Abstract
The diagnosis of peripheral T-cell and NK-cell lymphomas (PTNKCL) is difficult with few standards for required ancillary studies. We evaluated a series of PTNKCLs using a tiered approach to immunohistochemistry and molecular genetic characterization to document diagnostic accuracy and clinical relevance. Seven hematopathologists reviewed 374 cases that included PTNKCL and non-PTNKCL cases to mimic diagnostic practice. Cases received tier 0, 1, and 2 diagnoses by 3 independent pathologists, on the basis of hematoxylin and eosin stains and progressive immunohistochemistry panels. A tier 2b diagnosis was rendered when gene rearrangement data were available, and a final consensus diagnosis was rendered after discussion of each case. Across all 374 cases, consensus agreement was 92.5%. For PTNKCLs, World Health Organization subclassification was possible in 16.5%, 37.1%, 82.8%, and 85.9% of individual reviewer diagnoses at tier 0, 1, 2, and 2b, respectively. Gene rearrangement contributed to a change in diagnosis in 51 of 647 (8%) individual reviews. Following this algorithm may provide prognostic information on the basis of individual marker expression in common PTNKCL types (CD4 in peripheral T-cell lymphoma, not otherwise specified and PD-1 in angioimmunoblastic T-cell lymphoma). This evidence-based approach to the diagnosis of PTNKCL informs practicing pathologists, clinical trial designers, and policy-makers regarding required ancillary studies.
Keywords: peripheral T-cell lymphoma, diagnosis, accuracy, evidence-based medicine, pathology, guidelines
Peripheral T-cell and NK-cell lymphomas (PTNKCLs) are a heterogenous group of lymphomas with different clinicopathologic features, and, in the Western world, they make up only 10% to 20% of non-Hodgkin lymphomas.1 Cutaneous T-cell lymphomas, in particular mycosis fungoides, are among the most common type of T-cell lymphoma, and their clinical and pathologic characteristics have been fairly well characterized.2,3 Diagnosis and subclassification of noncutaneous T-cell and NK-cell lymphomas can be more problematic because of their diversity of presentations and heterogeneity in histopathology and immunophenotype. The most common types of noncutaneous T-cell and NK-cell lymphomas are peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL). These are generally aggressive lymphomas with poor prognosis.4,5 Anaplastic large cell lymphomas (ALCLs) are also relatively common but have been segregated into ALK-positive (ALCL, ALK+) and ALK-negative (ALCL, ALK−) entities because of their distinctly different molecular pathogenesis and clinical behavior with conventional multiagent chemotherapy.5 Other rarer types of noncutaneous PTNKCLs in the 2008 World Health Organization (WHO) classification include entities such as hepatosplenic T-cell lymphoma, enteropathy-associated T-cell lymphoma, adult T-cell leukemia/lymphoma, Epstein-Barr virus–positive T-cell lymphoproliferative disorders of childhood, extranodal NK/T-cell lymphoma of nasal type, and subcutaneous panniculitis-like T-cell lymphoma.6 Because of their relative rarity, arriving at the correct diagnosis can be difficult for many pathologists. Overall diagnostic accuracy (reproducibility) among experts has been found to be approximately 81% in a series of cases submitted for review as putative T-cell and NK-cell lymphomas in a study conducted before the most recent iteration of the WHO classification; however, this ranged from 67% to 95% depending on the specific subtype.4
The past few years have witnessed the development of a range of therapeutic agents specifically approved for use in T-cell lymphomas such as denileukin diftitox, pralatrexate, vorinistat, brentuximab vedotin, and rhomedepsin.7 Thus accurate diagnosis of PTNKCLs will likely become even more important as new therapeutic options become available that may be subtype specific. Although expert-recommended guidelines exist for ancillary studies in the tissue diagnosis of PTNKCLs, evidence as to the performance is lacking.8 Indeed, accurate diagnosis and subclassification of PTNKCLs requires assessment and integration of clinical, histologic, immunophenotypic, and, in some cases, molecular genetic information. At the same time, pressure to limit use of ancillary testing will mount to curtail costs. The development of evidence-based practice is critical to justify the use of such testing. To this end, we sought to characterize the diagnostic accuracy and clinical relevance of a defined approach to the diagnosis and subclassification of PTNKCLs, focusing on noncutaneous lymphomas, using a retrospective, multicenter series of cases that included both PTNKCLs and their mimics to approximate “realworld” experience. Our data support the use of a clearly defined algorithm as a standardized approach to the tissue diagnosis of PTNKCLs.
METHODS
A prospectively defined set of 375 cases (75 cases/site) were requested from each site, consisting mainly of noncutaneous PTNKCLs (diagnosed at the accruing site) as well as up to 15% of total cases chosen to be mimics of PTNKCL (such as reactive lymphadenitis, Hodgkin lymphoma, or B-cell lymphoma) to approximate a realistic diagnostic situation. In total, 374 cases were received for analysis. The study was carried out with approval of each site’s Institutional Review Board.
