Abstract
Malaria is a parasitic infection of global importance. Although relatively uncommon in developed countries, where the disease occurs mainly in travellers who have returned from endemic regions, it remains one of the most prevalent infections of humans worldwide. In endemic regions, malaria is a significant cause of morbidity and mortality and creates enormous social and economic burdens. Current efforts to control malaria focus on reducing attributable morbidity and mortality. Targeted chemoprophylaxis and use of insecticide-treated bed nets have been successful in some endemic areas. For travellers to malaria-endemic regions, personal protective measures and appropriate chemoprophylaxis can significantly reduce the risk of infection. Prompt evaluation of the febrile traveller, a high degree of suspicion of malaria, rapid and accurate diagnosis, and appropriate antimalarial therapy are essential in order to optimize clinical outcomes of infected patients. Additional approaches to malaria control, including genetic manipulation of mosquitoes and malaria vaccines, are areas of ongoing research.
Malaria is a parasitic infection transmitted by mosquitoes that has afflicted humans over the millennia. Once endemic in the United States and Canada, it is now confined to more tropical and subtropical climates, particularly Africa. Despite advances in knowledge, malaria continues to cause significant morbidity and mortality worldwide.
The social and economic burden of malaria in endemic countries is immense. Already a disease of the poor, malaria further contributes to economic woes by debilitating people afflicted with the disease and imposing significant financial costs on affected people and governments.1 An estimated US$12 billion in economic revenue is lost annually in Africa because of malaria.2
Epidemiology
Malaria is one of the most prevalent human infections worldwide. Over 40% of the world's population live in malaria-endemic areas.3 Exact numbers are unknown, but an estimated 300 to 500 million cases and 1.5 to 2.7 million deaths occur each year.3 Ninety percent of deaths occur in sub-Saharan Africa, the majority involving children less than 5 years of age. Malaria disproportionately affects the poor, in whom higher morbidity and mortality can be largely attributed to lack of access to effective treatment; 60% of malaria deaths worldwide occur in the poorest 20% of the population.2 In addition to children, pregnant women (particularly primigravidae) and nonimmune people (e.g., travellers, foreign workers) are at highest risk of severe disease. However, all age groups may be at risk of severe disease during malaria epidemics, which occur either when changes in the physical environment (caused by climatic variation, agricultural projects or mining, for example) increase the capacity of mosquitoes to transmit the disease or when population displacements (natural disasters, war) expose nonimmune populations to infection. In Canada and other developed nations, malaria is an imported disease and a significant but preventable cause of morbidity and mortality in travellers to endemic areas.4
Plasmodia species are the parasites responsible for malaria. Only 4 of the over 100 species of plasmodia are infectious to humans. The majority of cases and almost all deaths are caused by Plasmodium falciparum. Plasmodium vivax, Plasmodium ovale and Plasmodium malariae cause less severe disease. Malaria is present to varying degrees in 105 countries (Fig. 1), the majority of which contain drug-resistant strains (Table 1). Over 90% of all malaria cases occur in Africa, and most are caused by P. falciparum. This species also predominates in Haiti and the Dominican Republic. In Mexico, Central and South America, the Mediterranean, Asia and Oceania, both P. falciparum and P. vivax are endemic. Disease caused by P. ovale and P. malariae is relatively rare.
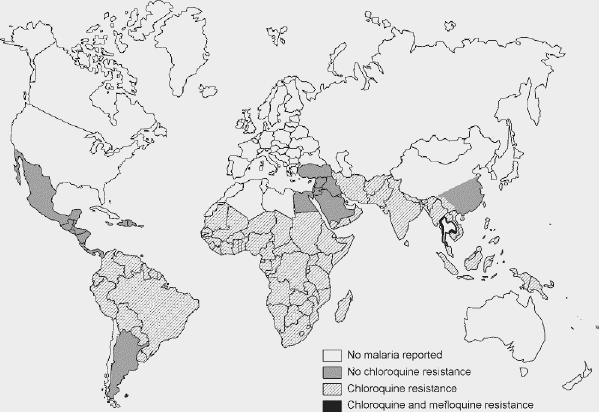
Fig. 1: Global distribution of malaria. Drug resistance is represented by the shaded areas (see legend). This map is intended as a visual aid only; online sources of country-specific malaria risk are provided in “Additional Resources.” Reproduced, with permission, from the Committee to Advise on Tropical Medicine and Travel, Health Canada. Canadian recommendations for the prevention and treatment of malaria among international travellers — 2003. Can Commun Dis Rep 2004;30(Suppl 1). In press.
