Abstract
BACKGROUND
The aim of the study is to investigate the prognostic role of pre-treatment of markers of the systemic inflammatory response (neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and albumin) in patients with oropharyngeal carcinoma treated with chemoradiotherapy.
METHODS
A total of 251 patients with oropharyngeal squamous cell cancer treated with chemoradiotherapy between 2004 and 2010 were retrospectively identified. NLR, PLR, and albumin were recorded from baseline blood parameters. NLR threshold of >5 and PLR thresholds of ≤150, >150 and ≤300, and >300 were used for analysis.
RESULTS
Median follow-up was 46 months (range 9–98). The 3 year overall survival, local control, regional control, and distant control were 70%, 85%, 87%, and 87%, respectively. On multivariate analysis, locoregional control was associated with T stage (HR 3.3 (95% CI 1.5–6.9), P = 0.002) and NLR (HR 2.1 (95% CI 1.1–3.9), P = 0.023). Overall survival was associated with T stage (HR 2.47 (95% CI 1.45–4.2), P = 0.001) and grade (HR 0.61 (95% CI 0.38–0.99), P = 0.048). PLR and albumin were not significantly associated with disease outcomes or survival.
CONCLUSIONS
The NLR is an independent prognostic factor for locoregional control in oropharyngeal cancer treated with chemoradiotherapy.
Keywords: oropharynx cancer, squamous cell cancer, chemotherapy, radiotherapy, neutrophil-to-lymphocyte ratio
Introduction
Concurrent chemoradiotherapy (CRT) is a standard organ-preserving approach for patients with locally advanced oropharyngeal cancers.1 Human papilloma virus (HPV)-related oropharyngeal squamous cell carcinomas are associated with a more favorable prognosis compared with HPV-negative disease.2 Treatment regimens for oropharyngeal carcinoma are associated with significant early and long-term toxicity.3 There is considerable interest in the development of additional prognostic biomarkers to allow the stratification of treatment intensity according to prognosis. The identification of a robust favorable prognosis group would allow investigation of approaches testing a de-escalation of treatment intensity with the aim of maintaining cure rates whilst reducing toxicity; conversely, studies of treatment intensification may be considered in patients with a poorer prognosis.
Host-related factors including performance status, weight loss, smoking, comorbidity, in addition to tumor pathology, play an important role in cancer outcomes.4 It has become evident that the cancer-associated systemic inflammatory response has an important influence on disease-related outcomes for many cancer sites.5,6 Systemic inflammatory responses are associated with alterations in circulating white blood cell counts, with a neutrophilia and relative lymphopenia.7 The neutrophil-to-lymphocyte ratio (NLR), a biomarker of the host systemic inflammatory response, has been shown to be highly promising in stratifying outcome in large cohorts of patients with cancers arising from unselected sites, with a higher pre-treatment ratio associated with a poorer prognosis.8,9 Other components of the systemic inflammatory response including platelet counts, albumin and C-reactive protein (CRP) levels are prognostically important in some studies.10 The platelet-to-lymphocyte ratio (PLR) is prognostically important in some tumor sites.11,12
There is a paucity of data with regard to the prognostic influence of the systemic inflammatory response in head and neck cancers. This is further complicated by the range of anatomical tumor sites and treatment approaches within the head and neck region. Here, we report the outcomes of patients with oropharyngeal carcinoma treated with concurrent-chemoradiotherapy and examine the prognostic influence of biomarkers of the host systemic inflammatory response including the NLR and PLR.
Methods
Study design
A total of 249 consecutive patients with oropharyngeal squamous cell carcinoma treated with radical chemo-radiotherapy with curative intent between January 2004 and December 2010 at St. James’s Institute of Oncology, Leeds, UK were retrospectively identified using electronic databases. Inclusion criteria for the current study were: histologically proven squamous cell carcinoma, oropharyngeal primary site, treatment with curative intent, documented intention to treat with concurrent chemoradiotherapy ± induction chemotherapy. Exclusion critieria were: local or regional surgery prior to chemoradiotherapy, unavailability of pre-chemotherapy blood results. Full blood counts were routinely measured at St. James’s Institute of Oncology prior to treatment. Pre-treatment hemoglobin, albumin, neutrophil, lymphocyte, and platelet counts were recorded from routine blood counts. NLR and PLRs were calculated. Tumor-node-metastasis (TNM) staging was routinely recorded in case notes according to the American Joint Committee on Cancer (AJCC) classification 2002 edition.