Immunohistochemical (IHC) panels were designed by consensus of the authors and after discussion and comparison of local practices. Specifically, an initial limited panel was constructed to assist in distinguishing B-cell lymphomas, Hodgkin lymphoma, and reactive processes from suspected T-cell lymphoma. A secondary panel was designed with the intent to aid in confirmation of lymphoma and subclassification. Gene rearrangement (GR) studies were then added (if available). Each case received tier 0, 1, and 2 diagnoses by 3 independent pathologists, on the basis of review of hematoxylin and eosin staining along with basic clinical and demographic data available to sites at the time of the biopsy, panel 1 IHC (CD3, CD5, CD10, CD20, CD21, CD30, CD45, PAX5), and panel 2 IHC (CD2, CD4, CD7, CD8, CD23, PD-1, CD56, EBER, ALK1, TIA1, TCRγ, TCRβF1), respectively. WHO 2008 criteria were used for diagnosis.6 Because this was a retrospective study, clinical data were of course nonuniform, but any existing clinical or laboratory data deemed relevant for diagnosis by the submitters were included. The percentage of large cells was classified as ≥70% versus < 70%. For all markers, except CD10, ALK1, and EBER, expression in > 20% of tumor cells was considered positive. Greater than or equal to 10% was used for CD10. Clear expression in any tumor cells was considered positive for ALK1, although in practice essentially all cells were positive. EBER was scored as positive in background cells if the number of positive cells in the tumor was judged to be more than that seen in latently infected tissues (estimated at > 0.1% of total cells). EBER was also assessed in tumor cells and considered positive when > 20% of tumor cells stained positive on IHC analysis. CD21 and CD23 were used primarily to assess for abnormal proliferations of follicular dendritic cells. Immunostaining was performed according local protocols on automated immunostainers (Ventana Benchmark: Ventana Medical Systems, Tucson, AZ; Dako Autostainer Plus: Dako, Carpinteria, CA; and Leica Bond: Leica Biosystems, Buffalo Grove, IL). For some cases, CD56 was used at one referral site (UCLA; Dako) and TCRβF1and TCRγ at another site (Mayo Clinic; Leica) according to local clinical protocols, as not all sites had these markers available at initiation of the study.
A tier 2b diagnosis was then rendered after GR results were made available. GR studies were performed according to clinical laboratory methods followed at the local site either as part of the initial diagnostic evaluation or later as part of this study if not initially performed (for cases with available tissue). Seventy-three cases had GR studies performed centrally using the Biomed2 protocol and interpreted using capillary electrophoresis fragment analysis according to published guidelines.9
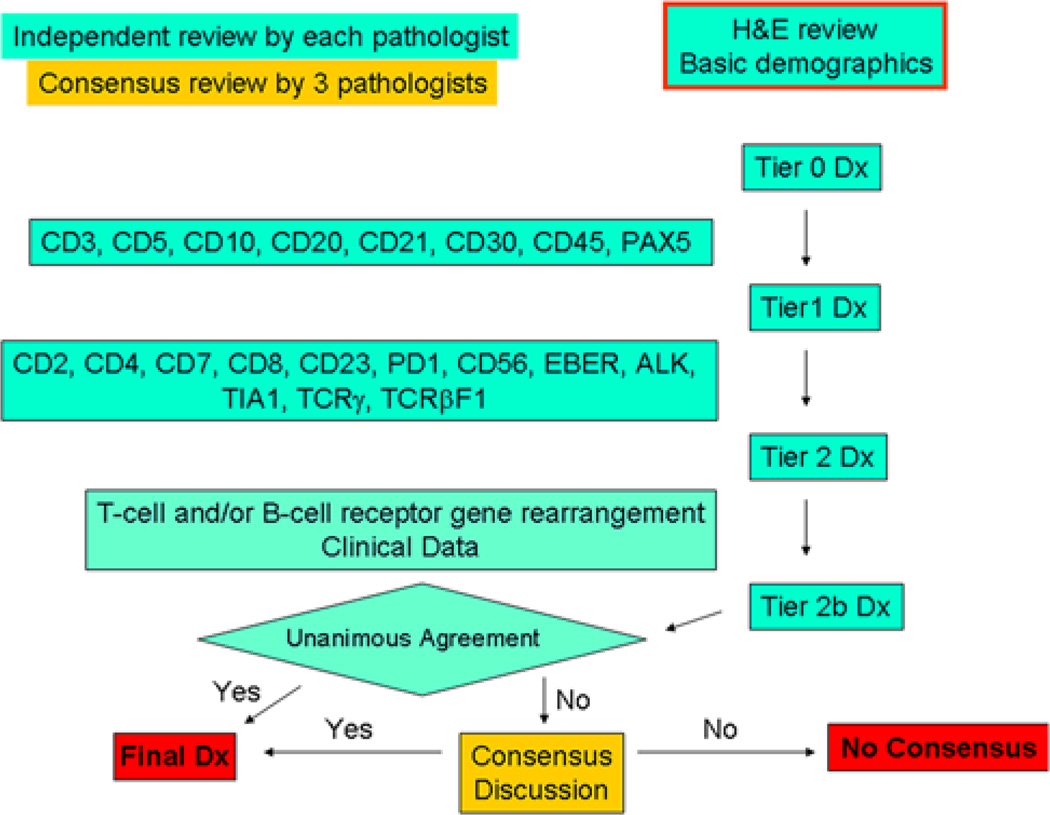
At this point pathologists were asked to independently rate (yes/no) whether they felt that the genetic data were required to reach their diagnosis. A final consensus diagnosis was rendered after full discussion of each case by the 3 reviewers with all available clinical data. Figure 1 summarizes the review protocol, and Table 1 lists the diagnostic categories available for reviewers. Entities such as blastic plasmacytoid dendritic cell neoplasm and mycosis fungoides were included as diagnostic categories in the event cases were incidentally submitted. Cases in which consensus (unanimity) was not achieved were noted as “no consensus.”
FIGURE 1.
Schematic of case review.
TABLE 1.
