Preface
Clinical trials of vaccines against Mycobacterium tuberculosis are in full swing and results are starting to come in, some not so encouraging as exemplified by the latest Aeras-422 and MVA85A trials. Other than empirically determining whether a vaccine reduces the number of cases of active tuberculosis, a daunting prospect given the chronic nature of the disease, we have no way of assessing vaccine efficacy. Therefore, investigators seek to identify biomarkers that predict vaccine efficacy. Historically, focus has been on CD4+ T cell production of interferon-γ, but this has not been a useful correlate of vaccine-induced protection. Here we discuss recent advances in our understanding of immune control of M. tuberculosis and how this knowledge could be used for vaccine design and evaluation.
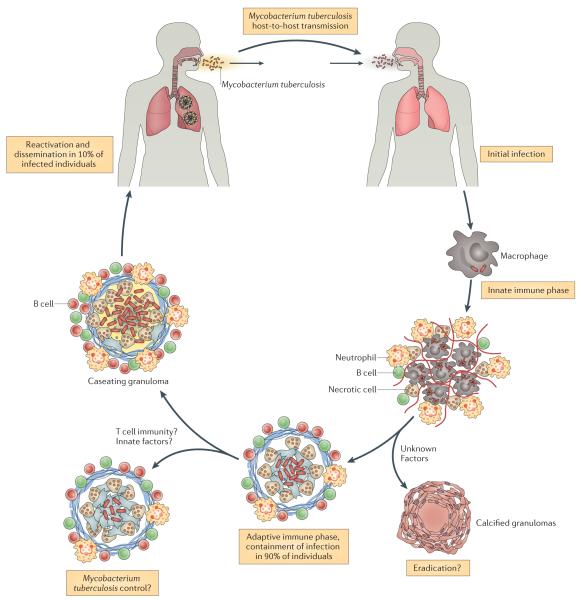
Tuberculosis (TB) is caused by the pathogenic bacterium Mycobacterium tuberculosis, which is transmitted between people via aerosol droplets containing bacteria. The droplets are inhaled and deposited in distal lung alveoli (Figure 1)1. M. tuberculosis is an intracellular bacterium and although it can infect different cell types, alveolar macrophages are its favorite niche. The initial stages of infection are characterized by innate immune responses involving the recruitment of inflammatory cells to the lung2; induction of an adaptive immune response occurs only later, after dissemination of M. tuberculosis to draining lymph nodes3-5. In the lymph node, presentation of bacterial antigens by dendritic cells leads to priming and expansion of antigen-specific T cells, which differentiate from naïve into effector T cells. The effector T cells then migrate to the infected lung and, in combination with other leukocytes, stimulate the formation of granulomas. Granulomas are organized structures containing macrophages, lymphocytes and fibroblasts6. Within the granuloma, macrophages are activated, for example, by IFNγ secreted by CD4+ T cells (Th1 cells), which is thought to restrict the dispersal and replication of M. tuberculosis.
Figure 1.
TB pathogenesis. Infection is initiated by inhalation of aerosol droplets containing bacteria. The initial stages of infection are characterized by innate immune responses involving recruitment of inflammatory cells to the lung. Following bacterial dissemination to the draining lymph node, dendritic cell presentation of bacterial antigens leads to T cell priming, and triggers an expansion of antigen-specific T cells, which are recruited to the lung. The recruitment of immune T cells, B cells, activated macrophages and other leukocytes leads to the establishment of granulomas, which can contain M. tuberculosis. The majority of infected individuals will remain in a “latent” state of infection, in which no clinical symptoms are present. A small percentage of these people will eventually progress and develop active disease, which can lead to the release of M. tuberculosis from granulomas eroded into the airways. When individuals with active TB cough, they can generate infectious droplets that propagate the infection.
Although the human immune system can control the infection, control does not invariably lead to sterilization. In fact, most people infected with M. tuberculosis are clinically asymptomatic, a state referred to as latent TB7. These latently infected people –estimated to be one third of the world’s population – represent an enormous reservoir of potential disease. Epidemiological studies find that 5-10% of people with latent TB will develop active disease sometime during their lives 8. Individuals with active TB cough and generate infectious droplets that propagate the infection (Figure 1).
An effective vaccine is needed to stop the ongoing pandemic. Mycobacterium bovis Bacille Calmette Guerin (BCG), an attenuated form of M. bovis, was introduced nearly a century ago as a vaccine against M. tuberculosis, but it has had little impact in eliminating TB. In part, this is because BCG efficacy against active pulmonary TB is extremely variable between populations, and BCG-induced protection is significantly lower in the developing world9. Remarkable progress has been made in the development of new vaccine candidates and several are now in clinical trials (Box 1). Although there is some pessimism about whether a vaccine can be developed that averts infection, the general consensus is that a vaccine that prevents the progression to active disease could reduce the prevalence of pulmonary TB and ultimately break the cycle of transmission.
Box 1. Tuberculosis vaccines.
Owing to the shortcomings of BCG vaccination in preventing TB, significant effort has been put into developing new vaccines. Currently more than 12 candidates are being tested in clinical trials137, 138. These candidates aim to replace BCG, or act as a booster vaccine following BCG. The vaccines include viral vectors expressing M. tuberculosis antigens; M. tuberculosis proteins with improved adjuvants; recombinant BCG strains and live attenuated M. tuberculosis vaccines. Unfortunately, some preliminary results have been disappointing: Aeras-422, a recombinant BCG strain failed because of safety concerns137 and MVA85A, a new vaccine consisting of Modified Vaccinia Ankara virus (MVA, a replicative-defective variant of Vaccinia virus) expressing the M. tuberculosis antigen 85A, and designed to enhance BCG-induced protection, showed no efficacy in a Phase 2b trial139.
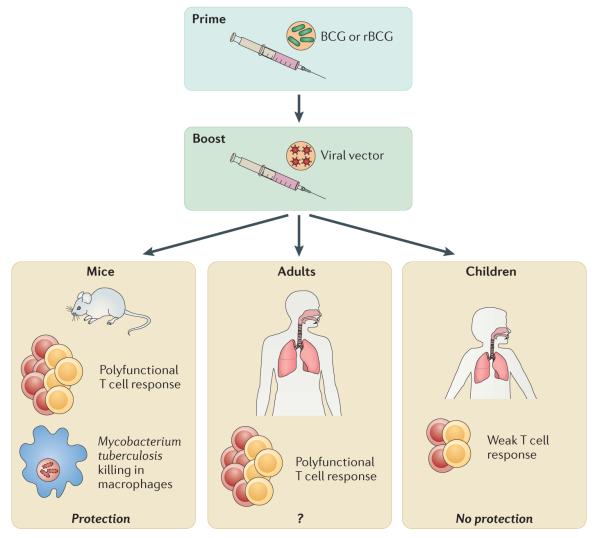
MVA85A, has been extensively investigated as a booster following BCG vaccination, in what has become known as the ‘prime-boost’ strategy (see accompanying figure). MVA85A is effective in boosting BCG vaccination in a variety of M. tuberculosis animal challenge models. Initial studies with MVA85A in people showed promise, as significantly more antigen-specific T cells from the boosted group secreted IFNγ and were polyfunctional compared to vaccination with BCG alone140, 141. These effects were durable and lasted at least 24 weeks after the MVA85A boost140. However, the recent results of the phase 2b clinical trial indicate that MVA85A is not effective at preventing M. tuberculosis infection or tuberculosis139. Administered to infants ages 4-6 months as a booster to BCG vaccination given at birth, MVA85A elicited overall small numbers of CD4+ T cells that secrete IFNγ, IL-2, and TNF at 28 days after vaccination. Although slightly greater T cell responses were noted in the vaccinated group, no differences in protection from TB were observed in a 2-year follow-up139.