Induction chemotherapy
Induction chemotherapy was used based upon clinician preference, patient and tumor factors; in general induction chemotherapy was considered for patients with bulky disease. Regimens consisted of 2–4 cycles of cisplatin 80 mg/m2 day 1 and 5-fluorouracil (5-FU) 800 mg/m2 days 2–5, three times weekly.13 From 2006, docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, and 5-FU 750 mg/m2 days 2–5, three times weekly was available for selected fit patients.14
Concurrent chemotherapy
Patients <70 years old were considered for concurrent chemotherapy. Standard concurrent chemotherapy was cisplatin 100 mg/m2 days 1 and 29. Carboplatin AUC 4 was substituted for cisplatin if creatinine clearance was <55 mL/min.
Radiotherapy
The majority of the patients in this study were treated with a two-phase conformal technique of two lateral parallel opposed 6 MV photon fields with multiple field-in-fields, with a matched anterior neck field and posterior electron fields, as previously described.14 The clinical target volume included primary site and bilateral level Ib, II, III, IV, and V lymph nodes. Retropharyngeal lymph nodes were variably included depending upon tumor site and stage. Conventionally fractionated intensity modulated radiotherapy (IMRT) was used to treat patients from late 2009; target volume delineation was based upon a compartmental approach previously described for oropharyngeal cancer in the UK parotid sparing oropharynx trial.15 IMRT was delivered with a 5–7 angle step and shoot IMRT technique. Standard dose during this period was 70 Gy in 35 fractions, with alternate regimens used at clinician discretion.
Response assessment and follow-up
Tumor response was routinely assessed 3–4 months after the completion of the treatment. Response assessment routinely included clinical examination, panendoscopy, and imaging (CT, MRI, and/or PET-CT). Examination under anesthetic and biopsies were performed in the event of any clinical or radiological suspicion. A neck dissection was considered only if there was an incomplete nodal response. Subsequently, patients were followed up with physical examination every 6–8 weeks in the first year after treatment, every 3 months for an additional 2 years, and every 6 months until discharge at 5 years.
Statistical analysis
Time elapsed to treatment failure or death was calculated using the first day of treatment as the starting point. Local and regional controls were defined as no evidence of recurrence or progression of the primary tumor and neck lymph nodes, respectively. Distant control was defined as the absence of distant metastases. In the overall survival estimates, deaths due to all causes are included in the calculations. For the purposes of analysis, an NLR of ≥5 is allocated a score of 1, and <5 a score of 0. A PLR of ≤150 is given a score of 0, >150 and ≤300 a score of 1, and a score >300 is given a score 2. Univariate and multivariate Cox regression (backward elimination based on a likelihood ratio test) analyses were performed for each endpoint. The following factors were included in the multivariate model: age, gender, smoking status, tumor grade, T stage, N stage, use of induction chemotherapy, number of concurrent chemotherapy cycles, albumin, NLR, and PLR. Statistical significance was declared at P < 0.05. Analysis was performed using SPSS software (Version 19.0.0. SPSS Inc., Chicago, IL, USA).
Results
A total of 251 patients with oropharyngeal squamous cell carcinoma treated with concurrent chemoradiotherapy ± induction chemotherapy were identified. Two patients were excluded as blood results were unavailable; no patients with active systemic inflammation were identified. Median follow-up for living patients was 46 months (range 9–98). Demographics and patient characteristics (Table 1) and treatment details are analyzed (Table 2).
Table 1.
Patient demographics and disease characteristics.
