Abstract
FMS-like tyrosine kinase III (FLT3) mutations occur in one-third of acute myeloid leukemia (AML) patients and predict poor outcome. The incidence and impact of FLT3 in myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) is unknown. We conducted a retrospective review to identify WHO MDS and CMML patients with FLT3 mutations at diagnosis. A total of 2,119 patients with MDS and 466 patients with CMML were evaluated at MD Anderson between 1997 and 2010. Of these, FLT3 mutation analysis was performed on 1,232 (58%) MDS and 302 (65%) CMML patients. FLT3 mutations were identified in 12 (0.95%) MDS patients: 9 (75%) had FLT3-ITD mutation and 3 had FLT3-tyrosine kinase domain (TKD) mutation. MDS patients with FLT3 mutations were younger (P = 0.02) and presented as RAEB (P = 0.03) more frequently. Median overall survival (OS) for FLT3-mutated MDS patients was 19.0 months versus 16.4 months for FLT3-nonmutated MDS patients (P = 0.08). FLT3 mutations were identified in 13 (4.3%) CMML patients: 8 had FLT3-ITD mutation and 5 had FLT3-TKD mutation. There were no significant differences in demographic and disease characteristics among CMML patients with and without FLT3 mutations. Median OS for FLT3-mutated CMML patients was 10.8 months versus 21.3 months for FLT3-nonmutated CMML patients (P = 0.12). FLT3 occurs in MDS and CMML at a lower frequency than AML and does not predict poor outcome.
Introduction
FLT3 (FMS-like tyrosine kinase III) is a transmembrane tyrosine kinase that belongs to the Class III family of receptor tyrosine kinases (RTKs; other members of this family include receptors for KIT, FMS, and PDGF) [1]. Signaling via RTKs is frequently deregulated in hematological malignancies [2]. FLT3 is expressed on the leukemic cells of 70–100% of patients with acute myeloid leukemia (AML) [3]. Additionally, activating mutations in FLT3 are observed in ~30% of adult AML patients [4]. The two leading types of mutations found in AML include internal tandem duplications in the juxtamembrane domain (ITD, 17–34%) and mutations in the tyrosine kinase domain (TKD) activation loop (~7%) [5]. FLT3 stimulates survival and proliferation of leukemic blasts [6]. Studies suggest that patients with FLT3-ITD have significantly elevated peripheral blood white cell counts and increased bone marrow blasts at diagnosis [5,7]. Furthermore, they have a significantly higher induction death rate, increased relapse risk, inferior event-free survival (EFS), and decreased overall survival (OS) [5,7,8]. FLT3-TKD mutations have unknown prognostic and predictive significance in AML [9].
The incidence and impact of FLT3 in myelodysplastic syndrome (MDS) remains poorly defined [9–12]. We conducted a retrospective review at MDACC to identify the incidence, prognostic, and predictive impact of FLT3 mutations (ITD and TKD) in patients with MDS (per WHO classification) or chronic myelomonocytic leukemia (CMML). We included CMML, because from a practical approach, they are treated as MDS. A higher frequency of FLT3 mutations in CMML compared to MDS has been previously reported [12].
Methods
We conducted a retrospective review of patients with MDS and CMML evaluated at MDACC between January 1997 and December 2010. The study was conducted following institutional guidelines. A departmental database was used to identify patients with WHO classification MDS or CMML who had documented FLT3 mutation (either ITD or TKD) at diagnosis.
Variables collected on all patients (FLT3 mutated and FLT3 nonmutated) at diagnosis included the following: age, gender, performance status, white blood cell count, absolute neutrophil count (ANC), platelet count, hemoglobin, bone marrow blast percentage, karyotype, and history of a prior malignancy. The IPSS risk score was calculated to determine a patient’s risk of leukemic transformation and survival [13].
Detection of FLT3 mutations
FLT3 analysis has been routinely performed on all patients with MDS and CMML evaluated at MDACC since 2003. However, FLT3 analysis has also been performed retrospectively on stored MDS and CMML bone marrow specimens at MDACC dating back to 1997. Hence, we were able to include FLT3 mutation status data on MDS and CMML patients from January 1997 to December 2010.