A recurring question is whether the cytokines measured in these studies are useful predictors of vaccine protection, or whether specific markers exist that could have predicted a lack of protection. Another issue is whether the immature immune systems of infants, compromises potential vaccine efficacy. These findings raise the question of whether MVA85A should be evaluated in adults.
Most antiviral vaccines that have proven to be effective are based on antibody-mediated immunity. As is the case for many intracellular bacteria, M. tuberculosis is able to avoid most antibacterial effects mediated by antibodies by living and growing inside macrophages. Thus, based on the substantial experimental foundation that T cell immunity is required to control primary M. tuberculosis infection, the consensus among vaccinologists is that vaccine-induced T cell mediated immunity will be required to prevent clinical TB. However, despite significant advances in defining how the immune system responds to M. tuberculosis, our understanding of protective immunity following infection (natural immunity) is incomplete. Furthermore, little is known about the mechanisms of vaccine-induced immunity, and whether it differs from natural immunity, and studies to answer these questions have not kept pace with the speed with which new vaccines are entering clinical trials. It is unknown which immunological parameters or biomarkers predict who will control the infection and who will develop clinical disease both in the setting of natural and of vaccine-induced immunity. Such knowledge would revolutionize our approach to surveillance, control, and treatment of TB and it would greatly accelerate vaccine design and evaluation. However, identifying biomarkers of vaccine protection is difficult: until there is a successful vaccine that induces protective immunity, how can such a biomarker be identified? As it stands now, any success or failure of TB vaccines will be largely empiric and difficult to predict.
In this Opinion, we discuss immune defenses against M. tuberculosis infection. T cells predominantly mediate protective immunity and recent results begin to clarify how different T cell subsets and functions restrict bacterial growth. Finally, we will discuss how one might use knowledge about these different mechanisms to develop new vaccine strategies to prevent tuberculosis.
The “central dogma” of protective immunity
Establishing the importance of IFNγ
During the past four decades, the predominant paradigm in both basic and clinical research has been that IFNγ production by CD4+ T cells is the major driver of immunity to TB. Research in the 70’s found that T cells, and not antibodies, are required for host resistance to TB, and established the mouse as a useful model of tuberculosis10. The T cell hypothesis was further refined in the 80’s with the identification of CD4+ T cells producing IFNγ (Th1 cells) as the dominant T cell subset participating in the immune response to M. tuberculosis11, 12. The use of knockout mice in the 90’s established a crucial role for CD4+ T cells, with additional roles for CD8+ T cells, iNKT cells and γδ+ T cells13, 14. The discovery that AIDS, a condition often associated with TB, was caused by HIV, a virus that infects and kills CD4+ T cells, supported a key role for CD4+ T cells in immunity against M. tuberculosis in people15.
A central role for IFNγ, a cytokine involved in the response against viruses and intracellular bacteria, in anti-mycobacterial immunity is based on the extreme susceptibility of mice that lack IFNγ16, 17. IFNγ activates macrophages to kill intracellular bacteria by activating downstream antimicrobial effector pathways including iNOS, IFNγ inducible GTPases, phagosomal maturation and acidification, autophagy, and Vitamin D receptor signaling18-23. Genetic studies confirm a role for IFNγ in people: families with mutations in the IL-12/IFNγ/STAT1 axis develop disseminated infections caused by BCG and non-tuberculous mycobacteria (NTM) species. This inherited susceptibility, called Mendelian Susceptibility to Mycobacterial Disease (MSMD), reveals the crucial nature of this signaling pathway, which was first described in mice16, 17, 24, 25.
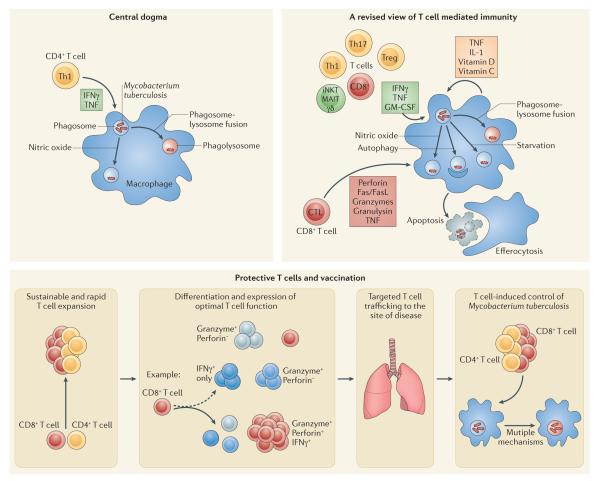
These discoveries helped to define the ‘central dogma’ of TB immunity, namely that T cell production of IFNγ activates macrophages to kill intracellular M. tuberculosis (Figure 2a). Indeed, detection of IFNγ produced by T cells is the most widely used method for detecting immune responses following infection or vaccination.
Figure 2.
Paradigms of protective immunity to TB.
a. The “central dogma” of protective immunity to TB is that CD4+ T cells produce IFNγ, which synergizes with TNF (produced by the T cell or the macrophage), and together these activate macrophage antimicrobial activity capable of restricting M. tuberculosis growth. Two pathways activated by IFNγ that are capable of killing M. tuberculosis are nitric oxide (NO) production and phagolysosome fusion, which acidifies the bacterial phagosome.
b. “A revised view of protective T cell immunity” incorporates additional T cell subsets (CD4+, CD8+, and unconventional T cells – γδ T cells, MAIT cells and CD1-restricted T cells), and includes additional mechanisms by which T cells mediate killing of M. tuberculosis. These include additional cytokines (for example, possibly GM-CSF) and cytolysis of infected macrophages. The cytolytic mechanisms vary and can include cytotoxic granules, which can deliver antimicrobial peptides such as granulysin, but can also deliver granzymes, which can trigger apoptotic cell death. CTL activity mediated by FasL/Fas or TNF can also lead to apoptosis. Apoptosis can have a beneficial effect on the outcome of infection as infected apoptotic cells can be engulfed by bystander macrophages, which are capable of destroying the apoptotic cells including any intracellular bacteria. Finally, several components of the innate response, including IL-1 and vitamins, can synergize with cytokines produced by T cells.
c. “Protective T cells and vaccination” focuses on the desired features of protective T cell responses. Rationale vaccine design should aim to elicit protective T cells by optimizing their action on infected cells in several ways. Vaccine-elicited memory T cells must rapidly expand and generate secondary effector T cells that undergo sustained proliferation following activation. While the functions of primary effector T cells are expressed heterogeneously (broken arrow), vaccination (solid arrow) can lead to more homogenous expression of effector functions during the recall response. Such T cells, often identified as multifunctional T cells, may have a greater protective potential. Primed effector and memory T cells should efficiently traffic to sites of infection, but the kinetics of the response must be balanced with respect to T cell subsets, and limit the potential for T cell exhaustion, excessive inflammatory pathology, or an ineffective response that hinders T cell - target contact.