Diagnostic Categories for Reviewers
| Benign/reactive | ALCL (ALK−) | NK/T-cell lymphoma, nasal type |
| Atypical lymphoid infiltrate | ALCL (ALK+) | BPDCN |
| Lymphoma (unable to classify) | ALCL | MF |
| B-cell NHL (specify type) | PTCL-NOS | T-cell or NK-cell lymphoma (unable to specify further) |
| Hodgkin lymphoma (specify type) | ATLL | Other T-cell lymphoma (specify WHO type) |
| T-precursor LBL | EATL | Other (specify) |
| AITL | HSTCL |
ATLL indicates adult T-cell leukemia/lymphoma; BPDCN, blastic plasmacytoid dendritic cell neoplasm; EATL, enteropathy-associated T-cell lymphoma; HSTCL, hepatosplenic T-cell lymphoma; MF, mycosis fungoides.
Overall survival (OS) was defined as the time from diagnostic tissue sampling until death due to any cause. Patients still alive were censored at the date of last contact. Associations between OS and clinical factors, including lymphoma subtype and IHC markers, were assessed using Kaplan-Meier (KM) curves and Cox proportional hazard models.
RESULTS
Cases
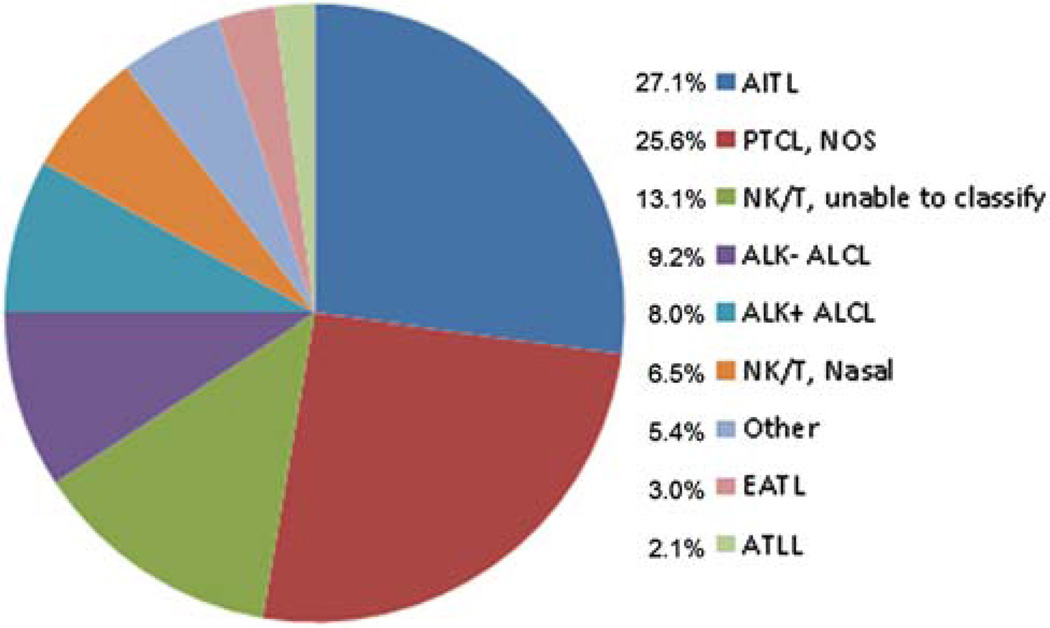
A total of 374 cases were submitted with a final study diagnosis of PTNKCL (336 cases), non-PTNKCL (33 cases: 10 benign/reactive processes and 23 non-PTNKCLs), or “no consensus” in which agreement between lymphoma versus benign could not be reached (5 cases). For the 336 cases of PTNKCL, 54% were nodal biopsy sites, 42% were extranodal, 2% were both nodal and extranodal, and 2% were unknown location. The relatively high frequency of extranodal sites likely reflects the propensity of PTNKCL for extranodal involvement as part of high-stage disease and/or intrinsic biology of specific PTNKCL entities (such as extranodal NK/T-cell lymphoma). Considering the extranodal cases alone, the most common sites were cutaneous/subcutaneous (19%), nasopharyngeal (13%), small bowel (10%), lung (8%), spleen (7%), and soft tissue (5%). Other sites were less frequent. It is noteworthy that the cutaneous/subcutaneous lymphomas were largely secondary to systemic PTNKCL, with only 1 case of primary cutaneous ALCL. For the 336 PTNKCLs, there were 4019 individual diagnoses rendered across the tiers and multiple reviewers. Ninety-two percent (309/336) of PTNKCL cases had complete phenotypic data, and 65% (220/336) had GR data available for review. Of these, 159 (74%) were positive. There was no bias toward a particular PTNKCL subtype with regard to missing data points. Figure 2 illustrates the diagnostic categories that resulted from the review process. As expected, the dominant T-cell lymphoma categories were PTCL-NOS, ALCL (both ALK− and ALK+), and AITL. Thus, the disease distribution reflected a Western T-cell lymphoma population.
FIGURE 2.
Distribution of PTNKCL diagnoses that resulted from the review. ATLL indicates adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma.
