Abstract
Objective
To describe the association of systolic hypotension during the first 6 hours after successful resuscitation from pediatric cardiopulmonary arrest (CA) with in-hospital mortality.
Design
Retrospective cohort study
Setting
Fifteen children's hospitals associated with the Pediatric Emergency Care Applied Research Network
Patients
Patients between 1 day and 18 years of age who had a cardiopulmonary arrest, received chest compressions > 1 minute, had a return of spontaneous circulation (ROSC) > 20 minutes and had a systolic blood pressure documented within 6 hours of arrest.
Interventions
None.
Measurements and Main Results
Three hundred eighty three patients had complete data for analysis. Patients with a documented minimum systolic blood pressure < 5th percentile for age and sex within the first 6 hours following ROSC were considered to have early post-resuscitation hypotension. Two hundred fourteen patients (56%) had early post-resuscitation hypotension. One hundred eighty four patients (48%) died prior to hospital discharge. After controlling for patient and CA characteristics, hypotension in the first 6 hours following ROSC was associated with a significantly increased odds of in-hospital mortality (adjusted OR=1.71; 95%CI, 1.02–2.89; P=0.042) and odds of unfavorable outcome (adjusted OR=1.83; 95%CI, 1.06–3.19; P=0.032).
Conclusions
In the first six hours following successful resuscitation from pediatric cardiac arrest, systolic hypotension was documented in 56%, and was associated with a higher rate of in-hospital mortality and worse hospital discharge neurologic outcomes.
Keywords: Children, heart arrest, resuscitation, hypotension, shock
Introduction
Cardiac arrest is a major public health problem with >500,ooo cardiac arrests in adults and >10,oo0 in children each year in the US.(1–4) Survival rates are less than 10% following out-of-hospital cardiac arrests in adults and children.(1–4) During the decade from 2000 to 2009, risk-adjusted survival rates following in-hospital cardiac arrests have increased in adults from 14%to 22% and in children from 14% to 44%.(5, 6)
After successful initial resuscitation following a cardiac arrest, most patients die in the post-cardiac arrest period prior to hospital discharge.(1, 4, 6–10) Over the last two decades, a Post-Cardiac Arrest Syndrome characterized by myocardial dysfunction, systemic ischemia-reperfusion response, brain injury, and multi-organ dysfunction has been described.(9, 11, 12) Recent adult data suggest that early hypotension after cardiac arrest is associated with a higher mortality rate. (8, 10)
In a large multicenter observational cohort study of children with successful resuscitation following either out-of-hospital cardiac arrest or in-hospital cardiac arrest, most children in each group died prior to hospital discharge.(13) Using this rich investigational database, our goals are to: 1) determine the prevalence of hypotension in the first 6 hours among children following successful resuscitation from cardiac arrest and 2) evaluate whether systolic hypotension in the first 6 hours following resuscitation from cardiac arrest was associated with a higher rate of post-cardiac arrest in-hospital mortality and/or unfavorable neurologic outcome.
Methods
Study Population
Our study was conducted using an existing public access dataset from the Pediatric Emergency Care Applied Research Network (PECARN). The PECARN database was created with support from the NICHD (HD044955) to plan the current NHLBI-funded Therapeutic Hypothermia For Pediatric Cardiac Arrest (THAPCA) Trials (NCT00880087 and NCT00878644). The database was derived from a retrospective cohort study of in-hospital (IH) and out-of-hospital (OH) cardiac arrest (CA) conducted between July 1, 2003 and December 31, 2004 at 15 children's hospitals associated with the PECARN. Patients from one day (24 hours) to 18 years of age (inclusive) who experienced CA requiring at least one minute of chest compressions and who had ROSC for a minimum of 20 minutes were eligible for inclusion. Out-of-hospital classification was assigned if chest compressions were initiated prior to hospital arrival; IH classification was assigned if chest compressions were initiated in the emergency department or other hospital setting. Patients with cardiac arrests in a neonatal ICU and those who had planned CA in the operating room as part of congenital heart disease surgical repair were excluded. Identification of patients and database management for the PECARN database has been previously described.(13–15)
Variables collected in the original database included: patient characteristics, such as age, sex, race, ethnicity, and pre-existing chronic conditions; pre-event characteristics, such as presence and types of vascular access, endotracheal intubation, and monitoring devices; event characteristics, such as location and timing of CA, first and subsequent monitored cardiac rhythms, defibrillation, and drugs administered during the arrest; etiology of CA; hospital course characteristics, such as use of extracorporeal membrane oxygenation (ECMO), therapeutic hypothermia, other intensive care monitoring devices and interventions, drug therapies, seizures, and subsequent cardiac arrests; physiologic and laboratory data, such as pre-arrest lactate and blood pH, glucose blood urea nitrogen and creatinine concentrations in the first 6 hours post-arrest; and outcome data, such as survival to hospital discharge and Pediatric Cerebral Performance Category (PCPC) scores at hospital discharge.