Shortcomings of the “central dogma”
Although IFNγ and CD4+ T cells are key components of the immune responses against mycobacteria, the intricacies of immunity to M. tuberculosis require that we reassess their roles. For instance, the risk of active TB significantly increases during the first year after HIV infection despite normal CD4+ T cell counts26, and progression to AIDS, which is characterized by a substantial loss of CD4+ T cells, does not correlate with the development of active TB26, 27. HIV infection induces a number of immunological abnormalities, some that are apparent even before CD4+ T cell numbers decline28. It is possible that alterations in CD4+ T cell function secondary to HIV infection increase TB susceptibility even before CD4+ T cell numbers fall. However, this pattern of susceptibility is clearly different from other opportunistic infections whose incidence correlates with the peripheral blood CD4+ T cell count in HIV patients29.
Similar complexity is observed for MSMD patients: over 300 cases of MSMD have been described, but M. tuberculosis infection was present in only four cases; the rest were BCG or NTM species24. Although such bias might reflect the relative exposure to BCG or NTM compared to M. tuberculosis, IFNγ-activated pathways might be more important for immunity against NTM than against M. tuberculosis24, 30. Although these rare cases of TB and immunodeficiency are instructive, most people that develop active TB have no obvious defects within their T cell compartment and generate M. tuberculosis-specific IFNγ responses. Thus, whereas HIV and MSMD patients establish that T cells and IFNγ are required for immunity against M. tuberculosis, T cells producing IFNγ do not appear to be sufficient to prevent active disease.
The shortcomings of the “central dogma” also apply to disease progression and vaccine-induced protection in otherwise healthy people, as more T cells secreting IFNγ or greater IFNγ levels do not correlate with protection31. In fact, patients whose T cells produce greater amounts of IFNγ are more likely to progress to active disease than patients with weaker responses32, supporting the idea that IFNγ levels correlate better with bacterial burden than disease control. Such a correlation between increased M. tuberculosis bacterial burden and increased T cell IFNγ production has been observed in humans, non-human primates (NHP), and mice32-35.
Similar conclusions can be drawn from vaccination studies36. BCG vaccination can elicit protective T cells in experimental animals, but IFNγ production by these T cells has not been predictive of vaccine-induced protection36, 37. The only predictor of protection in mice vaccinated with BCG is an increased number of antigen-specific CD8+ T cells36. In some human studies, increased IFNγ production by T cells has been observed after BCG vaccination or adult re-vaccination, but protection was not evaluated38-41. One study in South African infants vaccinated with BCG addressed the relationship between vaccine-induced protection, T cell frequency and their cytokine profile, but found no correlation between the number of BCG-elicited T cells producing IFNγ or multiple cytokines (IFNγ, IL-2, and TNF) and the development of culture positive TB42.
These data raise the question: if CD4+ T cells and IFNγ are important, why doesn’t IFNγ production by CD4+ T cells correlate with protection? The idea that, because CD4+ T cells produce IFNγ, their IFNγ production must be important is an assumption and one with little supporting data. Several studies have shown that CD4+ T cells protect mice against M. tuberculosis independently of IFNγ43-47. Transgenic CD4+ T cells, which are specific for the M. tuberculosis antigen ESAT6 retain their ability to protect mice against M. tuberculosis even when unable to produce IFNγ or TNF 44. Similarly, the ability of IFNγ−/− memory T cells to mediate protection is only slightly diminished compared to wild type memory T cells45, 48. These studies demonstrate that although CD4+ T cells and IFNγ are important for M. tuberculosis control, T cell functions other than IFNγ production can mediate protection. Furthermore, it is unknown how much IFNγ is needed, which cells are required to produce it, and whether more is better49. Also, the inflammatory microenvironment in which IFNγ is produced might be important, as the balance between IFNγ and different cytokines, such as IL-10 and other Th2 cytokines, is likely to influence disease outcome50. Therefore, it is crucial to identify factors that are required for resistance and correlate with susceptibility in individuals with intact immune systems, as opposed to components of the immune response that are necessary for protection but don’t predict clinical outcome or disease state.
The notion that IFNγ is necessary but not sufficient for bacterial control following mycobacterial infection is supported by multiple studies in mice. For example, several knockout mice (such as TNF−/−, GM-CSF−/−, IL-1−/−, and IL-6−/−) die rapidly following M. tuberculosis infection, similar to IFNγ−/− animals51-54. Since these mice produce IFNγ, their failure to control M. tuberculosis indicates that additional pathways besides IFNγ are essential for immunity. Although the data from knockout mice and MSMD families is irrefutable, more mechanistic insights into the protective pathways that lead to M. tuberculosis control are needed. It is also important to remember that although IFNγ inhibits M. tuberculosis replication in murine macrophages55, it is not sufficient to control M. tuberculosis growth in human macrophages56, 57. Similarly, nitric oxide (NO) production by murine macrophages can kill M. tuberculosis, but its production by human alveolar macrophages and its role in controlling M. tuberuclosis in these cells is controversial 58, 59. These observations reinforce the idea that we must look beyond the CD4+ T cell/IFNγ central dogma to identify other immunological functions that protect against M. tuberculosis.
Reassessing protective immunity
In recent years, many studies have looked past the central dogma and revealed different pathways involved in protective immunity during TB. These studies reveal characteristics of protective T cells that should be incorporated in the design of new vaccines against M. tuberculosis (Figure 2b).
Other mediators that activate macrophages
The cytokines TNF, GM-CSF and IL-1β and vitamins C and D are all implicated as mediators that activate macrophages to control M. tuberculosis growth. Mice that lack TNF are highly susceptible to M. tuberculosis infection and TNF production by T cells has been shown to be important for resistance against M. tuberculosis51, 60. TNF synergizes with IFNγ in stimulating NO production by macrophages, maintains granuloma structure, and limits immunopathology, possibly through modulation of IL-10 levels, inhibition of Th2 responses, and limiting neutrophil infiltration61-63. The widespread use of TNF blockers to treat patients with autoimmune diseases for which TNF is a pathogenic factor, such as rheumatoid arthritis, has resulted in numerous cases of reactivated latent TB, establishing TNF as an important mediator of resistance to M. tuberculosis in people64.
Mice lacking GM-CSF are highly susceptible to M. tuberculosis and GM-CSF treatment of human macrophages restricts intracellular growth of M. tuberculosis and M. avium53, 65, 66. GM-CSF is produced by a multitude of cells including leukocytes, epithelial cells and fibroblasts, and loss of this cytokine leads to abnormalities in surfactant recycling and the development of a lung disease that resembles human pulmonary alveolar proteinosis67. Overexpressing GM-CSF in epithelial cells reverses these lung abnormalities but the susceptibility to M. tuberculosis remains, suggesting that GM-CSF production by other cells, perhaps T cells, contributes to protection in mice. This idea is supported by the observation that iNKT cell production of GM-CSF contributes to host resistance against tuberculosis 68. Additionally, the presence of anti-GM-CSF autoantibodies that block GMCSF function has been linked to both cryptococcal meningitis and pulmonary TB in otherwise healthy subjects indicating that GM-CSF has an important role in host defense against infection in people69.