FLT3 mutations were analyzed in the clinical molecular diagnostic laboratory at MDACC. FLT3 mutation status was determined in DNA from initial bone marrow aspirate samples. Genomic DNA from bone marrow samples was isolated using the Autopure extractor (QIAGEN/Gentra, Valencia, CA). FLT3 mutation was analyzed as previously described [14].
Statistical analysis
Differences among variables were evaluated by the χ2 test and Mann–Whitney U test for categorical and continuous variables, respectively. All P values were two-sided, and P < 0.05 was significant. Survival distributions were estimated using the Kaplan–Meier method, and the differences were compared using the log-rank test. OS was defined as the time from presentation to the MDACC leukemia service to death from any cause or the last follow-up. Time to progression (TTP) was the time from diagnosis to progression to AML by WHO criteria (i.e., ≥20% blasts).
Results
There were 2,119 patients with MDS and 466 patients with CMML evaluated at MDACC between January 1997 and December 2010. FLT3 mutational analysis was performed on 1,232 (58%) of the MDS patients and 302 (65%) of the CMML patients. FLT3 mutations were identified in 12 (0.95 %) MDS patients and 13 (4.3%) CMML patients.
Patient characteristics
Demographic and disease characteristics were compared between the 12 FLT3-mutated MDS patients and 1,220 FLT3-nonmutated MDS patients (Table I). FLT3 ITD and FLT3 TKD mutations were present in 9 (8%) and 3 patients (25%), respectively. The MDS patients with FLT3 mutations were younger (60 vs. 68 years; P = 0.02) and tended to present as RAEB more frequently than MDS patients without FLT3 mutations (P = 0.03). No other significant differences were identified between the two groups. Median white count (WBC), hemoglobin, platelet count, and ANC for the FLT3-mutated MDS patients at diagnosis were 3.5 × 109/l (range, 1.2–14.5), 9.9 g/l (range, 8.3–11.4), 47 × 109/l (range, 15–101), and 1.5 × 109/l (range, 0.5–7.2), respectively. Karyotype in FLT3-mutated MDS patients was diploid in 9 (75%); −5/−7 in 1 (8%), 11q in 1 (8%), and other cytogenetic aberrations in 1 patient (8%). FLT3-mutated MDS patients by IPSS: 1 patient had low risk (8%), 6 had intermediate-1 (50%), 4 had intermediate-2 (34%), and 1 had high-risk disease (8%).
TABLE I.
MDS: FLT3-Mutated Versus FLT3-Nonmutated
| FLT3-mutated N (%)/median (range) |
FLT3-nonmutated N (%)/median (range) |
P | |
|---|---|---|---|
| Number | 12 | 1220 | |
| Age (years) | 60 [20–74] | 68 [16–92] | 0.02 |
| Sex | |||
| Female | 3 (25%) | 432 (35%) | 0.45 |
| Male | 9 (75%) | 788 (65%) | |
| WBC ↔ 109/L | 3.5 [1.2–14.5] | 3.2 [0.3–159] | 0.8 |
| Hemoglobin (g/dL) | 9.9 [8.3–11.4] | 9.8 [4.0–17.5] | 0.85 |
| Platelets ↔ 109/L | 47 [15–101] | 73 [1–1040] | 0.08 |
| ANC ↔ 109/L | 1.5 [0.5–7.2] | 1.4 [0–77.4] | 0.69 |
| Diagnosis | |||
| RA | 1 (8%) | 559 (46%) | 0.03 |
| RAEB | 11 (92%) | 640 (52%) | |
| Deletion 5q | 0 | 21 (2%) | |
| Cytogenetics | |||
| Diploid, -Y | 9 (75%) | 583 (48%) | 0.26 |
| Deletion 5/7 | 1 (8%) | 323 (26%) | |
| Other | 2 (17%) | 251 (21%) | |
| Indeterminate | 0 | 60 (5%) | |
| Not done | 0 | 3 (0.2%) | |
| IPSS score | |||
| High | 1 (8%) | 110 (9%) | 0.71 |
| INT-1 | 6 (50%) | 474 (39%) | |
| INT-2 | 4 (34%) | 347 (28%) | |
| Low | 1 (8%) | 258 (21%) | |
| Not available | 0 | 31 (3%) |
Demographic and disease characteristics were compared between the 13 CMML FLT3-mutated patients and 289 FLT3-nonmutated patients (Table II). FLT3 ITD and FLT3 TKD mutations were present in 8 (62%) and 5 patients (38%), respectively There were no significant differences in demographic and disease characteristics among CMML patients with and without FLT3 mutations. Median WBC, hemoglobin, platelet count, and ANC for the FLT3-mutated CMML patients at diagnosis were 31.3 × 109/l (range, 2.6–211.8), 10.1 g/l (range, 8.7–12.7), 120 × 109/l (range, 23– 429), and 13.3 × 109/l (range, 0.5–108), respectively. Karyotype in FLT3-mutated CMML patient was diploid in 10 (77%), 20q-in 1 (8%), and others in 2 patients (15%).