| NUMBER (%) (TOTAL = 249) | |
|---|---|
| Male | 200 (80.3%) |
| Female | 49 (19.7%) |
|
| |
| Age (median (range)) | 55 (31–78) |
|
| |
| Smoking | |
| Never | 71 (28.5%) |
| Ex/current | 166 (66.7%) |
| Not recorded | 12 (4.8%) |
|
| |
| Primary tumour site: | |
| Tonsil | 154 (61.3%) |
| Base of tongue | 82 (32.9%) |
| Soft Palate | 8 (3.2%) |
| Oropharynx, not classified | 5 (2.0%) |
|
| |
| T stage | |
| TX | 1 (0.4%) |
| T1 | 47 (18.9%) |
| T2 | 70 (28.1%) |
| T3 | 37 (14.9%) |
| T4 | 94 (37.8%) |
|
| |
| N stage | |
| N0 | 25 (10.0%) |
| N1 | 28 (11.2%) |
| N2a | 31 (12.4%) |
| N2b | 87 (34.9%) |
| N2c | 54 (21.7%) |
| N3 | 24 (9.6%) |
|
| |
| Grade | |
| Well differentiated | 12 (4.8%) |
| Moderately differentiated | 68 (27.3%) |
| Poorly/undifferentiated | 133 (53.4%) |
| Unclassified | 36 (14.5%) |
Table 2.
Treatment details.
| NUMBER (%) (TOTAL = 249) | |
|---|---|
| Induction chemotherapy | |
| None | 95 (38.2%) |
| Docetaxel/cisplatin/5-fluorouracil | 55 (22.1%) |
| Cisplatin or carboplatin with 5-fluorouracil | 96 (38.6%) |
| Other | 3 (5.2%) |
|
| |
| Concurrent chemotherapy | |
| Cisplatin | 216 (86.7%) |
| Carboplatin | 21 (8.4%) |
| Planned but not given after induction chemotherapy | 12 (4.8%) |
|
| |
| No. Cycles concurrent chemotherapy | |
| 0 | 12 (4.8%) |
| 1 | 70 (28.1%) |
| 2 | 145 (58.2%) |
| 3 | 22 (8.8%) |
|
| |
| Radiation dose | |
| 70 Gy in 35 fractions | 186 (74.7%) |
| 68 Gy in 34 fractions | 13 (52.2%) |
| 66 Gy in 33 fractions | 8 (3.2%) |
| 65 Gy in 30 fractions | 19 (7.6%) |
| 56 Gy in 20 fractions | 21 (8.4%) |
| Not received | 2 (0.8%) |
|
| |
| Neutrophil-to-lymphocyte ratio pre-treatment | |
| ≤5 | 196 (78.7%) |
| >5 | 50 (20.1%) |
| Missing data | 3 (1.2%) |
|
| |
| Platelet-to-lymphocyte ratio pre-treatment | |
| ≤150 | 109 (43.8%) |
| >150≤300 | 116 (46.6%) |
| >300 | 21 (8.4%) |
| Missing data | 3 (1.2%) |
Survival outcomes and the role of the NLR and PLR
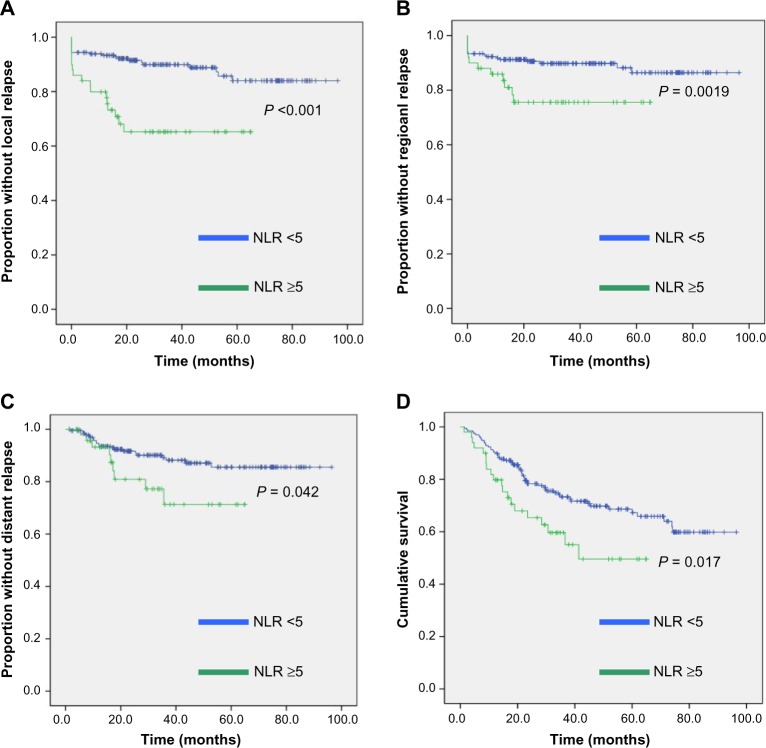
The 3 year overall survival, local control, regional control, and distant control were 70%, 85%, 87%, and 87%, respectively. Univariate analysis was performed for the following factors: gender, grade, T stage, N stage, number of cycles of concurrent chemotherapy, use of induction chemotherapy, NLR (<5 versus >5), PLR (≤150 versus >150 versus >300), and smoking status (Table 3). Kaplan–Meier curves illustrating the disease outcomes stratified by a NLR of ≤5 and >5 based on univariate analysis are shown in Figure 1. Multivariate analysis was performed including the following factors in the multivariate model: age, gender, smoking status, tumor grade, T stage, N stage, use of induction chemotherapy, number of concurrent chemotherapy cycles, albumin, NLR, and PLR (Table 4). Statistically significant prognostic factors for local control were T stage (P = 0.003) and the NLR (P = 0.009), for regional control the NLR (P = 0.009), for loco-regional control T stage (P = 0.002) and the NLR (P = 0.023), for distant control T stage (P = 0.021), and for overall survival T stage (P = 0.001) and grade (P = 0.048). The PLR was not significantly associated with any disease or survival outcomes on the multivariate analysis.