Mice lacking IL-1β, a pro-inflammatory cytokine produced by macrophages, or its receptor are highly susceptible to M. tuberculosis infection and IL-1β directly inhibits intracellular growth of M. tuberculosis47, 52, 70-72. Although mice lacking IL-1β die prematurely from infection, IL-1β can also be detrimental by recruiting pathogenic Th17 cells and neutrophils to the lung, resulting in tissue inflammation 46, 73, 74. IL-1β also activates human macrophages to control bacterial replication70, 72, 75.
Stimulation with either a ligand that triggers TLR2/1 or IFN-γ induces the nuclear vitamin D receptor (VDR) and enzymes that catalyze the conversion of vitamin D to its bioactive form22, 76. Signaling through VDR elicits production of the human cathelicidin LL-37, an antimicrobial peptide that directly kills M. tuberculosis77. Beyond its role in cathelicidin production, Vitamin D is involved in autophagy, phagolysosome fusion and IL-1β production22, 78, 79. Dissecting the role of vitamin D has been challenging. Multiple studies show decreased levels of bioactive vitamin D in TB patients, but whether this is a cause or an effect of TB is unknown; and whether Vitamin D supplementation benefits treatment is still uncertain80.
Vitamin C might be important for immunity against M. tuberculosis, as vitamin C affects M. tuberculosis survival and growth81. Given the established association between malnutrition and susceptibility to TB, it is important to determine whether specific nutritional deficiencies contribute to M. tuberculosis susceptibility.
Killing of infected macrophages
In addition to cytokine production, T cells, particularly CD8+ T cells, kill cells that they recognize as ‘foreign’. CD8+ T cells with the capacity to kill target cells are called cytotoxic T lymphocytes (CTLs). M. tuberculosis elicits CD8+ T cell responses in people and animal models and these CD8+ T cells behave as CTLs in vivo 82-85. Three different molecular pathways mediate CTL activity: exocytosis of cytotoxic granules containing proteins that cause lysis and apoptosis of target cells, such as perforin, granulysin and granzymes; Fas/FasL (CD95/CD95L), cell surface proteins that mediate death signaling; and TNF85. The increased susceptibility of Fas−/−, FasL−/− and perforin−/− mice to M. tuberculosis corroborate the importance of these pathways for immunity86, 87. Importantly, perforin is required for protection mediated by CTLs85. Human CD8+ T cells also require perforin to restrict M. tuberculosis growth, with granulysin being an important granule constituent88. Other than perforin, the crucial effector molecules for murine CD8+ T cells are unknown88.
How killing of infected macrophages by CD8+ T cells impairs M. tuberculosis survival is an active area of investigation. All three killing pathways induce target cell apoptosis, which is associated with reduced bacterial viability89. The engulfment of apoptotic, infected cells by uninfected macrophages – a process known as efferocytosis – leads to rapid association of the bacteria trapped in the phagocytosed apoptotic cell (the ‘efferosome’) with lysosomes and killing of M. tuberculosis 90.
T cells orchestrate granuloma formation
In addition to detecting infected macrophages, T cells have a key role in the formation of granulomas. T cell-derived cytokines (such as TNF) and chemokines (such as CCL3) recruit inflammatory macrophages, neutrophils and B cells to the granuloma91. IFNγ and TNF maintain granuloma architecture in mice and people17, 64, 92-94. The importance of CD4+ T cells in shaping the granuloma microenvironment is inferred from HIV+ subjects who form dysfunctional granulomas that fail to contain M. tuberculosis95 and by studies in guinea pigs and rabbits96, 97. Recent imaging studies in people and NHP indicate that granulomas behave autonomously and are more dynamic than previously appreciated8, 98. Granulomas change over time independently of each other with respect to size and metabolic activity – some shrink whereas others expand. Although CD4+ T cells promote granuloma formation early after M. tuberculosis infection, they also contribute to transmission by promoting granuloma necrosis accompanied by erosion into airways during later disease stages99.
Balancing pro- and anti-inflammatory signals
In many chronic infections, including TB, immune-mediated tissue injury is more detrimental than the pathogen itself. Therefore, mechanisms exist to counter-regulate pro-inflammatory immune cells and prevent the harmful effects of excessive inflammation; however, these effects might also dampen protective immunity.
Foxp3+ regulatory T cells (Tregs) suppress inflammation and limit immune responses by producing immunosuppressive cytokines such as IL-10 and TGF-β and by directly interacting with other cells via inhibitory cell surface molecules100. Tregs are generated following M. tuberculosis infection in humans, NHP and mice101-104. In mice, Treg elimination can enhance protective immunity, as observed by the survival of fewer bacteria; however, whether this occurs at the risk of greater tissue injury has not been addressed104-106.
Chronic antigen stimulation and exposure to inflammatory cytokines leads to a state of T cell exhaustion that is manifested by a progressive loss of T cell function over time, which has been best documented during chronic viral infection. There is great interest in the mediators of exhaustion because blocking them might ‘re-invigorate’ T cell immunity and promote pathogen clearance during chronic infection. One such mediator, PD-1, is a cell surface receptor expressed by antigen-activated T cells. Interaction of PD-1 with its ligands transduces a signal that inhibits T cell proliferation and cytokine production107. Disruption of the PD-1/ligand interaction, through the use of neutralizing antibodies or in knockout mice, increases the number and function of M. tuberculosis-specific T cells in the lungs of infected mice108-110. However, in the absence of PD-1 signaling, dysregulation of CD4+ T cells leads to increased bacterial burden, lung tissue destruction, and death of infected mice108, 110. These data suggest that T cell exhaustion might represent a beneficial regulatory mechanism that prevents overt immunopathology.
Neutrophils serve an early protective role against M. tuberculosis in the lung by producing IL-1β, TNF, defensins, cathelicidins, lipocalin, NADPH oxidase and superoxides77, 111-113. Neutrophils also participate in T cell priming including cross-presentation of class-I restricted antigens, a process important for the stimulation of CD8+ T cells by intracellular pathogens114. However, when the short-lived neutrophils die, the pro-inflammatory contents of their granules can be released; thus, an excess of neutrophils can promote tissue damage.
Although IFNγ is a pro-inflammatory cytokine, it also limits inflammation, at least in part through direct and indirect inhibition of neutrophils. IFNγ can have anti-proliferative effects on T cells and modulate their function, including inhibiting CD4+ T cell production of IL-17, a cytokine that drives neutrophilic inflammation115. In addition, IFNγ acts directly on neutrophils to inhibit their accumulation in the lung46, 73. In fact, we view neutrophil infiltrates in the lung as a sign of failed Th1 immunity, which leads to accelerated tissue destruction during chronic M. tuberculosis infection46. Similarly, NO restrains inflammation by inhibiting IL-1β production by macrophages. Whereas NO production by murine macrophages mediates the antimicrobial activity of IFNγ, NO also inhibits NLRP3 inflammasome assembly, which curtails the production of IL-1β116.
Collectively, these data support the notion that T cells are uniquely positioned to influence the balance of pro- and anti-inflammatory signals. These results strengthen the idea that the function of CD4+ T cells and IFNγ is broader than activating macrophages and is necessary for optimal immunity during M. tuberculosis infection. Thus, IFNγ acts as a key negative regulator of innate immunity including neutrophils and IL-1β, both of which might be beneficial early, but have detrimental effects if they persist into the chronic phase of M. tuberculosis infection. The anti-inflammatory role of T cells might prevent over-exuberant protective responses that cause harmful immunopathology and tissue damage during chronic infection46, 116. Measuring surrogates of pro- or anti-inflammatory signals, for example by expression profiling117 or measuring the monocyte/lymphocyte ratio118 in peripheral blood, could be useful to identify individuals who are at risk for active TB.