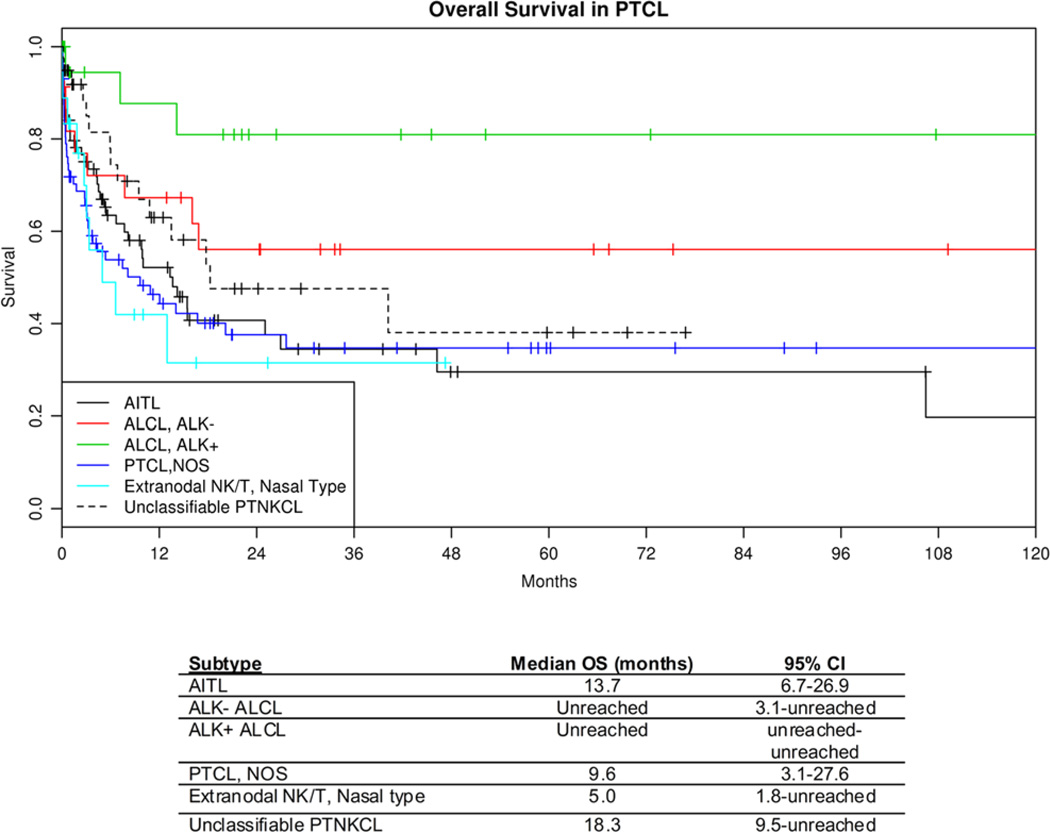
To further characterize these cases, we examined the OS of the major types of PTCL WHO diagnostic categories using the KM method. OS data were available in 274 cases, and 47% have died (median follow-up of 12mo for those still alive; range, 0 to 168 mo). This analysis showed that our patient population with diagnoses rendered according to the study plan appeared to have outcomes expected on the basis of other large series (Fig. 3).4 ALCL, ALK+ had the longest OS, followed by ALCL, ALK−, with PTCL-NOS, AITL, and NK/T-cell lymphomas of nasal type having shorter OS estimates.
FIGURE 3.
KM OS curves by diagnosis. ATLL indicates adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma.
Diagnostic Panel Performance
Across all 374 cases, consensus/unanimity was reached in 92.5% of cases, and only 28 cases lacked consensus after discussion (7.5%). In terms of individual reviewer independent agreement (combining all nonneoplastic diagnoses together as 1 category), the rate increased from 39% to 65% from tier 1 to tier 2b, and consensus discussion was required in 35% of cases. Of the 28 cases that lacked consensus, 23 were issues related to refinement of diagnosis, and the 3 most common issues reflected diagnostic gray zones between PTCL-NOS and AITL (5), ALCL, ALK− (4), and unclassifiable PTNKCL (4). This latter situation arose because of reviewers’ uncertainty about whether they had enough tissue to confidently exclude more specific types of PTCL, raising doubt about whether PTCL-NOS was the most appropriate category. Thus, in 98.7% of cases agreement was reached by all 3 reviewers that a case represented lymphoma or a benign condition. Only 5 cases (1.3% of total cases) were ultimately left in which unanimity could not be reached regarding lymphoma versus a benign diagnosis. When considering the 336 cases in which there was agreement on a PTNKCL, 94% could be assigned either a consensus WHO classification designation (87%) or consensus unclassifiable PTNKCL designation (7%).
Table 2 shows the individual marker results for the main diagnostic T-cell lymphoma subtypes. A few points are worth noting. First, although CD30 was by definition positive in ALCL cases, a substantial subset of AITL and PTCL-NOS expresses CD30 albeit usually less uniformly and extensively as ALCL. Second, as loss of pan-T-cell antigens can be used as evidence of an abnormal immunophenotype, CD7 was lost more frequently than CD2 and CD5 in AITL, ALK− ALCL, and PTCL-NOS, whereas CD3 was more frequently absent in ALK+ ALCL, and CD5 was most frequently absent in extranodal NK/T-cell lymphoma, perhaps reflecting the NK cell lineage of the latter. PD-1 was seen most frequently in AITL, as expected. However, a significant minority of PTCL-NOS had some degree of PD-1 expression.
TABLE 2.