TABLE II.
CMML: FLT3 Mutated Versus FLT3 Nonmutated
| FLT3 mutated N (%)/ median (range) |
FLT3 nonmutated N (%)/ median (range) |
P | |
|---|---|---|---|
| Number | 13 | 289 | |
| Age (years) | 65 [55–81] | 70 [32–91] | 0.4 |
| Sex | 0.45 | ||
| Female | 5 (38%) | 83 (29%) | |
| Male | 8 (62%) | 206 (71%) | |
| WBC ↔ 109/L | 31.3 [2.6–211.8] | 13.2 [1.1–149.8] | 0.39 |
| Hemoglobin (g/dL) | 10.1 [8.7–12.7] | 10.6 [5.1–16.4] | 0.64 |
| Platelets ↔ 109/L | 120 [23–429] | 89 [6–820] | 0.36 |
| ANC ↔ 109/L | 13.3 [0.5–108.0] | 6.9 [0.3–6.9] | 0.68 |
| Diagnosis | |||
| CMML-1 | 9 (69%) | 199 (69%) | 0.98 |
| CMML-2 | 4 (31%) | 90 (31%) | |
| Cytogenetics | |||
| Diploid, -Y | 10 (77%) | 206 (72%) | 0.73 |
| Deletion 5/7 | 0 | 21 (7%) | |
| Other | 3 (23%) | 53 (18%) | |
| Indeterminate | 0 | 4 (1%) | |
| Not done | 0 | 5 (2%) |
Outcomes
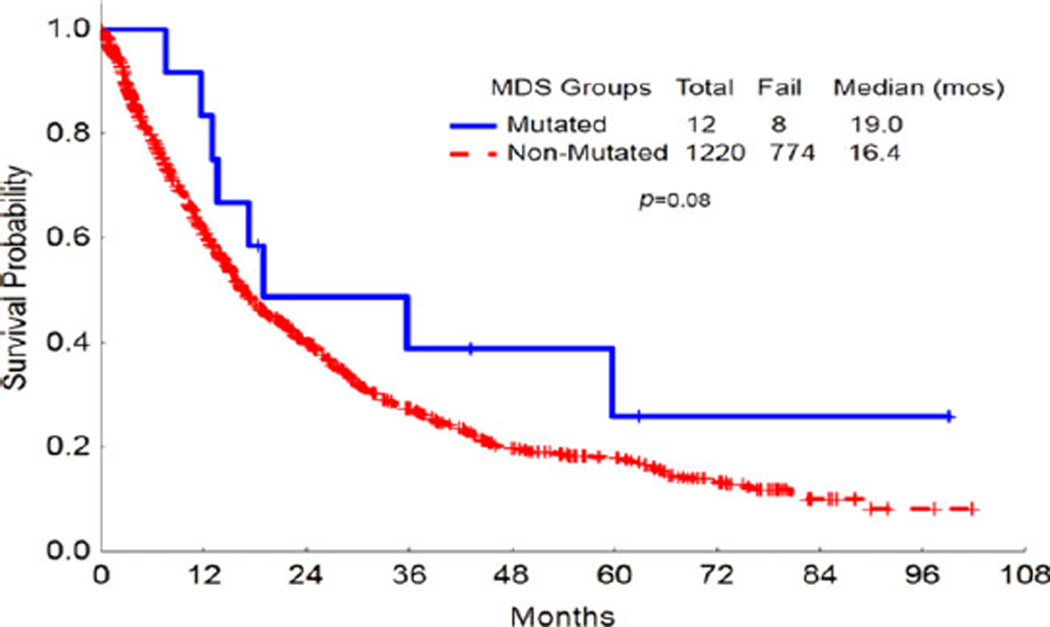
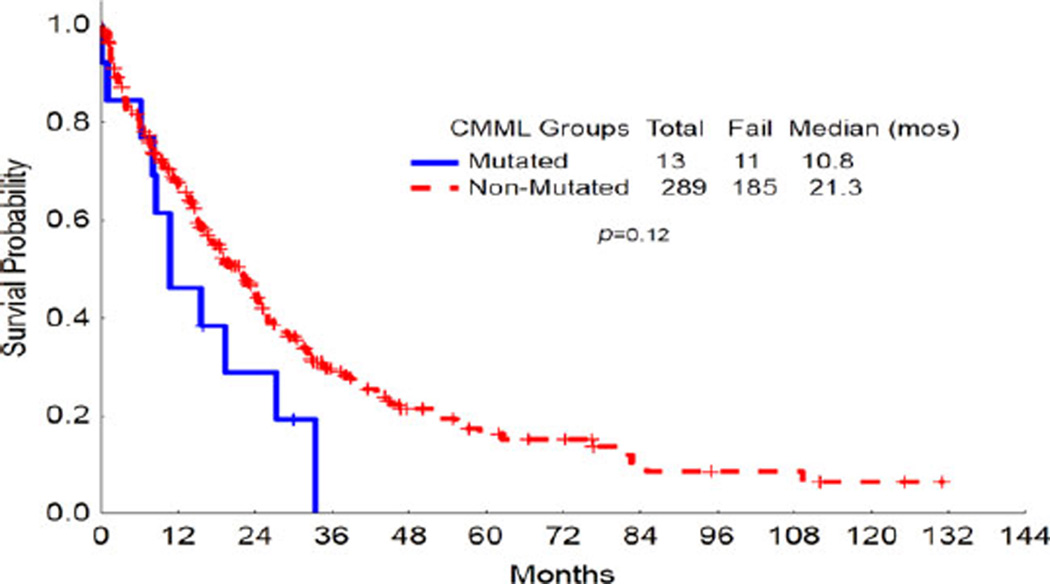
Median OS for MDS patients with FLT3 mutation was 19 months compared to 16 months for those without FLT3 mutation (P = 0.08; Fig. 1). Median OS for CMML patients with FLT3 mutation was 12 months compared to 21 months for those without FLT3 mutation (P = 0.12; Fig. 2). At the time of analysis, 9 of 25 patients had progressed to AML and median TTP was 9 months (range, 1–36).
Figure 1.