Other cells participate in the immune response to M. tuberculosis
Although it is generally accepted that conventional CD4+ and CD8+ T cells mediate protection against M. tuberculosis, many other cell types participate in the immune response (see Box 2 for the contribution of non-conventional T cells). The TB mouse model is CD4+ T cell centric and it is difficult to prove a role even for conventional CD8+ T cells. Other T cell subsets are not present or are qualitatively different in the mouse compared to humans. Similarly, the contribution of B cells and antibody-mediated immunity needs further clarification119. Thus, these different cell types need to be investigated in other models. Both CD8+ T cells and non-conventional T cells appear to have a quantitatively greater role in immunity to M. tuberculosis in NHP than in other animal models82, 120. Understanding the roles of these different cells types during M. tuberculosis infection might provide opportunities to discover new protective effector functions, and to develop methods to augment their function as part of new vaccination or treatment strategies.
Box 2. A role for non-conventional T cells in immunity to tuberculosis.
Box 2.
A role for non-conventional T cells in immunity to tuberculosis
| Cell Type | iNKT cell | Group I CD1- restricted T cell |
Mucosal- associated invariant T (MAIT) cell |
γδ T cell | Th17 cell |
|---|---|---|---|---|---|
| Recognizing | CD1d | CD1a, CD1b, CD1c |
MR1 | Butyrophilin142 | MHC II |
| Antigen Type | Lipids | Lipids | Riboflavin metabolites |
Phosphoantigens | Peptides |
|
Required for murine M. tuberculosis- immunity? |
No 14, 87, 143, 144 | Not found in mice |
Unknown | No | No145 |
|
Detected in M. tuberculosis- infected people? |
Yes 146-148 | Yes 149-151 | Yes 152, 153 | Yes 154 | Yes 155 |
|
Respond to M. tuberculosis- infected macrophages? |
Yes 156 | Yes 149 | Yes 157 | Yes | Yes 158 |
|
Stimulation leads to enhanced antimycobacterial function? |
Yes 130, 159 (αGalCer) |
Unknown | Unknown | Yes 160 (IL-2 + phosphoantigen) |
Unknown |
iNKT cells
iNKT cells are a T cell subset that recognize lipid and glycolipid antigens. Subjects with active TB have reduced iNKT cell numbers in peripheral blood compared to latently infected or healthy individuals146-148. Treatment of infected mice with αGalCer, a potent activator of iNKT cells, improves disease outcome and synergizes with antibiotics130, 159. αGalCer also stimulates human iNKT cells to lyse M. tuberculosis-infected macrophages and kill intracellular bacteria in vitro161. Murine iNKT cells cultured with M. tuberculosis-infected primary macrophages restrict bacterial growth and adoptive transfer of iNKT cells limits bacterial growth in vivo156. Activated iNKT cells also have adjuvant-like properties and conjugation of αGalCer to BCG augments its efficacy as a vaccine162. Why iNKT cells are dispensable in the intact mouse yet exert a major protective role once activated needs investigation. Also, the use of αGalCer in human TB still has not been explored.
Group I CD1-restricted T cells
CD1-restricted T cells that recognize the mycobacterial lipid glucose monomycolate or C32-phosphomycoketide can be detected in the peripheral blood of M. tuberculosis patients149-151. The effector function of these T cells and whether they can be elicited by vaccination is still not fully understood.
γδ T cells
Human γδ T cells recognize small organic phosphate antigens and alkylamines and expand in response to M. tuberculosis infected cells in vitro. Exciting data indicate that they generate a recall response following BCG vaccination and M. tuberculosis challenge in NHP120, 154, 163, 164. Although γδ T cells are not required for bacterial control in mice, they are the main source of IL-17 in the lung during M. tuberculosis infection13, 165. Activation of γδ T by IL-2 and phosphoantigen treatment results in reduced bacterial burdens and attenuated lesions in the lungs of NHP infected with M. tuberculosis160.
MAIT cells
MAIT cells, which are found in human lung and peripheral blood, recognize M. tuberculosis-infected cells152, 157. MAIT cells can have antimicrobial activity against bacteria and yeast, but their role during M. tuberculosis infection still requires investigation153.
Th17 cells
IL-17 has an early role in the recruitment of antigen-specific IFNγ-secreting Th1 cells, particularly after BCG vaccination164, 166, 167 as well as early granuloma formation. However, persistence of Th17 cells can be detrimental. IL-17 promotes neutrophil recruitment and inflammation, and if not ultimately suppressed by IFNγ, can exacerbate tissue damage46, 74, 115, 165.
Lessons for developing T cell vaccines
Is natural immunity against TB sufficient?
Many pathogens do not elicit protective immunity, including common ones that cause urinary tract infections (enteric gram-positive and gram-negative bacteria), sexually transmitted infections such as Chlamydia trachomatis, Neisseria gonorrhea and Treponema pallidum, and pharyngitis caused by group A streptococci; others such as poxviruses induce long term protection, an observation that is the basis of vaccination. It is still unclear why some pathogens, but not others, induce protective immunity against reinfection. What is the case for TB? If only around 10% of infected people develop active disease during their lifetime, one must concede that natural immunity works well, even if it doesn’t lead to sterilization. What about the 10% that develop symptomatic disease? Although progression to symptomatic disease can sometimes be attributed to acquired immunodeficiency (AIDS, TNF blockade, corticosteroids, autoantibodies, etc.) in many cases, immunocompetent individuals also develop active TB, which indicates a failure of their immune systems to control infection. Why does the immune system fail to enforce latency and allow active disease to emerge in immunocompetent individuals?
People previously treated for TB are at higher risk of developing additional episodes of disease121-125. Can this be attributed to relapse after inadequate treatment? Or, do these individuals have a defect in immunity that might explain why they developed disease in the first place? If these are the subjects that we are trying to protect by vaccination, we need to understand why they are susceptible to TB. This is important, as vaccines that aim to augment typical immune responses might fail to protect people with immune defects. Such people might not respond normally to vaccines or they might be resistant to their effects, suggesting that natural immunity in these individuals is defective.
As an example, after aerosol M. tuberculosis infection, C3HeB/FeJ mice develop necrotic granulomatous lesions and die rapidly. However, these mice have robust T cell responses126, 127. The genetic basis for their susceptibility has been mapped to several loci and the dominant one, Ipr1, is preferentially expressed by macrophages and alters their death modality128. Macrophages expressing the resistant allele of Ipr1 are more prone to apoptosis following intracellular infection, whereas macrophages expressing the susceptible allele undergo necrosis, which is associated with higher bacterial loads and more tissue destruction. This is an important insight as some people might develop active TB because their macrophages are unable to control intracellular M. tuberculosis growth, rather than because they have dysfunctional T cells. Similarly, an increase in type-I and type-II interferon-inducible genes is found in the peripheral blood of individuals with active pulmonary tuberculosis117. Surprisingly, this gene signature is mostly accounted for by changes in neutrophil gene expression. These data support that, just as for macrophages, alterations in neutrophil functions can have an impact on disease susceptibility and progression. Thus, even vaccines that elicit strong T cell responses might not be effective at protecting such people from TB because their macrophages, neutrophils or other cell types cannot respond appropriately to the T cell signals. Without understanding why people are susceptible to disease, we cannot predict how to protect them.