Dates and times of important clinical events were recorded, and relevant time intervals were determined. Utstein-style definitions were used for variables for which such definitions exist.(16, 17) Time 0 represented the time that chest compressions were initiated. Both physiologic and laboratory data were collected as minimum and maximum values obtained from 0–6 hours. If there was only one value provided for a time interval, it was assigned to both the minimum and maximum. If there was no documented value within a given time period it was considered missing.
This study was exempted by The Children's Hospital of Philadelphia Institutional Review Board because it was a de-identified, publicly available data set.
Inclusion and Exclusion Criteria
Patients were excluded from analysis if they were missing information on age, sex or systolic blood pressure during the 0–6 hours interval following ROSC (see Exposures below). Patients treated with ECMO or patients without clear documentation regarding ECMO use in the first 2 hours following ROSC were excluded because of the limited time for hypotension in light of full mechanical support so soon after arrest. Patients who died within the first 6 hours were also excluded because they may have had persistent hypotension that was untreated or undertreated (e.g., if they were moribund). Patients were excluded from the secondary analysis of neurologic outcome if they were unable to have a functional outcome category assigned to them based on missing PCPC scores (see Outcomes below).
Exposures and Outcomes
Hypotension was defined as a minimum systolic blood pressure (SBP) < 5th percentile derived from normative age and sex data (Table 1).(18) Arrest times were categorized as night or weekend vs. weekdays.(13, 19) Arrest location was stratified by location (IH vs. OH) and witnessed status.
Table 1.
Fifth percentile systolic blood pressures by age and gender for the 50% height percentile. (https://sites.google.com/a/channing.harvard.edu/bernardrosner/pediatric-blood-press)
| Age (years) | 5%ile Male | 5%ile Female |
|---|---|---|
| 1 | 71 | 72 |
| 2 | 73 | 74 |
| 3 | 75 | 75 |
| 4 | 77 | 77 |
| 5 | 78 | 78 |
| 6 | 80 | 80 |
| 7 | 82 | 81 |
| 8 | 84 | 82 |
| 9 | 85 | 83 |
| 10 | 86 | 85 |
| 11 | 88 | 88 |
| 12 | 89 | 90 |
| 13 | 92 | 91 |
| 14 | 94 | 92 |
| 15 | 96 | 92 |
| 16 | 97 | 93 |
| 17 | 99 | 94 |
The primary outcome was in-hospital mortality. The secondary outcome was neurologic outcome, determined by the Pediatric Cerebral Performance Category (PCPC). The PCPC is a six-point classification system to define cognitive function: 1 = normal; 2 = mild disability; 3 = moderate disability; 4 = severe disability; 5 = coma or vegetative state; and 6 = death.(20) Favorable neurologic outcome was defined as a PCPC score of 1 or 2 at hospital discharge, or no change from pre-arrest to hospital discharge.(21) Unfavorable neurologic outcome was defined as a discharge PCPC score of 3, 4, 5 or 6 and a change from pre-arrest PCPC score ≥ 1. If patients were missing a pre-arrest PCPC score, but had a discharge PCPC score of 1 or 2, they were determined to have a favorable neurologic outcome. If patients were missing a pre-arrest PCPC score, but died, they were included in the unfavorable neurologic outcome group. If patients were missing a pre-arrest PCPC score, but had a discharge PCPC score of 3, 4 or 5, they were excluded from the analysis of functional outcome because the appropriate group (favorable versus unfavorable neurologic outcome) could not be determined.