Table 1

In the United States in 2000, 1402 cases of malaria were reported; of these, 59% were known to have been acquired in Africa, 20% in the Americas and 18% in Asia.5 Of 418 cases reported in Canada in 2001, 53% were determined to have been caused by P. falciparum (most from sub-Saharan Africa), 15% by P. vivax (the majority from the Indian subcontinent) and 9% by P. ovale. In Canada about 400 cases are reported annually, with 15 severe cases and 1 or 2 deaths each year (Dr. Anne McCarthy, Chair, Malaria Subcommittee, CATMAT, personal communication, 2003). This annual figure represents a drop of almost 60% from a peak of 1036 cases in 1997,6 but may be considerably underestimated owing to underreporting.
Efforts to eradicate malaria have clearly been unsuccessful in many regions of the world, and current efforts to control the disease focus on reducing attributable morbidity and mortality. The Roll Back Malaria program — a global partnership established by the World Health Organization, the World Bank, the United Nations Development Programme and UNICEF — has been expanded to include national governments, nongovernmental organizations, private sector groups and researchers. The 4 main components of the program include improving access to effective treatment, preventing malaria during pregnancy, reducing mosquito–human contact by widespread use of insecticide-treated bed nets and ensuring timely and appropriate action during malaria epidemics.1
Pathophysiology
Our understanding of the transmission and pathogenesis of this disease has evolved considerably from the 18th-century belief that malaria was miasmatic in origin (mal, bad; aria, air). An appreciation of the life cycle and transmission of plasmodia and the pathophysiology of infection is key to understanding the disease process.
The life cycle of the malaria parasite, spanning its mosquito and human hosts, is shown in Fig. 2. The parasite is transmitted by night-biting Anopheles mosquitoes. Warm climates with high humidity and abundant rain create favourable conditions for mosquitoes by increasing breeding areas and prolonging survival, thereby facilitating transmission. Domestically acquired malaria can occur but is rare. Such cases include “runway” or “airport” malaria, in which local transmission of disease has been attributed to an infected mosquito that was transported on a long-haul flight,7,8 transmission by local mosquitoes that have acquired the infection from migrants or visitors,9 transfusion-acquired infection10 and congenital infection.11
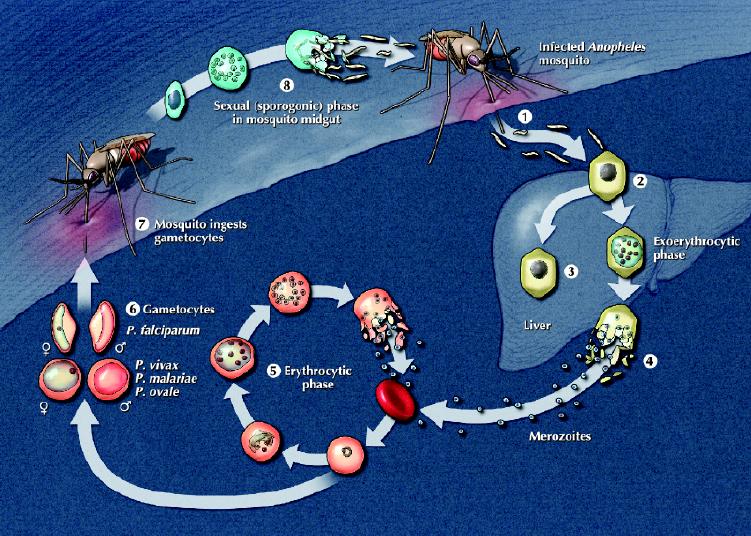
Fig. 2: The plasmodia life cycle. The human (asexual) stage of the life cycle begins with the exoerythrocytic phase. When an infected mosquito bites a human, sporozoites in the mosquito's saliva enter the bloodstream (1). The sporozoites travel to the liver, where they invade hepatocytes (2); over a period of up to 4 weeks, the infected hepatocytes mature into schizonts. In Plasmodium vivax and P. ovale infections only, some schizonts may remain dormant as hypnozoites (3) for weeks to years before causing clinical relapses. With schizont rupture, merozoites are released into the bloodstream (4). In the erythrocytic phase, merozoites invade erythrocytes and either undergo an asexual cycle of reproduction (5) or develop into nonmultiplying sexual forms (gametocytes) (6). These gametocytes are crucial for perpetuating the life cycle, as they are ingested by a feeding mosquito (7) and undergo sexual reproduction within the mosquito midgut; thousands of infective sporozoites (8) are produced, which then migrate to the salivary glands, ready to initiate another life cycle. Photo: Lianne Friesen and Nicholas Woolridge
The pathogenesis of malaria is best understood for P. falciparum infection. Several factors contribute to the severity of clinical disease. High parasite burdens combined with the unique ability of infected erythrocytes to adhere to host endothelium contribute to microvascular occlusion, metabolic derangement and acidosis, which lead to the manifestations of severe malaria (acute respiratory distress syndrome, renal insufficiency and cerebral malaria).12 In addition, a vigor ous cytokine response to parasite proteins released during schizont rupture can contribute to adverse clinical outcomes.13 Manifestations of disease may also be related to intravascular hemolysis and parasite consumption of glucose. Host factors such as sickle cell disease and glucose-6-phosphate dehydrogenase deficiency can modify the severity of disease.14,15 Infections caused by P. vivax, P. ovale and P. malariae are generally milder than falciparum malaria; symptoms are related to parasite burden and cytokine release, since vaso-occlusive phenomena do not occur.