Table 3.
Univariate analysis.
| LOCAL CONTROL | REGIONAL CONTROL* | LOCOREGIONAL CONTROL* | DISTANT CONTROL* | DFS (INCLUDES DEATH AS EVENT) | OS | |
|---|---|---|---|---|---|---|
| P value | ||||||
| Gender M vs F |
0.450 | 0.115 | 0.113 | 0.579 | 0.331 | 0.213 |
| Grade 1/2 vs 3 |
0.150 | 0.905 | 0.106 | 0.366 | 0.04 | 0.017 |
| T stage 1/2 vs 3/4 |
0.000 | 0.174 | 0.000 | 0.020 | 0.000 | 0.000 |
| N stage 0/1 vs 2/3 |
0.718 | 0.089 | 0.775 | 0.550 | 0.4290 | 0.226 |
| No. concurrent cycles of chemotherapy 0/1 vs 2/3 |
0.099 | 0.249 | 0.149 | 0.605 | 0.117 | 0.133 |
| Induction chemotherapy None vs induction |
0.019 | 0.028 | 0.011 | 0.568 | 0.602 | 0.957 |
| NLR <5 vs ≥ 5 |
0.000 | 0.019 | 0.001 | 0.042 | 0.012 | 0.017 |
| PLR ≤150 vs >150 vs >300 |
0.073 | 0.894 | 0.457 | 0.520 | 0.187 | 0.043 |
| Smoking status Non-smoker vs ex/current |
0.207 | 0.146 | 0.113 | 0.012 | 0.043 | 0.053 |
Note:
Note: death not included as event.
Figure 1.
Kaplan–Meier curves showing (A) local control, (B) regional control, (C) distant control, and (D) overall survival, according to a NLR of ≤5 or >5. P values are those from univariate analysis.
Table 4.
Multivariate analysis.
| VARIABLE | HAZARD RATIO | 95% CONFIDENCE INTERVAL | P VALUE |
|---|---|---|---|
| Local control | |||
| T stage 3/4 vs 1/2 | 3.958 | 1.609–9.736 | 0.003 |
| NLR ≥ 5 vs <5 | 2.516 | 1.262–5.017 | 0.009 |
| Regional control | |||
| NLR ≥ 5 vs <5 | 2.762 | 1.286–5.933 | 0.009 |
| Locoregional control | |||
| T stage 1/2 vs 3/4 | 3.263 | 1.536–6.929 | 0.002 |
| NLR <5 vs ≥ 5 | 2.072 | 1.105–3.886 | 0.023 |
| Distant control | |||
| T stage 3/4 vs 1/2 | 2.959 | 1.181–7.416 | 0.021 |
| Overall survival | |||
| Grade 3 vs 1/2 | 0.613 | 0.378–0.995 | 0.048 |
| T stage 3/4 vs 1/2 | 2.474 | 1.453–4.210 | 0.001 |
Discussion
The systemic inflammatory response appears to be associated with poorer cancer survival independent of tumor stage in multiple tumor sites.4,6 Several biochemical and hematological parameters, including white blood cells, neutrophil and platelet counts, albumin, and CRP levels, have been examined as possible biomarkers for a systemic inflammatory response.4 The NLR is one of the most promising biomarkers, being shown to stratify outcome in a host of tumor sites, including gastro-esophageal, pancreatic, lung, colorectal, renal, and breast tumors.5,16 The prognostic influence of the NLR has been reported in the context of a variety of treatments, including surgery,17 neoadjuvant chemotherapy,18 chemotherapy,19 radiotherapy,5 and during follow-up.20 Despite the heterogenous nature of the studies performed, the NLR has a consistent prognostic impact, suggesting an association with more aggressive tumor biology.5
We report the analysis of the outcomes from our experience of managing a large cohort of patients with locally advanced oropharyngeal carcinoma with chemo-radiotherapy in relation to the prognostic influence of biomarkers of the host inflammatory response. On multivariate analysis, a raised NLR was associated with poorer local and regional control. Although there was an association with distant control and overall survival on univariate analysis (Fig. 1), this was not significant on multivariate analysis. The PLR and albumin levels were not prognostically significant.