Statistical Analysis
Standard descriptive statistics were used to summarize patient and CA event characteristics, stratified by hypotension status and survival to discharge. Fisher's exact tests or Wilcoxon rank-sum tests were used to determine differences between groups. Univariable logistic regression models were used to estimate the association between hypotension over the first 6 hours after CA and odds of in-hospital mortality (primary) and odds of unfavorable neurologic outcome (secondary). Multivariable models included patient and CA event characteristics based on a priori clinical rationale or a posteriori evidence for potential confounding. Variables included were: age (cubic splines), pre-existing conditions (lung or airway; hematologic, oncologic, or immune compromised; genetic metabolic; neurologic), total number of vasopressors before arrest, night or weekend arrest, arrest location, first documented rhythm, and total doses of epinephrine at arrest. A final parsimonious model eliminated variables from the multivariable model using a stepwise variable-selection procedure that minimized the Akaike information criterion (AIC). The potential for modification of the effect of hypotension on in-hospital mortality by vasopressor use during the first six hours after ROSC was evaluated by including an interaction term between hypotension and an indicator variable for any vasopressor use in the parsimonious model. For the primary outcome, P<0.05 was used to determine statistical significance; for the secondary outcome, P<0.05 was considered to suggest significance; and for interaction analyses, P<0.1 was considered to suggest significance. All analyses were completed using R 2.15.2 (R Development Core Team, Vienna, Austria).
Results
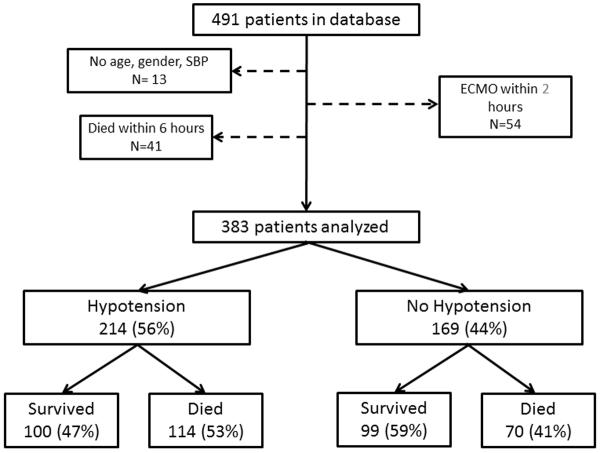
Four hundred ninety one patients were enrolled in the database. After applying exclusion criteria, 383 patients were eligible for analysis (Figure 1). Patients were stratified by minimum SBP in the first 6 hours following arrest by age and sex derived percentiles. Two hundred fourteen patients (56%) had documented hypotension (minimum SBP <5th percentile for age and sex) in the first 6 hours following ROSC.
Figure 1.
Consort diagram patients with exposure to post-cardiac arrest hypotension and outcomes.
In a sensitivity analysis, we treated minimum systolic blood pressure as a continuous variable, indexed to the 50%ile for age and sex. The strong association of hypotension with mortality remained (p=0.003) but there was some evidence that the association was non-linear (p=0.036).
Patients with documented early post-resuscitation hypotension were less likely to have pre-existing lung or airway disease, but more likely to be on pre-arrest vasopressors and less likely to have had an OHCA. Patients with early post-resuscitation hypotension received more epinephrine during resuscitation and were on more vasopressors following ROSC (Supplemental Digital Content - Table 2). Patients with post-resuscitation hypotension had lower post-resuscitation median minimum pH and higher post-resuscitation median maximum creatinine.
One hundred eight four patients died prior to hospital discharge. Patients who died were more likely to have an out-of-hospital arrest, an initial rhythm of PEA or asystole, a longer duration of CPR and more doses of epinephrine (Supplemental Digital Content - Table 3). Non-survivors also had a lower post-resuscitation median minimum pH, a lower post-resuscitation median minimum plasma bicarbonate concentration, and higher post-resuscitation median maximum glucose.