Clinical presentation
The “nonimmune” host
More than 85% of travellers with malaria will experience symptoms only after they return from an endemic area.5,16,17 Symptoms of P. falciparum infection are usually apparent within 8 weeks of return,5,17,18 whereas those caused by P. vivax may be delayed for several months;5,17 however, most infections will be symptomatic within 1 year of return regardless of the infecting species. Semi-immune people such as immigrants and visitors from endemic areas and those taking chemoprophylaxis may have delayed onset of illness and mild symptoms.19
Travellers who have visited malaria-endemic areas and experience fever should be instructed to seek medical attention immediately; a patient with this presentation should be considered to have malaria until proven otherwise. Misdiagnosis or delayed diagnosis of malaria is common and may lead to catastrophic results4,16 as nonimmune people are at higher risk of developing severe or complicated malaria (see below). Fever or a recent history of fever (usually above 38°C) is almost always present, but rarely in the classic tertian (occurring every 48 hours) or quartan (every 72 hours) pattern;18 more commonly, the fever pattern is hectic. Other nonspecific symptoms include chills, malaise, headache, myalgias, cough and gastrointestinal symptoms.
Fever and splenomegaly are the most frequent physical findings on examination. Less often, hepatomegaly, jaundice and abdominal tenderness are noted. The presence of rash and lymphadenopathy should suggest an additional or alternate diagnosis.
The “immune” host
Although previous infection does produce demonstrable cellular and humoral immune responses, these are not fully protective, as evidenced by the repeated infections experienced by people living in endemic areas. However, the clinical presentation of malaria among such “immune” people is typically less severe than in nonimmune people; a significant proportion (up to 80%) of immune people with parasitemia may be completely asymptomatic.20,21
Fever is not a reliable indicator of malaria in endemic areas, but malaria must always be considered in the presence of fever. Among Montagnard (Vietnamese) refugees to the United States who were screened for malaria, for example, only 17% of the 58% who were parasitemic reported a history of recent fever.22 Headache, a feeling of cold and arthralgias are common presenting symptoms in children.23 Anemia, splenomegaly and hepatomegaly are commonly associated with malaria.
Severe and complicated malaria
Severe malaria, as defined by the World Health Organization, refers to a parasitemic person with 1 or more of the following: prostration (inability to sit up without help), impaired consciousness, respiratory distress or pulmonary edema, seizures, circulatory collapse, abnormal bleeding, jaundice, hemoglobinuria or severe anemia (hemoglobin < 50 g/L or hematocrit < 15%).24 Prostration and altered consciousness occur frequently in both children and adults with severe disease; respiratory distress, seizures and severe anemia are more common in children, whereas renal failure and jaundice occur more frequently in adults.24 Acute respiratory distress syndrome, an immune-mediated complication, often occurs during the second to fourth day of treatment, even when parasitemia is decreasing.25 Severe malaria usually occurs with parasitemia of 5% or more, and even with optimal management, the mortality rate exceeds 20%.26
At highest risk of complications from malaria are nonimmune people and children and pregnant women who live in endemic regions. Complications generally involve the central nervous, pulmonary, renal and hematopoietic systems. Hypoglycemia occurs because of parasite consumption of glucose and treatment with quinine; acidosis is another common metabolic derangement. Severe anemia, acute renal failure, respiratory failure, intravascular hemo-lysis and coagulopathies, and shock may develop. Bacterial infection may occur as a complication of malaria itself (e.g., aspiration pneumonia) or may be iatrogenic. One of the most serious complications is cerebral malaria, manifested by altered level of consciousness, focal neurologic findings and seizures. Mortality is high (15% to 25%), and survivors may have residual neurologic deficits.
Although semi-immune people and those living in endemic regions tend not to experience severe malaria, they may still experience complications from recurrent infections. In children, severe anemia is the most common complication of chronic malaria, with hematocrits not infequently approaching 15%. Massive splenomegaly causing abdominal pain may be associated with both bone marrow and immune dysfunction (hyperactive malarial splenomegaly). Nephrotic syndrome has also been attributed to falciparum malaria in endemic areas,27 as has splenic lymphoma.28
Laboratory findings
Hematologic abnormalities are common: thrombocytopenia (platelet count < 150 х 109/L) occurs in up to 70% of patients and anemia in 25%. The leukocyte count is normal or low; leukocytosis is seen in less than 5% of cases and is a poor prognostic factor.24 Liver function test results are often abnormal; transaminase levels are elevated in about 25% of cases, bilirubin in one-third and lactose dehydrogenase in up to 80%. An elevated bilirubin level in the face of a high lactate dehydrogenase level suggests hemolysis and is often a clue to diagnosis. Electrolyte abnormalities, especially hyponatremia, and an elevated creatinine level may be present. Hypoglycemia is rare on presentation except in those with very high parasitemias. Metabolic acidosis is usually associated with severe disease. Radiologic investigations are often unremarkable, although noncardiogenic pulmonary edema is not uncommon with severe malaria.