Individual Features in PTNKCLs
| Tier | Stain | Cutoff (%) | AITL (N=91) (%) |
ALK− ALCL (N=31) (%) |
ALK+ ALCL (N=27) (%) |
PTCL-NOS (N=86) (%) |
Extranodal NK/T, Nasal Type (N=22) (%) |
|---|---|---|---|---|---|---|---|
| Tier 1 | CD3 | > 20 | 100.0 | 45.2 | 11.5 | 95.4 | 100.0 |
| Tier 1 | CD5 | > 20 | 93.2 | 19.4 | 36.0 | 67.1 | 25.6 |
| Tier 1 | CD10 | > 10 | 56.0 | 0.0 | 0.0 | 2.4 | 0.0 |
| Tier 1 | CD20 | > 20 | 2.2 | 0.0 | 0.0 | 3.5 | 0.0 |
| Tier 1 | CD21 | Tumor > 20 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tier 1 | CD21 | Background abnormal meshworks | 72.7 | 6.7 | 4.0 | 8.6 | 0.0 |
| Tier 1 | CD30 | > 20 | 11.2 | 100.0 | 100.0 | 23.3 | 18.2 |
| Tier 1 | CD45 | > 20 | 90.0 | 58.6 | 48.0 | 78.8 | 81.0 |
| Tier 1 | PAX5 | > 20 | 0.0 | 3.2 | 0.0 | 2.4 | 0.0 |
| Tier 2 | CD2 | > 20 | 96.6 | 58.1 | 22.2 | 91.8 | 90.0 |
| Tier 2 | CD4 | > 20 | 82.4 | 67.7 | 46.2 | 56.6 | 14.3 |
| Tier 2 | CD7 | > 20 | 47.2 | 16.1 | 22.2 | 57.1 | 36.4 |
| Tier 2 | CD8 | > 20 | 4.5 | 16.1 | 7.7 | 19.3 | 40.9 |
| Tier 2 | CD23 | Tumor > 20 | 0.0 | 3.2 | 0.0 | 0.0 | 0.0 |
| Tier 2 | CD23 | Background abnormal meshworks | 80.2 | 3.2 | 0.0 | 11.0 | 0.0 |
| Tier 2 | PD-1 | > 20 | 92.0 | 3.2 | 0.0 | 31.3 | 0.0 |
| Tier 2 | ALK | Any | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Tier 2 | TIA1 | > 20 | 0.0 | 26.7 | 53.9 | 41.7 | 90.5 |
| Tier 2 | TCRβF1 | > 20 | 59.0 | 17.2 | 23.1 | 74.1 | 38.1 |
| Tier 2 | EBER | Tumor > 20 | 2.3 | 0.0 | 0.0 | 1.2 | 100.0 |
| Tier 2 | EBER | Background abnormal | 76.1 | 7.1 | 0.0 | 17.1 | 0.0 |
| Tier 2 | CD56 | > 20 | 0.0 | 3.2 | 4.0 | 11.6 | 42.9 |
| Tier 2 | TCRγ | > 20 | 1.2 | 0.0 | 0.0 | 9.6 | 4.6 |
In the tiered approach to diagnosis, addition of incremental immunophenotypic data resulted in an increase in ability of reviewers to reach a diagnosis of lymphoma and ultimately to a specific WHO subtype of PTNKCL. For the 1008 individual reviews on the 336 PTNKCLs, Table 3 shows that the ability to reach a WHO subtype increased from 16.5% to 82.8% from tier 0 through 2. GR (tier 2b) allowed for a small increase in WHO subclassification (82.8% to 85.9%). Of interest, GR information was felt to contribute to only 8% of the individual reviewer’s diagnostic decisions.
TABLE 3.
Change in Diagnosis by Tier by Individual Pathologist (336 Cases)
| Tier | % Specific WHO Diagnoses |
|---|---|
| 0 | 16.5 |
| 1 | 37.1 |
| 2 | 82.8 |
| 2b | 85.9 |
Clinical Correlates
Survival analysis (Fig. 3) yielded several observations of interest. First, it is evident from the survival curves that cases of unclassifiable PTNKCL cases had poor OS, comparable to PTCL-NOS. Thus, in the setting of detailed phenotypic and genotypic data, confident diagnosis of PTNKCL, unclassifiable, provides clinically relevant information given that these patients had an aggressive clinical course. It is noteworthy that the IHC panels do permit exclusion of entities with historically superior OS, namely ALCL, ALK+ and, likely, ALCL, ALK−. Second, although not statistically significant (perhaps because of lack of sufficient numbers), it appears that ALCL, ALK− has a prognosis intermediate between ALCL, ALK+ and PTCL-NOS. Comparison of OS of ALCL, ALK− and PTCL-NOS showed a trend for longer OS for ALCL, ALK− (hazard ratio [HR] = 0.35, 95% confidence interval [CI], 0.10–1.31, P = 0.12), supporting the distinction of ALCL, ALK− from PTCL-NOS.10
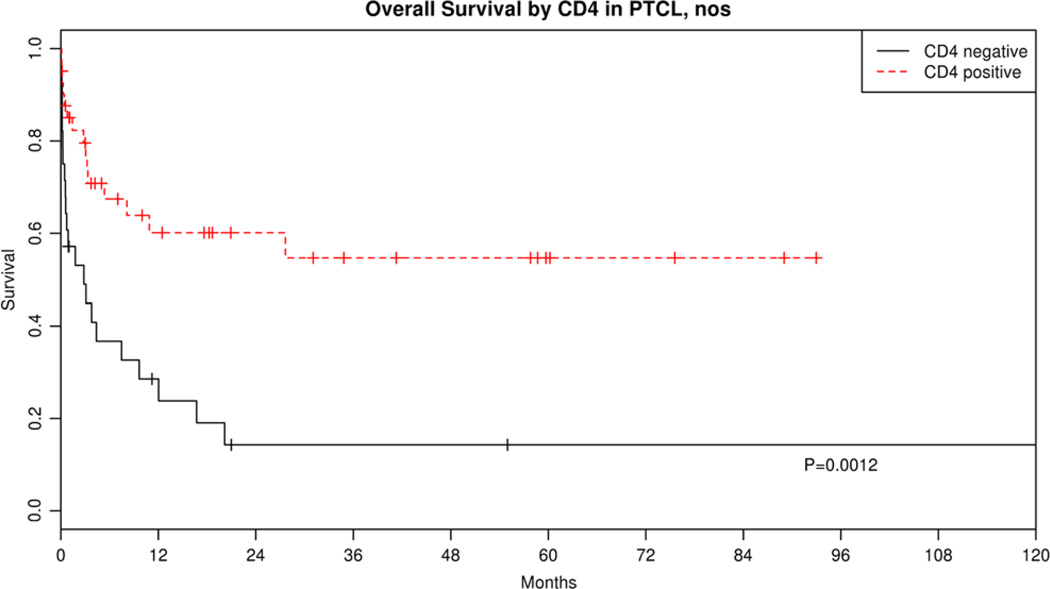
Finally, we had the opportunity to evaluate the association of specific phenotypic markers in particular subtypes of lymphoma as a hypothesis-generating exercise. For PTCL-NOS and AITL subtypes, we speculated that T-cell subset markers in our diagnostic panel might have prognostic importance. For PTCL-NOS, we tested whether CD4, CD8, cytotoxic molecule (TIA1), TCRβF1, or TCRγ expression or large cells ≥70% was associated with OS. We found that only CD4 expression was associated with good prognosis in our cohort of PTCL-NOS (HR = 0.34, 95% CI, 0.17–0.65, P = 0.001; Fig. 4). AITL has recently been shown to be a lymphoma of follicular helper T cells (Tfh).11 Several markers of Tfh cells appear useful in recognition of this cell type and diagnosis of AITL, including CD10, CXCL13, BCL6, ICOS, and PD-1.12–17 However, whether expression has any impact on outcome is unknown. Analysis of CD10 and PD-1 expression within AITL in our series suggests that PD-1 expression (> 20%) was associated with poor OS (HR = 7.24, 95% CI, 0.99–53.0, P = 0.05).
FIGURE 4.
KM OS curves of PTCL-NOS by CD4 expression.