Overall survival of FLT3-mutated MDS patients versus FLT3-nonmutated MDS patients. Kaplan–Meier analysis of overall survival (OS) for FLT3-mutated MDS patients versus FLT3-nonmutated MDS petients. The OS did not differ significantly for FLT3-mutated versus FLT3-nonmutated patients. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
Overall survival of FLT3-mutated MDS patients versus FLT3-nonmutated CMML patients. Kaplan–Meier analysis of overall survival (OS) for FLT3-mutated MDS patients versus FLT3-nonmutated CMML petients. The OS did not differ significantly for FLT3-mutated versus FLT3-nonmutated patients. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The FLT3 gene is located on chromosome 13q12 [1]. FLT3 plays a crucial role in normal hematopoiesis and cellular growth in primitive hematopoietic stem and progenitor cells [15]. FLT3 is activated following binding of FLT3 ligand, leading to the activation of downstream signaling pathways including Stat5, RAS, and PI3 kinase [2,6]. Mutations occur in ~30% [4] of adult and pediatric AML cases and are particularly common among cytogenetically normal (44%: 32% with FLT3-ITD and 12% with FLT3-TKD) [16] or t(15;17) AML (40%) [17]. In the former setting, FLT3-ITD is associated with increased blasts, inferior EFS, and OS [5,7,18]. Prognosis is even worse when wild-type FLT3 allele is lost [8] and FLT3-ITD mutations negate the positive influence expressed by NPM1 mutation [19]. The prognostic relevance of FLT3-TKD mutations remains controversial [9].