Patients who had hypotension were more likely to die prior to hospital discharge (53% vs. 41%; OR=1.61; P=0.022) (Table 4). After controlling for patient and event characteristics, hypotension in the first 6 hours following ROSC was associated with a significantly increased odds of in-hospital death (adjusted OR=1.71; 95%CI, 1.02–2.89;P=0.042) and odds of unfavorable neurologic outcome (adjusted OR=1.83; 95%CI, 1.06–3.19; P=0.032).
Table 4.
Associations of hypotension during first six hours post-cardiac arrest with outcomes. Hypotension defined as a minimum systolic blood pressure < 5th percentile; percentiles derived from normative age and sex data.
| No hypotension | Hypotension | Unadjusted | Fully Adjusted* | Parsimonious** | |
|---|---|---|---|---|---|
| N (%) | N (%) | OR (95% CI); P | OR (95% CI); P | OR (95% CI); P | |
| In-hospital mortality | 70/169 (41) | 114/214 (53) | 1.61 (1.07, 2.43); 0.022 | 1.71 (1.02, 2.89); 0.042 | 1.71 (1.07, 2.76); 0.026 |
| Unfavorable outcome | 82/160 (51) | 128/207 (62) | 1.54 (1.02, 2.34); 0.043 | 1.83 (1.06, 3.19); 0.032 | 1.78 (1.08, 2.95); 0.024 |
OR, odds ratio; CI, confidence interval
Adjusted for age (cubic splines), pre-existing conditions (lung or airway; hematologic, oncologic, or immune compromised; genetic metabolic; neurologic), total number of vasopressors before arrest, night or weekend arrest, arrest location, first documented rhythm, and total doses of epinephrine at arrest; 26 patients removed due to missing data
Adjusted for arrest location, pre-existing conditions (hematologic, oncologic, or immune compromised; genetic metabolic), and total doses of epinephrine at arrest; 24 patients removed due to missing data
Of the fifty two patients excluded due to receiving early ECMO support, in-hospital death occurred in 19/38 (50%) of hypotensive patients and 9/14 (64%) of non-hypotensive patients. An unfavorable outcome occurred in 20/29 (69%) hypotensive patients and 9/12 (75%) non-hypotensive patients.
The impact of post-ROSC vasopressor use during the first six hours after ROSC on the association between hypotension and in-hospital mortality was also evaluated. Of children with early post-resuscitation hypotension, 88/214 (41%) received vasopressor infusions within the first six hours after ROSC. Among patients who received post-ROSC vasopressors, there was no difference in discharge outcomes between hypotension and no hypotension groups (p=0.18). However, among patients who did not receive vasopressors within six hours post-ROSC, those with no post-ROSC hypotension were less likely to die than those with hypotension (OR=2.12; 95% CI, 1.18–3.81).
Thirty three patients (8.6%) were initiated on a new vasopressor following resuscitation. Three hundred fifty patients did not have the initiation or addition of a new vasopressor following ROSC. The mortality rate for both groups was 48%.
Of the 214 with early post-resuscitation hypotension, 73 continued to receive preexisting vasopressor support, while 15 were initiated on vasopressor support. More than half (126/214) were not treated with a continuous vasopressor infusion.
Discussion
This study establishes that early post-resuscitation hypotension is associated with increased hospital discharge mortality in children after successful resuscitation from cardiac arrest. Among children with documented early post-ROSC hypotension, 53% died in the hospital compared to 41% without documented early post-ROSC hypotension. Importantly, early post-resuscitation hypotension was common: 56% had documented hypotension in the first six hours post-ROSC. Interestingly, only 41% of the children with early post-ROSC hypotension received post-ROSC vasopressor infusions during those first six hours post-ROSC in this high-risk cohort with a 53% in-hospital mortality rate.