Diagnosis
A high degree of suspicion and rapid diagnosis are essential to optimize outcome. Thick and thin peripheral blood smears, stained with Giemsa stain (or, alternatively, Wright's or Field's stains), remain the “gold standard” for routine clinical diagnosis (Fig. 3). Malaria smears permit both species identification and quantification (expressed as a percentage of erythrocytes infected or as parasites per microlitre) of parasites. Malaria should not be excluded until at least 3 negative blood smears have been obtained within 48 hours. However, processing and interpretation of malaria smears require appropriate equipment as well as considerable training and expertise, factors that limit their use in endemic regions. Furthermore, accurate interpretation of malaria smears remains problematic in many established clinical laboratories, especially those outside major referral centres.16,29
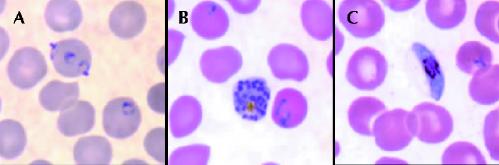
Fig. 3: Stages in the life cycle of Plasmodium falciparum. A: Ring forms (early trophozoites). B: Mature schizont, rarely seen in peripheral blood smears because of microvascular sequestration. C: Gametocyte, demonstrating the classic banana shape. Source: Division of Parasitic Diseases, US Centers for Disease Control and Prevention, Atlanta. Photo: CDC
Rapid malaria tests, which require minimal skill to perform and interpret, have been developed to overcome the problems of malaria smears. The most practical of these are the rapid antigen detection tests (RDTs), which detect parasite proteins in finger-prick blood samples.30 RDTs currently available can identify only P. falciparum and P. vivax, however. Malaria rapid test, manufactured by Makro Medical (Pty) Ltd., is the only test currently licensed for use in Canada. Important shortcomings of RDTs include their inability to quantitate parasitemia and suboptimal test performance with low-level parasitemia. Furthermore, because antigenemia may persist for prolonged periods even after treatment,31 some RDTs are unreliable as tests of cure. Nonetheless, their simplicity may make them attractive and useful alternatives to blood smears, particularly in laboratories where expertise in reading blood films is lacking or in centres where malaria is infrequently encountered. Based on clinical studies involving both travellers to and residents of endemic areas, the overall sensitivity and specificity of RDTs for the detection of falciparum malaria are over 90%.32,33 However, sensitivity falls dramatically with low-level parasitemia,34,35 and at present RDTs cannot be used alone to exclude malaria.
Polymerase chain reaction (PCR) is a sensitive (> 90%) and highly specific (almost 100%) test.36 It can detect extremely low numbers of parasites (and thus may be particularly useful in smear-negative cases) and is species- specific. However, most PCR assays do not have sufficiently rapid turnaround times to be clinically useful; therefore, PCR remains largely an investigational tool. Recent advances in real-time detection and automated DNA extraction may soon become available for routine laboratory diagnosis.37 Acridine orange staining (fluorescence microscopy) is technically difficult, requires special equipment and training, and is not widely used.30 Other diagnostic methods include automated detection of malaria pigment38and flow cytometry.39 Serologic tests have no role in the diagnosis of acute malaria.
Treatment
The treatment of malaria depends on the infecting plasmodia species, the geographic area of acquisition (which affects the likelihood of drug resistance) and the severity of infection. Falciparum malaria in the nonimmune person is a medical emergency and requires rapid initiation of antimalarial therapy. If the species cannot be immediately identified, the patient should be assumed to have drug-resistant falciparum malaria until proven otherwise. Hospital admission is advised for those with falciparum malaria or in whom the infecting species cannot be identified, and for those who are severely ill.
Current guidelines and drug dosages for treatment of uncomplicated malaria are outlined in Table 2. Uncomplicated falciparum malaria may be treated with oral therapy. The choice of agent is determined by the likelihood of infection with a drug-resistant strain (Table 1). Although mefloquine alone can be used for chloroquine-resistant strains, it is not well tolerated and combination therapy using atovaquone plus proguanil or quinine–doxycycline is preferred (Table 2).