Finally, instead of mimicking natural immunity, vaccine induced protection against TB might require ‘uncommon’ or ‘unnatural’ immunity, as recently discussed by the Gates Foundation (www.grandchallenges.org/grantopportunities/pages/tbvaccineaccelerator.aspx). An example of such ‘unnatural’ protective immunity is that induced after tetanus toxoid vaccination, which is not observed after natural infection with Clostridium tetani129. An example for ‘unnatural’ immunity to M. tuberculosis is iNKT cells (see Box 2). These cells are dispensable for protection against primary infection in immunocompetent mice, but their activation can prolong the survival of inbred strains of susceptible mice130. It might be possible to induce such ‘unnatural’ or ‘uncommon’ immunity; for example, by engineering BCG to express the bacterial toxins listeriolysin or perfringolysin, which alters the route of antigen presentation, and leads to more efficient stimulation of CD8+ T cells 131-133. Incorporating such strategies that stimulate a broader immune response may have a greater effect on induction of protective immunity than promoting a stronger response to a single antigen.
Quantity versus quality
The goal of vaccination is to elicit a population of long-lived memory T cells that, after M. tuberculosis challenge, will rapidly proliferate, acquire optimal effector functions, traffic to the lung, recognize M. tuberculosis-infected cells, control bacterial replication and lead to sterilization (Figure 2c). We assume that successful vaccination will elicit CD4+ and CD8+ T cells, which are specific for one or more mycobacterial antigens and whose functions will include IFNγ production (Th1 response). However, Th1 responses are unable to sterilize the host during active disease and because we cannot define protective immunity, there is no way to measure successful immunization, other than empirically quantifying changes in pathogen burden after challenge, or in people, natural exposure, an approach that is slow and cumbersome.
For infections that can be prevented by humoral immunity, antibody titers correlate with protective immunity. For T cell-based vaccines, we are not sure whether the number of elicited T cells will correlate with protection or whether a change in one of the many functions that T cells perform will be more useful. For example, re-exposure to antigen in vivo induces CD8+ T cells to more frequently and persistently co-express effector molecules (such as perforin, granzyme A and B, Fas-Ligand, and IFNγ) and to more efficiently kill than CD8+ T cells stimulated by antigen the first time134. This suggests that an important function of T cell vaccination is to induce and coordinate gene expression of effector molecules. Similarly, several different types of intermediate to long-term antigen-specific T cells (central, effector and tissue resident memory cells) persist after infection or vaccination with the potential to rapidly respond to infectious challenge135; however, it is unclear which population(s) are most effective in preventing TB.
Collectively, the data summarized in this Review suggest that vaccines that elicit large numbers of T cells with the capacity to only produce IFNγ might not be optimal for protecting against TB. We should be looking for changes in T cell function, rather than numbers, as key factors that will lead to a significant breakthrough in vaccine design. Furthermore, because several studies have revealed different pathways involved in protective immunity against TB (such as those mediated by IL-1β, GM-CSF, vitamins C and D and cytolysis), vaccine design should aim at arming T cells with the capacity to modulate such pathways in cells infected with M. tuberculosis (Figure 2c). Finally, we must avoid the trap of thinking that there exists one type of T cell that will mediate protection alone. The host response to M. tuberculosis elicits many different types of T cells and even if all of them do not kill M. tuberculosis, it is likely that they all have a role in orchestrating a successful immune response.
CONCLUSION
An important obstacle to vaccine development is our incomplete understanding of what constitutes protective immunity against M. tuberculosis. It is difficult to define the goals of vaccination without first knowing what the immune system is capable of. Although a vaccine that prevents infection is everyone’s first choice, the consensus seems to be that a vaccine that enforces latency and prevents transmission is a more realistic goal. However, would we feel different if we understood why some people do not become infected despite repeated exposure? Or why some granulomas behave autonomously with some of them apparently able to control and eradicate M. tuberculosis and others not136?
This lack of knowledge supports our main suggestion for vaccine design: that characterization of additional immune mediators and cell types, even ones that appear to have minor roles during natural infection, is an essential first step. An effective vaccine might need to engage multiple immune mechanisms activated during a typical infection and might need to skew the host response in ways not seen during natural infection.
TB is a chronic disease, and M. tuberculosis evades detection by antibodies by occupying an intracellular niche. Thus, a vaccine that generates CD4+ and CD8+ central memory T cells with high proliferative potential, as well as a cohort of potent CD8+ effector memory and resident memory T cells that are poised to rapidly kill infected cells in the lung can be expected to be an ideal T cell vaccine candidate for disease prevention. Alternative approaches that, for example, stimulate unconventional T cell subsets and B cell/antibody responses in concert with conventional T cells should be further investigated. However, we believe that continuing to develop T cell vaccines aimed at boosting childhood BCG vaccination by solely varying the antigen will likely continue to fail. It is not enough to target specific antigens without a better understanding of how vaccines modulate T cell subsets, function and trafficking. IFNγ production by CD4+ T cells is essential in certain situations but it will likely not be sufficient as a protective response after vaccination. We believe that the premise that CD4+ T cell production of IFNγ is required for protection during infection has never been shown conclusively and its role in vaccine-induced immunity is based on over-interpretation of published data. In addition, we believe it is important to make vaccines that elicit multiple T cells subsets that express diverse protective functions. For example, we predict that a vaccine that elicits CD4+ T cells producing GM-CSF and IFNγ, and CD8+ T cells that function as cytolytic effectors in addition to producing IFNγ, would be more protective than vaccines that elicit only IFNγ. Finally, we must broaden the ways in which vaccine candidates are evaluated and the biomarkers used to measure their effect. Without defined correlates of protection, this will be challenging. Ongoing efforts to expand the ways in which vaccine candidates are evaluated and to embrace the diversity and heterogeneity of T cells need to be supported.
Online summary.
Tuberculosis remains a major health threat worldwide, with estimated 8.7 million new cases and 1.4 million deaths in 2011. New vaccines are needed to stop this pandemic.
The only current vaccine in use – BCG – provides variable protection against pulmonary tuberculosis. Additionally, new vaccine candidates have failed at preventing M. tuberculosis infection or tuberculosis.
Vaccine development has been hampered by the lack of immunological correlates of protection. Although IFNγ production by CD4+ T cells has been widely used to measure vaccine efficacy, it does not correlate with vaccine-induced protection.
Many studies have found additional immunological mechanisms that lead to M. tuberculosis control. These include those mediated by other T cell subsets (such as CD8+ and non-conventional T cell subsets) and immune mediators such as TNF, IL-1β, GM-CSF, and vitamins C and D.
New vaccination strategies should focus on modulating T cell function, rather than numbers, as well as targeting other aspects of the immune system.
Broadening our understanding of the immune pathways that provide protection against M. tuberculosis and how they function in concert will both increase the number of targets for vaccination as well as improve our evaluation of future vaccine candidates.
Fig 3.
