clinical status as antineoplastic agents. Cancer Invest. 2005;23:635–642. doi: 10.1080/07357900500283119. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 11.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]