There is considerable heterogeneity of the NLR threshold utilized in reported series. Drawing conclusions based upon the use of varied NLR cut-offs is challenging. A significant majority of studies have used a threshold ratio of >5, and it has been recommended that future work should use this most commonly used threshold.5 Therefore, for the purpose of this study, we have used a threshold of >5. For similar reasons, we have utilized PLR thresholds commonly used elsewhere.21
In this study, pre-treatment variables have been analyzed in relation to outcome measures. Pre-treatment prognostic variables are of potential clinical importance in guiding decision making. We have not performed a similar analysis based upon blood counts acquired during treatment with either induction chemotherapy or concurrent chemotherapy. Such an analysis would have been of little value in our historical retrospective cohort as blood tests were not performed at consistent time points. In addition, the use of varied induction chemotherapy regimens for a significant proportion of patients would limit useful analysis. It therefore remains unclear as to whether NLR or PLR variables acquired during treatment have any prognostic significant. However, it might be expected that the bone marrow suppressive effect of chemotherapy with subsequent influence on NLR and PLR values would confound with any relationship with outcome measures.
The main limitation of this study is that we do not have HPV data available for this large retrospective cohort dating back to 2004. HPV-related oropharyngeal cancers typically occur in younger patients with less smoking history, are generally histopathologically poorly differentiated, and carry a more favorable prognosis with a 3 year survival of 82 versus 57% in the series reported by Ang et al.2 The influence of the NLR ratio upon outcome was present despite the inclusion of tumor grade and smoking status in the multivariate analysis. The relationship between the NLR and HPV status remains uncertain.
CRP levels have been combined with albumin levels into the modified Glasgow prognostic score as an index of the systemic inflammatory condition.6 We do not routinely check CRP levels prior to chemo-radiotherapy; therefore, it was not possible to analyze the prognostic value of this variable.
The NLR was not associated with distant control or overall survival on multivariate analysis. However, as shown in Figure 1, on univariate analysis, poorer outcomes were significantly associated with raised NLRs for these outcomes. The loss of statistical significance on multivariate analysis may result from the limited number of events in this sample size.