The epidemiologic and clinical features of FLT3 mutations in MDS and CMML are less known [20]. To date, this is the largest analysis of FLT3 mutations in MDS and CMML, either as individual diseases or as combined diagnosis. Case series have reported very low frequencies, both in adults and children [10,21,22]. Horiike et al. [10] first reported FLT3-ITD mutations in 7 of 92 patients with MDS (3%). Similarly, Shih et al. [12] analyzed 150 patients with MDS (RAEB-T not included) and identified FLT3-ITD mutations in only 5 (2.5%) patients [12]. The largest reported series of FLT3 mutations in MDS was published by Bacher et al. [23] in 2007. Of 367 patients with MDS, FLT3-ITD mutations were identified in eight cases (2.2%), all with an underlying diagnosis of RAEB, and FLT3-TKD mutation was identified in one patient (0.4%). Interestingly, in the same study, FLT3-ITD frequency was 12% in both secondary and therapy-related AML, 22% in de novo AML and up to 27% in relapsed AML [22]. In all three studies, FLT3 mutations were more frequent among cases progressing to AML and were associated with a trend to worse prognosis and decreased event free and OS. However, in our study, we did not see significant difference in OS in FLT3-mutated MDS and CMML patients when compared with those without FLT3 mutations. On analyzing the groups separately, we noted a trend toward decreased OS in FLT3-mutated CMML patients when compared with CMML patients without FLT3 mutations. Conversely, in MDS, we noted a trend to improve survival in the FLT3-mutated group. Advent of hypomethylating agents, improved supportive care, and better transplant options for MDS post-2005 may be responsible for the improved survival noted in the FLT3-mutated MDS patients.
The extremely low incidence of FLT3 mutations in IPSS low and intermediate-1 MDS coupled with increased incidence of FLT3 mutations in RAEB and AML as noted by Horiike et al. [10] and Bacher et al. [9] led to the suggestion that FLT3 mutation plays a role in progression from MDS to AML [24]. Two retrospective studies attempted to address this question by performing serial FLT3 analysis on MDS patients. Shih et al. [12] reviewed 70 patients with MDS that progressed to AML [11]. They identified three patients with FLT3-ITD at diagnosis and seven patients that acquired FLT3-ITD mutation during AML evolution. They noted that the incidence of FLT3-ITD at diagnosis of MDS was significantly lower than that at AML transformation and FLT3-ITD-mutated patients progressed to AML more rapidly than FLT3-ITD wild-type patients (2.5 ± 0.5 vs. 11.9 ± 1.5 months, P = 0.114). FLT3-ITD-mutated patients also had a significantly shorter survival than nonmutated patients (5.6 ± 1.3 vs. 18.0 ± 1.7 months, P = 0.0008) [11]. Similar data have been reported by Georgiou et al. [25] in 2006. Our data seem to support this hypothesis. Eleven of 12 of our FLT3 MDS patients had RAEB. In fact, when we compared disease characteristics of FLT3-mutated MDS patients versus nonmutated patients, the only statistically significant difference between FLT3-mutated and FLT3-nonmutated patients was that MDS patients with FLT3 mutations were younger and tended to present as RAEB more frequently than MDS patients without FLT3 mutations. Prospective studies are warranted to confirm this hypothesis.
Finally, interest in this mutation is not only derived from its value as a prognostic biomarker but also because it can serve as a therapeutic target [26,27]. A number of agents with FLT3 inhibitory activity are being developed [28–31] although experience in MDS is limited.
In conclusion, there was no significant difference in OS in MDS or CMML patients who were FLT3 mutated versus those without FLT3 mutations. This would suggest that FLT3 mutations in MDS and CMML may not carry the same negative prognostic impact as FLT3 mutations in AML. However, our retrospective analysis is limited by the relatively small number of patients of informative patients. The therapeutic value of FLT3 inhibitors should be explored in mutated patients.