DISCUSSION
Our understanding of the diversity of PTCLs has evolved substantially over the past 20 years and is reflected in the evolution of classification schemes. It was not until 1994 that histopathologic, immunologic, and genetic features were included in classification criteria.18 Since that time the addition of new entities in the WHO 2001 and 2008 classifications has expanded the number of peripheral T-cell and NK-cell neoplasms (including provisional types) from 10 to 21.6,18,19 Thus, pathologists are confronted with a wide variety of tumor types that must be distinguished from one another within the group of PTNKCLs, aside from the myriad other mimics including B-cell lymphomas, Hodgkin lymphoma, and reactive lesions that must be considered.
We undertook this study to document the performance of a standardized approach to the diagnosis and subclassification of PTNKCLs, focusing on noncutaneous types. We also attempted to conduct it in a manner that would approximate clinical practice in which other lymphoma types and reactive processes would be real possibilities. In this context we demonstrated that the overall ability to arrive at a specific WHO diagnosis improved from 16% to 86% after all available data were present. Furthermore, tier 1 studies should often be sufficient to exclude the diagnosis of T-cell or NK-cell lymphoma in the case of a non-PTNKCL (80% of reactive conditions were identified as such by reviewers at tier 1, data not shown). This would prevent unnecessary performance of tier 2 markers, which are generally most helpful for PTNKCL subclassification and often not needed for the workup of non-PTNKCLs. It is noteworthy that GR was helpful in only a small subset (8%) of cases, which argues against the practice of routine GR testing in most PTNKCLs.
Despite the overall good performance of the algorithm used in diagnostic workup, there was a small set of cases in which consensus could not be reached (7.5% of cases). The minority (only 5 of 374 cases, or 1.3%) included those in which agreement over the nature of the infiltrate (benign vs. lymphoma) was in question. The majority included those in which there were problems in refinement of a specific subtype of T-cell or NK-cell lymphoma. Most of these were due to disagreement among the PTNKCL subtypes, which reflects our incomplete understanding of the borders between PTCL-NOS and other lymphoma subtypes. These latter problems reflect issues related to diagnostic subtype uncertainty and shed light on areas that require future research.
The accuracy in diagnosis for PTNKCLs has been addressed in a prior landmark study by the International T-cell Lymphoma Project and has been estimated at 81%; however, the range varied for different types. For ALCL, ALK+, the reproducibility of an expert reviewer with a consensus diagnosis was 93%, whereas for AITL and PTCL-NOS it was 81% and 75%, respectively.4 However, the use of ancillary studies was not uniform, and the study was done before the wider use of some markers used in the current study such as Tfh and TCRγ markers, or adoption of more standardized GR protocols. In addition, cases submitted to the International T-cell Lymphoma Project study were submitted as known lymphomas.4 Our intent was to further refine accuracy estimates using a prospectively defined diagnostic workup in a series of cases that included non-PTNKCL entities to more closely mimic a diagnostic setting to minimize participant bias toward a PTNKCL diagnosis by the reviewer. Recommendations exist on the pathologic studies useful in the diagnosis of PTNKCLs. The National Comprehensive Cancer Center Network has put forth recommended phenotypic markers, which represents expert opinion grounded in literature pertaining to each entity; however, data on performance in practice are lacking.8 Our data suggest that the ability to arrive at consensus diagnosis was 92.5% for all cases submitted. Only 1.3% of submitted cases still provided diagnostic difficulty in which agreement could not be reached regarding a reactive versus lymphomatous process. Our progressive approach demonstrated an ability to arrive at a consensus in 94% and a specific WHO designation in 87% of PTNKCLs. One might argue about the exact content of our selected IHC tier contents. For example, TCRβF1 and TCRγ may not be required in all cases but does allow for complete characterization of a T-cell lymphoma. Nevertheless, our approach was designed by group consensus, and this study provides evidence for its performance.