Table 2
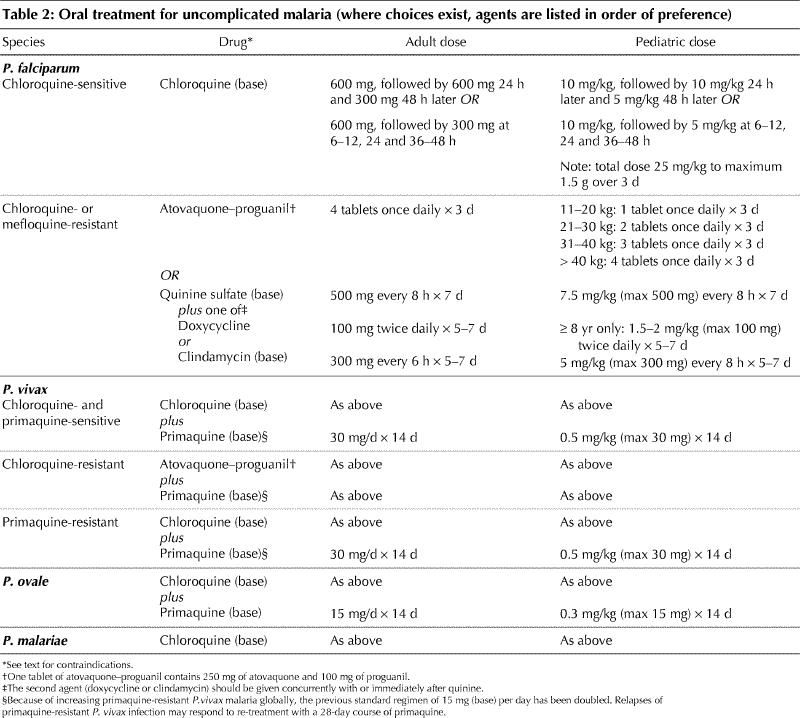
For P. vivax (except that acquired in New Guinea) and P. ovale infection, treatment involves a course of chloroquine, followed by 14 days of primaquine to eradicate hypnozoites and prevent relapses of disease. Chloroquine- and primaquine-resistant strains of P. vivax have been reported and require alternative therapies for treatment (Table 2). Infection caused by P. malariae is easily treated with chloroquine alone except possibly in Indonesia, where resistant strains have recently been reported.40
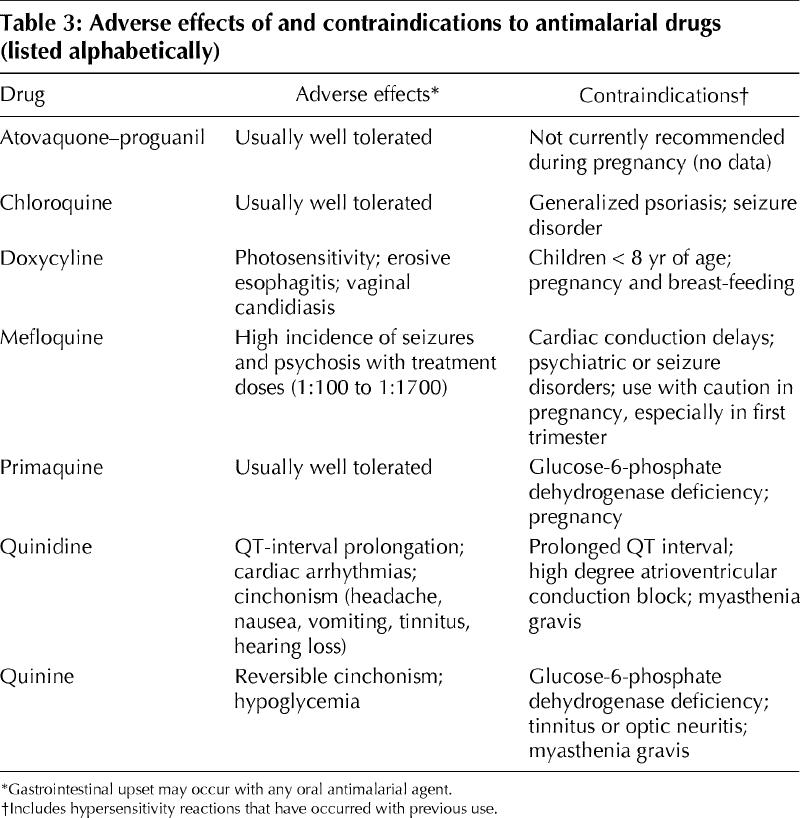
Adverse effects of and contraindications to antimalarial medications are listed in Table 3. Pregnant women should not receive doxycycline, primaquine (because the glucose-6-phosphate dehydrogenase status of the fetus is unknown), mefloquine (particularly during the first trimester) or atovaquone–proguanil (there are currently no data regarding safety in pregnancy). Doxycycline is contraindicated during breast-feeding.