Footnotes
Conflict of interest: Nothing to report
References
- 1.Baldus CD, Mrozek K, Marcucci G, et al. Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: A concise review. Br J Haematol. 2007;137:387–400. doi: 10.1111/j.1365-2141.2007.06566.x. [DOI] [PubMed] [Google Scholar]
- 2.Grundler R, Miething C, Thiede C, et al. FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood. 2005;105:4792–4799. doi: 10.1182/blood-2004-11-4430. [DOI] [PubMed] [Google Scholar]
- 3.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leuk: Off J Leuk Soc Am Leuk Res Fund UK. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 4.Odenike O, Thirman MJ, Artz AS, et al. Gene mutations, epigenetic dysregulation, and personalized therapy in myeloid neoplasia: Are we there yet? Semin Oncol. 2011;38:196–214. doi: 10.1053/j.seminoncol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 8.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 9.Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: The combination matters—An analysis of 3082 patients. Blood. 2008;111:2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 10.Horiike S, Yokota S, Nakao M, et al. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leuk: Off J Leuk Soc Am Leuk Res Fund UK. 1997;11:1442–1446. doi: 10.1038/sj.leu.2400770. [DOI] [PubMed] [Google Scholar]
- 11.Shih LY, Huang CF, Wang PN, et al. Acquisition of FLT3 or N-ras mutations is frequently associated with progression of myelodysplastic syndrome to acute myeloid leukemia. Leuk: Off J Leuk Soc Am Leuk Res Fund UK. 2004;18:466–475. doi: 10.1038/sj.leu.2403274. [DOI] [PubMed] [Google Scholar]
- 12.Shih LY, Lin TL, Wang PN, et al. Internal tandem duplication of fms-like tyrosine kinase 3 is associated with poor outcome in patients with myelodysplastic syndrome. Cancer. 2004;101:989–998. doi: 10.1002/cncr.20440. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 14.Lin P, Jones D, Medeiros LJ, et al. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am J Clin Pathol. 2006;126:530–533. doi: 10.1309/JT5BE2L1FGG8P8Y6. [DOI] [PubMed] [Google Scholar]
- 15.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lymphomyeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 17.Shih LY, Kuo MC, Liang DC, et al. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98:1206–1216. doi: 10.1002/cncr.11636. [DOI] [PubMed] [Google Scholar]
- 18.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Schnittger S, Weiss T, Haferlach C, et al. Prognostic impact of FLT3 mutation load in NPM1 mutated AML. Blood. 2009;114:341–341. [Google Scholar]
- 20.Nolte F, Hofmann WK. Molecular mechanisms involved in the progression of myelodysplastic syndrome. Future Oncol. 2010;6:445–455. doi: 10.2217/fon.09.175. [DOI] [PubMed] [Google Scholar]
- 21.Yokota S, Kiyoi H, Nakao M, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leuk: Off J Leuk Soc Am Leuk Res Fund UK. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Taki T, Yang HW, et al. Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol. 1999;105:155–162. [PubMed] [Google Scholar]
- 23.Bacher U, Haferlach T, Kern W, et al. A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica. 2007;92:744–752. doi: 10.3324/haematol.10869. [DOI] [PubMed] [Google Scholar]
- 24.Davids MS, Steensma DP. The molecular pathogenesis of myelodysplastic syndromes. Cancer Biol Ther. 2010;10:309–319. doi: 10.4161/cbt.10.4.12612. [DOI] [PubMed] [Google Scholar]
- 25.Georgiou G, Karali V, Zouvelou C, et al. Serial determination of FLT3 mutations in myelodysplastic syndrome patients at diagnosis, follow up or acute myeloid leukaemia transformation: Incidence and their prognostic significance. Br J Haematol. 2006;134:302–306. doi: 10.1111/j.1365-2141.2006.06171.x. [DOI] [PubMed] [Google Scholar]
- 26.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 27.Mrozek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes J, Foran J, Ghirdaladze D, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML Study. Blood. 2009;114:264–264. [Google Scholar]
- 29.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]