With regard to immunophenotypic details in the different types of PTNKCLs studied, 2 points are worth emphasizing. CD30 expression has received renewed interest given the availability of a therapy targeting this molecule.20 Although it was expressed in all ALCLs as expected, we found expression in >20% of lymphoma cells in other entities such as AITL and PTCL-NOS. Whether this may predict response to such targeted therapy is uncertain and is the subject of ongoing trials. PD-1 was, as expected, seen in a very high proportion of AITL cases, consistent with its putative Tfh origin. However, it is not specific for AITL. Consistent with the finding of prior studies, other T-cell lymphomas such as PTCL-NOS express PD-1 at variable frequencies, and our data are similar to those of a recent study by O’Malley and colleagues.17,21–23
Although the patients were not uniformly treated, our analysis of OS shows that patients subclassified in this study followed a clinical course similar to what is seen in other series of PTCL, further validating the clinical relevance of this diagnostic approach.4 A few points deserve emphasis. It is interesting to note that the KM curve of cases of PTNKCL that could not be subclassified on the basis of the algorithm we applied apparently yielded clinically useful information in that outcomes were poor and similar to PTCL-NOS. This is likely due to the fact that cases with good prognosis such as ALCL of either type could be excluded with CD30 and ALK1 stains. The argument can fairly be made that our unclassifiable cases should be considered PTCL-NOS, as this can be considered a “waste basket” category containing > 1 as yet undefined entity. However, cases left as unclassifiable were done so because of (a) significant doubt existing in the opinion of the reviewer that a more specific entity was possible (recognizing diagnostic gray zones between some entities) or (b) lack of additional tissue or data in an individual case, precluding confident subclassification. In either event, a diagnosis of T-cell or NK-cell lymphoma was certain in the minds of the reviewers.
Prior studies have explored and suggested tissuebased/ phenotypic prognostic markers for PTCL-NOS including proliferation gene signature or markers,24,25 TP53 expression,26 Epstein-Barr virus RNA,27 the metastasis- suppressor gene product nm23-H1,28 CCR4,29 VEGF mRNA,30 and FOXP1.31 Although not a primary goal of the study, use of our immunophenotypic panel may also provide information on prognosis. We found that CD4 expression in PTCL-NOS was a favorable risk factor. This is similar to a prior study of PTCL, unspecified in the 2001 WHO classification derived from Asia in which CD4+/CD8− phenotype was found to be associated with longer 1- and 2-year OS compared with CD8+/CD4−.32 Indirectly supporting this was the finding that cytotoxic molecule expression as defined by TIA1 or granzyme B expression was an unfavorable prognostic factor in multivariate analysis.33 We were unable to confirm the cytotoxic phenotype as a poor risk factor possibly because of lack of granzyme B in our diagnostic panels. In a recent follow-up study to the International Peripheral T-cell Lymphoma Project, clinical and pathologic features of the PTCL-NOS patients were reported, and the authors found that the number of large cells (> 70%) was predictive of poor OS.34 However, neither CD4 nor cytotoxic phenotype (TIA1 expression) was associated with outcome in that study. In the current study, we were also unable to confirm the percentage of large cells as a prognostic factor. The reasons for the discrepancies are not clear; however, factors such as lack of uniform treatment, statistical power, and difficulty in accurately identifying the correct phenotype in T-cell lymphomas using single-parameter IHC may play a role.35 Certainly, validation of our findings should be carried out.
With regard to AITL, the other lymphoma subtype for which we had sufficient case numbers, we found that PD-1 expression was associated with poor prognosis. Whether this can be related to an immunosuppressive microenvironment favorable to tumor growth or survival is as yet uncertain. Given the association of increased IgA levels (> 400 mg/dL) with poor prognosis in AITL,36 it is interesting to speculate that PD-1-positive neoplastic cells are capable of providing help to B cells required for B-cell production of IgA.37
In summary, we have evaluated a multicenter series of PTNKCLs using a tiered approach to immunophenotypic and molecular genetic characterization and demonstrate that a high degree of diagnostic accuracy (> 90%) is possible with this approach. GR studies are not required in the great majority of cases. However, not all cases can be subclassified, likely reflecting our incomplete understanding and agreement upon the boundaries between certain entities. Potential prognostic markers were also identified. In the era of evidence-based medicine, these data will help inform pathologists in practice, those designing clinical trials with planned central review, and those interested in understanding diagnostic requirements and medical necessity of ancillary studies in the diagnosis of PTNKCLs.
ACKNOWLEDGMENTS
The authors acknowledge David Viswanatha, MD, for assistance in performing GR studies for a subset of cases in this study.