Author biographies
Cláudio Nunes-Alves is currently a post-doctoral fellow in Sam Behar’s lab at the University of Massachusetts Medical School, where he studies adaptive immune responses to tuberculosis. He also studies how thymic infection impacts ongoing immunity in collaboration with the laboratories of Margarida Correia-Neves (at the Life and Health Sciences Research Institute, Braga, Portugal) and Christophe Benoist (at Harvard Medical School, Boston, MA).
Matthew G. Booty is currently a Ph.D. candidate in the Immunology Program at Harvard University and is performing his dissertation research with Sam Behar at the University of Massachusetts Medical School. His work focuses on several aspects of immunity to tuberculosis, including the regulation of CD8+ T cells and the role of eicosanoid signaling in host susceptibility.
Stephen Carpenter is an infectious disease physician who joined the Behar Lab in 2011 during his fellowship training at the Massachusetts General Hospital and Brigham and Women’s Hospital in Boston. He is currently an Instructor at the University of Massachusetts Medical School, studying how memory T cells function in tuberculosis, and aspires to improve TB vaccine efficacy through a better understanding of T cell function during infection.
Pushpa Jayaraman is a Research Assistant Professor whose research interests focus on the regulation of T cell function during TB infection. She is currently involved in studies to evaluate the role of Tim-3, a molecule established as an exhaustion marker in viral infections, in regulating innate and adaptive immune responses during chronic M. tuberculosis infection.
Alissa Rothchild is currently a Ph.D. candidate in the Immunology Program at Harvard University. Her dissertation work focuses on the activation and effector function of invariant Natural Killer T cells and the role of GM-CSF during Mycobacterium tuberculosis infection in the laboratory of Dr. Sam Behar at the University of Massachusetts Medical School.
Samuel M. Behar is Professor of Microbiology and Physiological Systems at the University of Massachusetts Medical School in Worcester, Massachusetts, USA. He studies innate and T cell-mediated mechanisms of microbial immunity that are leveraged by the host to combat pulmonary infection with Mycobacterium tuberculosis, with the ultimate goal of informing vaccine design.
References
- 1.Russell DG, Barry CE, III, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunology. 2011;4:252–60. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf AJ, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp.Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiley WW, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc.Natl.Acad.Sci.U.S.A. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunology. 2011;4:271–8. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horsburgh CR, Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. The New England journal of medicine. 2011;364:1441–1448. doi: 10.1056/NEJMcp1005750. [DOI] [PubMed] [Google Scholar]
- 8.Barry CE, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature reviews Microbiology. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. The Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 10.North RJ. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973;7:166–76. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- 11.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–25. [PubMed] [Google Scholar]
- 12.Shimokata K, Kishimoto H, Takagi E, Tsunekawa H. Determination of the T-cell subset producing gamma-interferon in tuberculous pleural effusion. Microbiol Immunol. 1986;30:353–61. doi: 10.1111/j.1348-0421.1986.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 13.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7:268–75. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- 16.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper AM, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–9. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 21.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabri M, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 24.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 25.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenberg P, et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–8. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 27.Murray J, et al. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS. 2007;21(6):S97–S104. doi: 10.1097/01.aids.0000299416.61808.24. [DOI] [PubMed] [Google Scholar]
- 28.Miedema F, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–14. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geldmacher C, Koup RA. Pathogen-specific T cell depletion and reactivation of opportunistic pathogens in HIV infection. Trends Immunol. 2012;33:207–14. doi: 10.1016/j.it.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcais A, Fieschi C, Abel L, Casanova JL. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med. 2005;202:1617–21. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallis RS, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13:362–72. doi: 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- 32.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. 2011;183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 33.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol. 2011;186:3527–37. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarevic V, Nolt D, Flynn JL. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J Immunol. 2005;175:1107–17. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 35.Abebe F, Mustafa T, Nerland AH, Bjune GA. Cytokine profile during latent and slowly progressive primary tuberculosis: a possible role for interleukin-15 in mediating clinical disease. Clin Exp Immunol. 2006;143:180–92. doi: 10.1111/j.1365-2249.2005.02976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittrücker H-W, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA. 2007;104:12434–9. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollenkopf H-J, Hahnke K, Kaufmann SHE. Transcriptional responses in mouse lungs induced by vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Microbes and infection / Institut Pasteur. 2006;8:136–144. doi: 10.1016/j.micinf.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira ES, Marinho JM, Barbosa T, study g. Interferon-gamma production by mononuclear cells in Bacille Calmette-Guérin-revaccinated healthy volunteers predicted long-term antimycobacterial responses in a randomized controlled trial. Vaccine. 2013;31:3778–3782. doi: 10.1016/j.vaccine.2013.04.079. [DOI] [PubMed] [Google Scholar]
- 39.Soares AP, et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray RA, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol. 2006;177:5647–51. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 41.Andersen A, et al. The immunological effect of revaccination with Bacille Calmette-Guérin vaccine at 19 months of age. Vaccine. 2013;31:2137–2144. doi: 10.1016/j.vaccine.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 42.Kagina BM, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003;171:4689–99. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 44.Gallegos AM, et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller HE, Robinson RT. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect Immun. 2012;80:3828–41. doi: 10.1128/IAI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayaraman P, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343–54. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol. 2013;190:270–7. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittrucker HW, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104:12434–9. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitt JM, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 52.Mayer-Barber KD, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–30. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Juarrero M, et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–22. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 54.Ladel CH, et al. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–9. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung JY, et al. The Intracellular Environment of Human Macrophages that Produce Nitric Oxide Promotes Growth of Mycobacteria. Infect Immun. 2013 doi: 10.1128/IAI.00611-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg JB, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–95. [PubMed] [Google Scholar]
- 58.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aston C, Rom WN, Talbot AT, Reibman J. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. American Journal of Respiratory and Critical Care Medicine. 1998;157:1943–1950. doi: 10.1164/ajrccm.157.6.9705028. [DOI] [PubMed] [Google Scholar]
- 60.Saunders BM, Briscoe H, Britton WJ. T cell-derived tumour necrosis factor is essential, but not sufficient, for protection against Mycobacterium tuberculosis infection. Clin Exp Immunol. 2004;137:279–87. doi: 10.1111/j.1365-2249.2004.02518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohan VP, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–55. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bean AG, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]
- 64.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 65.Denis M, Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol Lett. 1990;24:203–6. doi: 10.1016/0165-2478(90)90049-v. [DOI] [PubMed] [Google Scholar]
- 66.Bermudez LE, Young LS. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990;48:67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- 67.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 68.Rothchild A. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathogens. 2013 doi: 10.1371/journal.ppat.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosen LB, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190:3959–66. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayaraman P, et al. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190:4196–204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Y, et al. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sada-Ovalle I, et al. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol. 2012;189:5896–902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–85. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cruz A, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–16. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novikov A, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–7. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 77.Martineau AR, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 78.Verway M, et al. Vitamin D Induces Interleukin-1beta Expression: Paracrine Macrophage Epithelial Signaling Controls M. tuberculosis Infection. PLoS Pathog. 2013;9:e1003407. doi: 10.1371/journal.ppat.1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuk JM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Ralph AP, Lucas RM, Norval M. Vitamin D and solar ultraviolet radiation in the risk and treatment of tuberculosis. Lancet Infect Dis. 2013;13:77–88. doi: 10.1016/S1473-3099(12)70275-X. [DOI] [PubMed] [Google Scholar]