Table 3
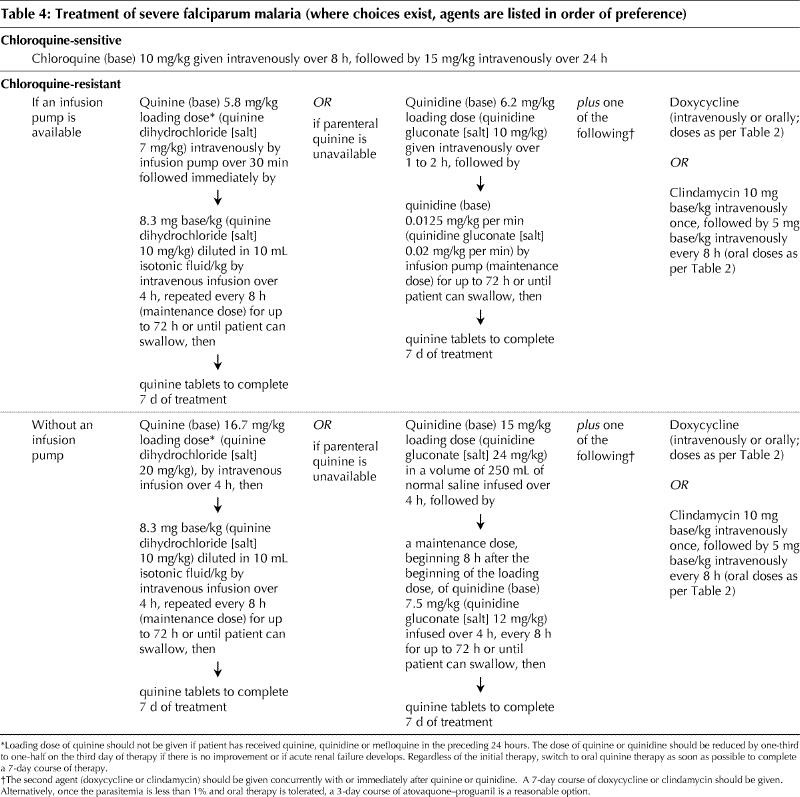
People with severe falciparum malaria and those unable to tolerate oral regimens require parenteral therapy, as outlined in Table 4. Quinine is the preferred parenteral therapy; if quinidine must be used, cardiac monitoring is required to watch for QT-interval prolongation. Quinine is not marketed in Canada but may be obtained by contacting the nearest Canadian Malaria Network centre (listing available online at www.travelhealth.gc.ca, by phone from the national coordinating centre, 613 737-7777 x2723, or after hours from the on-call pharmacy technician at 613 737-7777). Artemisinin derivatives, commonly used in Africa and Asia for the treatment of severe disease, are unavailable in Canada. The benefit of exchange transfusion is unclear;41 it may be considered when parasitemia is higher than 30%, when parasitemia higher than 10% is accompanied by clinical complications (pulmonary, renal or cerebral) or has not resolved after 12 to 24 hours of appropriate therapy, or if the prognosis is poor.24 Anticoagulants and systemic steroid therapy have no role in the treatment of severe malaria.
Table 4
The majority of patients will be cured with appropriate therapy; failures, when they occur, are usually evident within the first month after treatment. Repeat blood films are recommended 2, 7 and 28 days after therapy as tests of cure, and if symptoms recur.6
Prevention
Prevention in travellers
Malaria in travellers is preventable. No prophylactic regimen is 100% effective, however, and travellers must be told to seek medical attention immediately if they experience fever during or after travel, especially within the first 2 months after returning home. All travellers to malaria-endemic areas, including expatriates of such areas, should receive pretravel counselling from a qualified health care provider or travel medicine centre. A listing of travel medicine clinics in Canada is available online at www.travelhealth.gc.ca.