Source of Funding: E.D.H. received research funding for this project from Allos Therapeutics.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
REFERENCES
- 1.Cotta CV, Hsi ED. Pathobiology of mature T-cell lymphomas. Clin Lymphoma Myeloma. 2008;8:S168–S179. doi: 10.3816/CLM.2008.s.013. [DOI] [PubMed] [Google Scholar]
- 2.Jenni D, Karpova MB, Seifert B, et al. Primary cutaneous lymphoma: two-decade comparison in a population of 263 cases from a Swiss tertiary referral centre. Br J Dermatol. 2011;164:1071–1077. doi: 10.1111/j.1365-2133.2010.10143.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928–948. doi: 10.1002/ajh.22139. [DOI] [PubMed] [Google Scholar]
- 4.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 5.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK + ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. pp. 270–319. [Google Scholar]
- 7.Foss FM, Zinzani PL, Vose JM, et al. Peripheral T-cell lymphoma. Blood. 2011;117:6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 8.Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin’s lymphomas. [Accessed November 25, 2012];2012 Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nhl. [Google Scholar]
- 9.van Krieken JH, Langerak AW, Macintyre EA, et al. Improved reliability of lymphoma diagnostics via PCR-based clonality testing: report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia. 2007;21:201–206. doi: 10.1038/sj.leu.2404467. [DOI] [PubMed] [Google Scholar]
- 10.Pileri SA, Agostinelli C, Bacci F, et al. Pathobiology of ALK-negative anaplastic large cell lymphoma. Pediatr Rep. 2011;3(suppl 2):e5, 8–10. doi: 10.4081/pr.2011.s2.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogan A, Gaulard P, Jaffe ES, et al. Angioimmunoblastic T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. pp. 309–311. [Google Scholar]
- 12.Attygalle AD, Diss TC, Munson P, et al. CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2004;28:54–61. doi: 10.1097/00000478-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman DM, Brown JA, Shahsafaei A, et al. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Grogg KL, Attygale AD, Macon WR, et al. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19:1101–1107. doi: 10.1038/modpathol.3800625. [DOI] [PubMed] [Google Scholar]
- 16.Ree HJ, Kadin ME, Kikuchi M, et al. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30:403–411. doi: 10.1016/s0046-8177(99)90115-6. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Shahsafaei A, Dorfman DM. Germinal-center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma. Am J Clin Pathol. 2009;131:33–41. doi: 10.1309/AJCP62WRKERPXDRT. [DOI] [PubMed] [Google Scholar]
- 18.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 19.Jaffe ES, Harris NL, Stein H, et al. Tumours of Haematopoeitic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 20.Deng C, Pan B, O’Connor OA. Brentuximab vedotin. Clin Cancer Res. 2013;19:22–27. doi: 10.1158/1078-0432.CCR-12-0290. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan C, Warnke RA, Arber DA, et al. PD-1 expression in T-cell lymphomas and reactive lymphoid entities: potential overlap in staining patterns between lymphoma and viral lymphadenitis. Am J Surg Pathol. 2010;34:178–189. doi: 10.1097/PAS.0b013e3181cc7e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Malley DP, Chizhevsky V, Grimm KE, et al. Utility of BCL2, PD1, and CD25 immunohistochemical expression in the diagnosis of T-cell lymphomas. Appl Immunohistochem Mol Morphol. 2014;22:99–104. doi: 10.1097/PAI.0b013e31828ef1f7. [DOI] [PubMed] [Google Scholar]
- 23.Zhan HQ, Li XQ, Zhu XZ, et al. Expression of follicular helper T cell markers in nodal peripheral T cell lymphomas: a tissue microarray analysis of 162 cases. J Clin Pathol. 2011;64:319–324. doi: 10.1136/jcp.2010.084459. [DOI] [PubMed] [Google Scholar]
- 24.Cuadros M, Dave SS, Jaffe ES, et al. Identification of a proliferation signature related to survival in nodal peripheral T-cell lymphomas. J Clin Oncol. 2007;25:3321–3329. doi: 10.1200/JCO.2006.09.4474. [DOI] [PubMed] [Google Scholar]
- 25.Grierson HL, Wooldridge TN, Purtilo DT, et al. Low proliferative activity is associated with a favorable prognosis in peripheral T-cell lymphoma. Cancer Res. 1990;50:4845–4848. [PubMed] [Google Scholar]
- 26.Pescarmona E, Pignoloni P, Puopolo M, et al. p53 over-expression identifies a subset of nodal peripheral T-cell lymphomas with a distinctive biological profile and poor clinical outcome. J Pathol. 2001;195:361–366. doi: 10.1002/path.945. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d’Etude des Lymphomes de l’Adulte (GELA) study. Blood. 2006;108:4163–4169. doi: 10.1182/blood-2006-04-017632. [DOI] [PubMed] [Google Scholar]
- 28.Niitsu N, Nakamine H, Okamoto M. Expression of nm23-H1 is associated with poor prognosis in peripheral T-cell lymphoma, not otherwise specified. Clin Cancer Res. 2011;17:2893–2899. doi: 10.1158/1078-0432.CCR-10-2999. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10:5494–5500. doi: 10.1158/1078-0432.CCR-04-0371. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen JM, Sorensen FB, Bendix K, et al. Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors Flt-1, KDR, Flt-4 in different subtypes of non-Hodgkin lymphomas. Leuk Lymphoma. 2009;50:1647–1660. doi: 10.1080/10428190903156729. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Sato F, Xia H, et al. Forkhead box P1 overexpression and its clinicopathologic significance in peripheral T-cell lymphoma, not otherwise specified. Hum Pathol. 2012;43:1322–1327. doi: 10.1016/j.humpath.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Kojima H, Hasegawa Y, Suzukawa K, et al. Clinicopathological features and prognostic factors of Japanese patients with “peripheral T-cell lymphoma, unspecified” diagnosed according to the WHO classification. Leuk Res. 2004;28:1287–1292. doi: 10.1016/j.leukres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Asano N, Suzuki R, Kagami Y, et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol. 2005;29:1284–1293. doi: 10.1097/01.pas.0000173238.17331.6b. [DOI] [PubMed] [Google Scholar]
- 34.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 35.Geissinger E, Bonzheim I, Krenacs L, et al. Identification of the tumor cells in peripheral T-cell lymphomas by combined polymerase chain reaction-based T-cell receptor beta spectrotyping and immunohistological detection with T-cell receptor beta chain variable region segment-specific antibodies. J Mol Diagn. 2005;7:455–464. doi: 10.1016/s1525-1578(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837–2843. doi: 10.1182/blood-2011-08-374371. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]