Post cardiac arrest syndrome is a recently described clinical entity, manifested by myocardial dysfunction, systemic ischemia/reperfusion response, brain injury, and multi0rgan dysfunction.(9) The cardiovascular pathophysiology includes a sepsis-like syndrome associated with elevations in circulating cytokines, and concomitant myocardial dysfunction. This combination can cause hypotension and systemic hypoperfusion, resulting in further post-arrest end organ injury.(22) Notably, the children with early post-ROSC hypotension in this study had a lower minimum pH in the first six hours after ROSC and higher serum creatinine concentrations. This association may be due to a more severe cardiac arrest or the hypotension itself may be associated with secondary organ injury. Post-resuscitation myocardial dysfunction following adult OHCA is a myocardial stunning process that begins within hours of ROSC and resolves by 72 hours.(23) Although optimal management of post-ROSC myocardial dysfunction and hypotension has not been established, vasopressor support is recommended as part of the bundle of care to improve hemodynamic status, avoid secondary insults, and attempt to improve long-term survival and neurological outcomes. (24–27)
In this cohort of children successfully resuscitated from cardiac arrest, 56% had documented hypotension in the first six hours post-ROSC. These findings in children are similar to the 47–65% incidence of documented hypotension among adults admitted to an ICU after ROSC from cardiac arrest.(8, 10, 23) The adult studies included both out-of-hospital and in-hospital cardiac arrests, as does our study. Many of the adults had out-of-hospital cardiac arrests and may have had acute coronary syndromes. However, children and adults with in-hospital cardiac arrests have similar causes for their cardiac arrests: acute respiratory failure and acute circulatory shock.(21) For all of these groups of adults and children with an initially successful resuscitation the message is clear: post-ROSC hypotension is common. Therefore, frequent post-cardiac arrest hemodynamic assessment should be provided for these patients.
Most importantly, early post-ROSC hypotension is associated with worse outcomes. In our cohort, 53% of children with early post-ROSC hypotension died in the hospital compared with only 41% of children without early post-ROSC hypotension, and the adjusted OR for in-hospital mortality was 1.71 (95%CI, 1.02–2.89). Similarly, 38% of children with early post-ROSC hypotension had an unfavorable neurologic outcome compared to 49% of these children without early post-ROSC, and the adjusted OR for unfavorable neurological outcome was 1.83 (95%CI, 1.06–3.19). Our study extends the observation that early post-ROSC hypotension is associated with worse outcomes in adults to children without pre-existing coronary artery disease and acute coronary artery syndrome.(8, 10, 14)
This observational study cannot distinguish whether early post-ROSC hypotension resulted in worse outcomes versus the possibility that children with more severe pre-arrest and/or intra-arrest insults were more likely to have both early post-ROSC hypotension and higher mortality rates. However, the dangers of secondary injuries after initial hypoxic-ischemic insults are well established.(9) In addition, Trzeciak and colleagues showed that post-ROSC hypotension was common in adults (47% of 8736) and was associated with a higher in-hospital mortality rate (65% vs. 27%; adjusted OR 2.7; 95%CI, 2.5–3). They speculated that treatment to avoid this secondary insult might improve outcomes. Our findings among the sub-group of children in our study without early vasopressor therapy show that early hypotension was associated with worse outcomes. In contrast, among the sub-group with early vasopressor therapy, early hypotension was not significantly associated with worse outcomes. Perhaps the vasopressor therapy mitigated the severity of hypoperfusion, myocardial dysfunction, or allowed clinicians to simply titrate the vasopressor infusions to limit the duration and severity of hypotension. Similar to Trzeciak's findings, our data raise the possibility that closer hemodynamic monitoring, avoidance of hypotension, and prompt treatment of post-ROSC hypotension may improve post-resuscitation hemodynamics, may minimize secondary injuries, and may improve outcomes.
Our study had several limitations. Data was retrospectively collected and therefore limited to what was documented in the medical record. It was not documented in the database whether specific blood pressure measurements were obtained invasively or non-invasively, although 75% of patients were managed with an arterial catheter at some point during their post-resuscitation care. Normal blood pressure for children depends on age and sex, and blood pressure percentiles for age and sex are only available at threshold values. Therefore, it is difficult to evaluate blood pressure as a continuous variable across percentile groups. We do not have specific data regarding management of hypotension with respect to fluid resuscitation, electrolyte repletion or vasopressor titration in relation to specific blood pressure values. The database included lowest systolic blood pressure during the first 6 hours following ROSC, but did not indicate the duration of low blood pressure or when these lowest blood pressures occurred during the 6 hour post-ROSC time interval. Therefore, a patient categorized as having hypotension could have had only one documented low blood pressure without sustained hypotension. Nevertheless, post-ROSC hypotension was demonstrably associated with in-hospital mortality. Further, we excluded patients who were initiated on ECMO within 2 hours of ROSC or who died in the first 6 hours following ROSC and patients who did not have data for age, sex or ECMO treatment. Exclusion of early ECMO patients limits the generalizability of this study to this population. There were no available echocardiographic data or further details regarding myocardial function or vasopressor dosing. Finally, this retrospective study demonstrating associations of hypotension with increased mortality is hypothesis-generating and potentially clinically important, but the experimental design precludes establishment of causality.