Travellers should avoid outdoor activity after dusk to reduce exposure to night-biting Anopheles mosquitoes. If this is not feasible, then reducing risk with the use of long-sleeved shirts and pants (weather permitting) is advised (permethrin-impregnated clothing exists but is not yet available in Canada). A permethrin-impregnated bed net is recommended if the sleeping environment is not secure from mosquitoes.42 Insect repellents containing a maximum of 50% DEET (N,N-diethyl-m-toluamide, also known as N,N-diethyl-3-methylbenzamide) should be applied to exposed skin, especially after dusk; higher concentrations of DEET offer no additional advantage. DEET is safe for use in pregnant and breast-feeding women; lower concentrations and less frequent applications should be used with children to reduce the risk of neurotoxic events that may occur with excessive or prolonged use.43
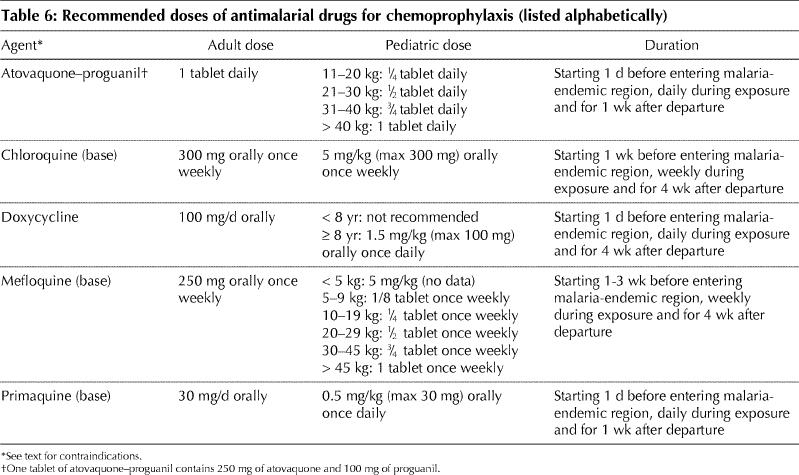
Chemoprophylactic agents should be selected on the basis of individual risk, known drug resistance patterns (Table 5) and other medical conditions that may limit the use of certain agents (Table 3). Doses are outlined in Table 6. Mefloquine appears to be safe in the second and third trimesters of pregnancy, and a risk–benefit decision must be made for women who require the drug during the first trimester. Standby therapy (self-treatment) does not replace the need for medical attention but may be life-saving in people who are unable to obtain medical care quickly; however, since adherence and follow-up are generally poor, it should be used only in very limited circumstances.44
Table 5
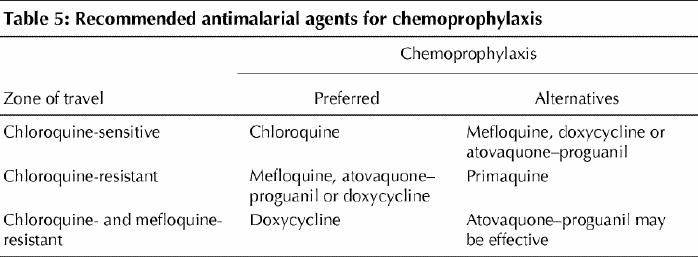
Table 6
Prevention in residents of endemic areas
Limited financial resources pose considerable barriers to malaria prevention and control efforts in endemic areas. Vector control has met with limited success. Targeted chemoprophylaxis and permethrin-impregnated bed nets have been successfully used against malaria in these regions.
Mass chemoprophylaxis in endemic areas is unfeasible for financial and other reasons. However, targeted chemoprophylaxis of those at highest risk of severe malaria (children and pregnant women, particularly primigravidae) has been successfully implemented in some regions, although drug cost and delivery may still be problematic. Anemia (which also contributes to childhood mortality) and malaria-related morbidity and mortality can be reduced in children less than 5 years of age who receive either intermittent or continuous chemoprophylaxis.45 Prevention of severe malaria results in fewer acute care visits and reduces hospital admission rates, and thereby improves utilization of health care resources.45 Although “rebound” episodes of malaria may occur after prophylaxis is discontinued, these do not appear to be associated with an increase in mortality, and clinical immunity also appears to be preserved.45 Chemoprophylaxis during pregnancy increases infant birth weight and survival, although this effect is largely limited to primigravidae.46,47
Insecticide-treated bed nets (ITNs) offer effective protection against malaria. In contrast to chemoprophylaxis, the benefits are not limited to individual users but may be conferred to communities as a whole.48,49 The use of ITNs in malaria-endemic regions significantly reduces the incidence of malaria and childhood mortality,42,50 as well as of anemia and malaria during pregnancy.51 In clinical trials, acceptance and usage rates have been high and are independent of prior experience with ITNs. Although a relatively inexpensive preventive measure by our standards, the cost of maintaining nets may be prohibitive to individual users in developing countries, and the overall costs associated with implementing and maintaining an ITN program are still unaffordable for many developing nations.
Future directions for malaria control and prevention
In addition to the initiatives outlined in the Roll Back Malaria program, another malaria control effort is the Malaria Genome Project. This is a collaborative effort to sequence the entire genome of P. falciparum, knowledge of which should help identify targets for improved diagnostic methods, new antimalarial drugs and vaccine development.52
There are currently no effective vaccines against malaria. As mentioned earlier, host immune responses to malaria confer only partial protection against subsequent infections. The abundance of possible antigenic targets, the lack of crossreactivity among these targets and the parasite's demonstrated ability to elude human immune responses pose tremendous challenges in the development of effective malaria vaccines.
Molecular manipulation of mosquito genomes to create mosquitoes that are incapable of becoming infected by and transmitting malaria (transgenic mosquitoes) is an interesting avenue for the prevention of malaria but has not yet been realized.
Summary
Malaria is a preventable infection that carries with it an enormous global burden. The majority of malaria cases and deaths occur in impoverished regions of the world in which malaria is endemic, and the disease poses a significant economic burden for these regions. More attention and additional resources must be paid to improving prevention, control and treatment if global control of malaria is to be achieved.