There is only limited data exploring the role of the systemic inflammatory response in head and neck cancer. Markers of the systemic inflammatory response in patients with head and neck cancer have not been compared to the normal population or to patients with non-malignant inflammatory conditions. The prognostic relevance of these biomarkers has not been previously reported in a cohort of oropharyngeal cancer patients. However, these findings are consistent with several other observations in head and neck cancer patients. One recent retrospective study of 273 patients with head and neck squamous cell cancers (including non-oropharyngeal sites) over a 10 year period and treatment approaches has reported that the PLR was an independent predictor of mortality and the NLR an independent predictor of recurrence.12 A raised NLR has been associated with a poorer prognosis in patients with nasopharyngeal carcinoma.22 Similarly, markers of blood leukocyte activation have been linked to survival in head and neck cancer.23 An important role for the host inflammatory response is also implied by one study demonstrating that elevated CRP levels were associated with poorer outcomes in operable oral squamous cell carcinoma.24
In multiple tumor types, the NLR appears to be related to more advanced disease stage and possible more aggressive tumor behavior.5 The underlying mechanism is unclear, although elements of the innate inflammatory response have been associated with upregulation of growth factors, angiogenesis, impaired cellular immunity, and cancer cachexia.4–6 Recent studies have found increased levels of pro-inflammatory cytokines in patients with an elevated NLR25,26 and an increased peri-tumor macrophage infiltration.25 These data suggest that a raised NLR reflects an up-regulated innate immune response.5 In addition to these data suggesting a role for the innate immune system in influencing the prognosis of oropharyngeal cancer, evidence is accumulating a role for the adaptive immune response. Ward et al recently showed that high levels of tumor infiltrating lymphocytes could stratify the prognosis of HPV-positive oropharyngeal cancers into high and low risk groups.27 In common with other tumor sites, it is becoming increasingly clear that both the innate and adaptive arms of the immune system appear to have a key role in influencing the behavior and outcome of head and neck cancers.28
Conclusion
In summary, the host systemic inflammatory response has been hypothesized to have an important role in cancer outcomes across a wide range of tumor sites. The NLR is a potential biomarker of the innate immune response. We retrospectively analyzed the outcomes of 249 patients with oropharyngeal carcinoma treated between 2004 and 2010 with concurrent chemoradiotherapy ± induction chemotherapy. The NLR was an independent prognostic factor for local and regional control. Further validation is required prior to incorporating the NLR into prognostic stratification.
Footnotes
Author Contributions
Conceived and designed the experiments: CY, LM, RP. Analyzed the data: CY, LM, HT, EK. Wrote the first draft of the manuscript: RP. Contributed to the writing of the manuscript: CY, LM. Agree with manuscript results and conclusions: EK, HT, MS. Jointly developed the structure and arguments for the paper: LM, MS, RP. Made critical revisions and approved final version: RP. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Ramos M, Benavente S, Giralt J. Management of squamous cell carcinoma of the head and neck: updated European treatment recommendations. Expert Rev Anticancer Ther. 2010;10(3):339–44. doi: 10.1586/era.10.6. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–63. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 5.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–6. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 7.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 8.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–41. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107(4):695–9. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer. 2013;119(12):2325–2. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 11.Szkandera J, Pichler M, Absenger G, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg. 2014:pii: S0002-9610(14)00043-9. doi: 10.1016/j.amjsurg.2013.10.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2013 Dec 13; doi: 10.1002/hed.23567. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121. doi: 10.1186/1748-717X-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prestwich RJ, Oksuz DC, Dyker K, Coyle C, Sen M. Feasibility and efficacy of induction docetaxel, cisplatin, and 5-fluorouracil chemotherapy combined with cisplatin concurrent chemoradiotherapy for nonmetastatic stage IV head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2011;81(4):e237–43. doi: 10.1016/j.ijrobp.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 17.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S. Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102(7):742–7. doi: 10.1002/jso.21725. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36(3):617–22. doi: 10.1007/s00268-011-1411-1. [DOI] [PubMed] [Google Scholar]
- 19.Chua W, Clarke SJ, Charles KA. Systemic inflammation and prediction of chemotherapy outcomes in patients receiving docetaxel for advanced cancer. Support Care Cancer. 2012;20(8):1869–74. doi: 10.1007/s00520-011-1289-3. [DOI] [PubMed] [Google Scholar]
- 20.Clarke SJ, Chua W, Moore M, et al. Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther. 2011;90(3):475–8. doi: 10.1038/clpt.2011.122. [DOI] [PubMed] [Google Scholar]
- 21.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–22. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 22.He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck. 2012;34(12):1769–76. doi: 10.1002/hed.22008. [DOI] [PubMed] [Google Scholar]
- 23.Millrud CR, Mansson Kvarnhammar A, Uddman R, Bjornsson S, Riesbeck K, Cardell LO. The activation pattern of blood leukocytes in head and neck squamous cell carcinoma is correlated to survival. PLoS One. 2012;7(12):e51120. doi: 10.1371/journal.pone.0051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF. Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol. 2011;36(2):147–53. doi: 10.1111/j.1749-4486.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 25.Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Kantola T, Klintrup K, Vayrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–36. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prestwich RJ, Errington F, Hatfield P, et al. The immune system–is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20(2):101–12. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]