- 81.Vilcheze C, Hartman T, Weinrick B, Jacobs WR., Jr Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun. 2013;4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen CY, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skinner MA, Parlane N, McCarthy A, Buddle BM. Cytotoxic T-cell responses to Mycobacterium bovis during experimental infection of cattle with bovine tuberculosis. Immunology. 2003;110:234–41. doi: 10.1046/j.1365-2567.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev.Immunol. 2006;26:317–352. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner J, et al. CD8- and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am J Respir Cell Mol Biol. 2001;24:203–9. doi: 10.1165/ajrcmb.24.2.4370. [DOI] [PubMed] [Google Scholar]
- 87.Sousa AO, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci U S A. 2000;97:4204–8. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stenger S, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 89.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–74. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin CJ, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saunders BM, et al. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–9. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- 92.Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171:3110–8. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- 93.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 94.Ehlers S, Kutsch S, Ehlers EM, Benini J, Pfeffer K. Lethal granuloma disintegration in mycobacteria-infected TNFRp55−/− mice is dependent on T cells and IL-12. J Immunol. 2000;165:483–92. doi: 10.4049/jimmunol.165.1.483. [DOI] [PubMed] [Google Scholar]
- 95.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–46. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 96.Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of Guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008;38:455–62. doi: 10.1165/rcmb.2007-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manabe YC, et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb) 2008;88:187–96. doi: 10.1016/j.tube.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Via LE, et al. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus) Infection and Immunity. 2013;81:2909–2919. doi: 10.1128/IAI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–76. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 102.Ribeiro-Rodrigues R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Green AM, et al. CD4(+) Regulatory T Cells in a Cynomolgus Macaque Model of Mycobacterium tuberculosis Infection. J Infect.Dis. 2010 doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scott-Browne JP, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kursar M, et al. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–5. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 106.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 108.Lazar-Molnar E, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–7. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jurado JO, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–25. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 110.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pedrosa J, et al. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–83. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rivas-Santiago B, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–41. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang CT, et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–12. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–9. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cruz A, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–20. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 116.Mishra BB, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berry MPR, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Naranbhai V, et al. Ratio of Monocytes to Lymphocytes in Peripheral Blood Identifies Adults at Risk of Incident Tuberculosis Among HIV-Infected Adults Initiating Antiretroviral Therapy. J Infect Dis. 2013 doi: 10.1093/infdis/jit494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676–86. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang D, et al. Immune distribution and localization of phosphoantigen-specific Vgamma2Vdelta2 T cells in lymphoid and nonlymphoid tissues in Mycobacterium tuberculosis infection. Infect Immun. 2008;76:426–36. doi: 10.1128/IAI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Millet JP, et al. Tuberculosis recurrence after completion treatment in a European city: reinfection or relapse? PLoS One. 2013;8:e64898. doi: 10.1371/journal.pone.0064898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Millet JP, et al. Tuberculosis recurrence and its associated risk factors among successfully treated patients. J Epidemiol Community Health. 2009;63:799–804. doi: 10.1136/jech.2008.077560. [DOI] [PubMed] [Google Scholar]
- 123.Crofts JP, Andrews NJ, Barker RD, Delpech V, Abubakar I. Risk factors for recurrent tuberculosis in England and Wales, 1998-2005. Thorax. 2010;65:310–4. doi: 10.1136/thx.2009.124677. [DOI] [PubMed] [Google Scholar]
- 124.Dobler CC, Crawford AB, Jelfs PJ, Gilbert GL, Marks GB. Recurrence of tuberculosis in a low-incidence setting. Eur Respir J. 2009;33:160–7. doi: 10.1183/09031936.00104108. [DOI] [PubMed] [Google Scholar]
- 125.van Rie A, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 126.Kamath AB, Alt J, Debbabi H, Behar SM. Toll-like receptor 4-defective C3H/HeJ mice are not more susceptible than other C3H substrains to infection with Mycobacterium tuberculosis. Infection and Immunity. 2003;71:4112–4118. doi: 10.1128/IAI.71.7.4112-4118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamath AB, Alt J, Debbabi H, Taylor C, Behar SM. The major histocompatibility complex haplotype affects T-cell recognition of mycobacterial antigens but not resistance to Mycobacterium tuberculosis in C3H mice. Infect Immun. 2004;72:6790–8. doi: 10.1128/IAI.72.12.6790-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan H, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–72. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson S. Antibody responses to clostridial infection in humans. Clin Infect Dis. 1997;25(Suppl 2):S173–7. doi: 10.1086/516220. [DOI] [PubMed] [Google Scholar]
- 130.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infection and Immunity. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun R, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 132.Rao M, et al. The Tuberculosis Vaccine Candidate Bacillus Calmette-Guérin ΔureC::hly Coexpressing Human Interleukin-7 or -18 Enhances Antigen-Specific T Cell Responses in Mice. PLoS ONE. 2013;8:e78966. doi: 10.1371/journal.pone.0078966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Desel C, et al. Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. The Journal of Infectious Diseases. 2011;204:1573–1584. doi: 10.1093/infdis/jir592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peixoto A, et al. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin PL. Sterilization of granulomas is common in both active and latent tuberculosis despite extensive within-host variability in bacterial killing. 2013 doi: 10.1038/nm.3412. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ottenhoff THM, Kaufmann SHE. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathogens. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brennan MJ, Stone MR, Evans T. A rational vaccine pipeline for tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16:1566–1573. doi: 10.5588/ijtld.12.0569. [DOI] [PubMed] [Google Scholar]
- 139.Tameris MD, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McShane H, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 141.Scriba TJ, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. European Journal of Immunology. 2010;40:279–290. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vavassori S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14:908–16. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 143.D’Souza CD, et al. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol. 2000;23:188–93. doi: 10.1165/ajrcmb.23.2.4063. [DOI] [PubMed] [Google Scholar]
- 144.Sugawara I, et al. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis (Edinb) 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]
- 145.Khader SA, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. The Journal of Immunology. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 146.Montoya CJ, et al. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin Immunol. 2008;127:1–6. doi: 10.1016/j.clim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 147.Sutherland JS, et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinb) 2009;89:398–404. doi: 10.1016/j.tube.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 148.Im JS, et al. Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin Immunol. 2008;127:214–24. doi: 10.1016/j.clim.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Rhijn I, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–13. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kasmar AG, et al. CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med. 2011;208:1741–7. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ly D, et al. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. 2013;210:729–41. doi: 10.1084/jem.20120624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gold MC, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 154.Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. Journal of Clinical Investigation. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scriba TJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. Journal of immunology (Baltimore, Md : 1950) 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gold MC, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6:35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. The Journal of Immunology. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 159.Sada-Ovalle I, Sköld M, Tian T, Besra GS, Behar SM. {alpha}-Galactosylceramide as a Therapeutic Agent for Pulmonary Mycobacterium tuberculosis Infection. American Journal of Respiratory and Critical Care Medicine. 2010 doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen CY, et al. Phosphoantigen/IL2 Expansion and Differentiation of Vgamma2Vdelta2 T Cells Increase Resistance to Tuberculosis in Nonhuman Primates. PLoS Pathog. 2013;9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gansert JL, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–61. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 162.Venkataswamy MM, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–56. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen Y, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Umemura M, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 165.Okamoto Yoshida Y, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–22. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 166.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 167.Gopal R, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364–73. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]