Conclusions
Following successful resuscitation from pediatric cardiac arrest, hypotension was documented in 56% of patients during the first six hours post-ROSC. Early post-ROSC hypotension was associated with a higher rate of in-hospital mortality and worse neurologic outcome at hospital discharge. These data raise the possibility that avoidance of post-ROSC hypotension and treatment of post-ROSC hypotension may improve post-ROSC hemodynamics, and improve clinically important outcomes.
Supplementary Material
Acknowledgment
This manuscript was prepared using the Hypothermia for Pediatric Cardiac Arrest Planning Grant Study Data Set obtained from the University of Utah, and does not necessarily reflect the opinions or views of the Hypothermia for Pediatric Cardiac Arrest Planning Grant study investigators, the Pediatric Emergency Care Applied Research Network (PECARN), or the Health Resources Services Administration (HRSA) Maternal Child Health Bureau (MCHB) Emergency Medical Services for Children (EMSC). The Hypothermia for Pediatric Cardiac Arrest Planning Grant Study was funded by NIH awards R21HD044955 and R34HD050531, and PECARN was funded by HRSA awards U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008.
Financial Support: Dr. Berg is funded by NIH award U10 HD063108.
Dr. Dean is funded by NIH awards K12HD047349, U01HD049934, U01HL094339, HRSA award U03MC00008, and the HA and Edna Benning Presidential Endowment.
Dr. Moler is funded by NIH awards U01HL094345, R01HL112745, U10 HD063106, and R34HD072101.
Dr. Sutton is funded by a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K23HD062629).
Dr. Topjian is funded by a NIH career development award K23NS075363 and U01HL094345
Dr. Nadkarni is funded by NIH award U01 HL107681, U01 HL094345, RO1 HL114484, R01 HL058669, AHRQ R18 HS022469, AHRQ R18 HS022464, and CIHR 2009-09-15
Dr. Topjian received support for article research from the National Institutes of Health and is employed by the University of Pennsylvania. Dr. Topjian's institution received grant support from NIH. Dr. French served as a statistical editor for JAMA Pediatrics and a Resuscitation Pediatric Research Task Force Member for the National Get with the Guidelines; and received support for article research from NIH. Dr. Sutton received grant support from the National Institutes of Health (NICHD Career Development Award). Dr. Moler received support for article research from NIH. Dr. Moler's institution received grant support from NIH. Dr. Dean received support for article research from NIH and is employed by the University of Utah. Dr. Dean's institution received grant support from NIH. Dr. Berg received grant support from NICHD (PI of Collaborative Pediatric Clinical Critical Care Research Network at CHOP).
Footnotes
Copyright Form Disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugate JE, Brinjikji W, Mandrekar JN, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 8.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79:410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 10.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37:2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. quiz 2904. [DOI] [PubMed] [Google Scholar]
- 11.Kern KB, Hilwig RW, Rhee KH, et al. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 12.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95:2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 13.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meert KL, Donaldson A, Nadkarni V, et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moler FW, Donaldson AE, Meert K, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaritsky A, Nadkarni V, Hazinski M, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the pediatric Utstein style. A statement for healthcare professionals from the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Circulation. 1995;92:2006–2020. doi: 10.1161/01.cir.92.7.2006. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B, Cook N, Portman R, et al. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167:653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 19.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. Jama. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 20.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 21.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Jama. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 22.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 23.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 24.HACA Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 25.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 26.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80:418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.