For travellers, improved education regarding malaria risk, prevention and treatment is required. Travellers to malaria-endemic areas should receive a thorough pretravel assessment from a properly qualified health care provider, since appropriate personal protective measures and chemoprophylactic regimens can significantly reduce the risk of malaria. Returned travellers who experience fever should seek medical attention immediately, and physicians assessing such patients should order urgent malaria smears and consider consultation with an expert in tropical diseases. Delays in seeking medical care, misdiagnosis and inappropriate or delayed treatment may result in disastrous outcomes. Physicians unfamiliar with the diagnosis and management of malaria should consult an infectious disease or tropical medicine specialist immediately if malaria is suspected.
Footnotes
This article has been peer reviewed.
Contributors: Kathryn Suh was the principal author. Kevin Kain and Jay Keystone contributed to writing the manuscript. All authors revised the manuscript and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Kathryn Suh, Division of Infectious Diseases, Children's Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa ON K1H 8L1; ksuh@cheo.on.ca
References
- 1.World Health Organization. Roll Back Malaria. Available: http://rbm.who.int (accessed 2003 Aug 6).
- 2.The World Bank. Malaria-at-a-glance. March 2001. Available: www1.worldbank.org/hnp/Malaria/index.asp (accessed 2003 Aug 6).
- 3.World Health Organization. World malaria situation in 1994. Parts I - III. Wkly Epidemiol Rec 1997;72:269-90.9293226
- 4.Kain KC, MacPherson DW, Kelton T, Keystone JS, Mendelson J, MacLean JD. Malaria deaths in visitors to Canada and in Canadian travellers: a case series. CMAJ 2001(5);164:654-9. [PMC free article] [PubMed]
- 5.Malaria surveillance — United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51(SS-5):9-23. [PubMed]
- 6.Committee to Advise on Tropical Medicine and Travel (CATMAT). Canadian recommendations for the prevention and treatment of malaria among international travelers 2000. Can Commun Dis Rep 2000;26(S2):1-42. [PubMed]
- 7.Conlon CP, Berendt AR, Dawson K, Peto TE. Runway malaria. Lancet 1990;335:472-3. [DOI] [PubMed]
- 8.Curtis CF, White GB. Plasmodium falciparum transmission in England: entomological and epidemiologic data relative to cases in 1983. J Trop Med Hyg 1984;87:101-14. [PubMed]
- 9.Zucker JR. Changing patterns of autochthonous malaria transmission in the United States: a review of recent outbreaks. Emerg Infect Dis 1996;2:37-43. [DOI] [PMC free article] [PubMed]
- 10.Slinger R, Giulivi A, Bodie-Collins M, Hindieh F, St. John R, Sher G, et al. Transfusion-transmitted malaria in Canada. CMAJ 2001(3);164:377-9. [PMC free article] [PubMed]
- 11.Malaria surveillance — United States, 2001. MMWRMorb Mortal Wkly Rep 2003;52(SS-05):1-14. [PubMed]
- 12.Cooke BM, Wahlgren M, Coppel RL. Falciparum malaria: sticking up, standing out and out-standing. Parasitol Today 2000;16:416-20. [DOI] [PubMed]
- 13.Jakobsen PH, Bate CAW, Taverne J, Playfair JH. Malaria: toxins, cytokines and disease. Parasite Immunol 1995;17:223-31. [DOI] [PubMed]
- 14.Allison AC. Protection afforded by sickle cell trait against subtertian malarial infection. BMJ 1954;1:290-4. [DOI] [PMC free article] [PubMed]
- 15.Ruwende C, Khoo SC, Snow RW, Yates SN, Kwiatkowski D, Gupta S, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995;376:246-9. [DOI] [PubMed]
- 16.Kain KC, Harrington MA, Tennyson S, Keystone JS. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis 1998;27:142-9. [DOI] [PubMed]
- 17.Svenson JE, MacLean JD, Gyorkos TW, Keystone J. Imported malaria. Clinical presentation and examination of symptomatic travelers. Arch Intern Med 1995;155:861-8. [DOI] [PubMed]
- 18.Winters RA, Murray HW. Malaria — the mime revisited: fifteen more years of experience at a New York City teaching hospital. Am J Med 1992;93:243-6. [DOI] [PubMed]
- 19.Lewis SJ, Davidson RN, Ross EJ, Hall AP. Severity of imported falciparum malaria: effect of taking antimalarial prophylaxis. BMJ 1992;305:741-3. [DOI] [PMC free article] [PubMed]
- 20.Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, et al. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg 2003;69:45-52. [PubMed]
- 21.Owusu-Agyei S, Koram KA, Baird JK, Utz GC, Binka FN, Nkrumah FK, et al. Incidence of symptomatic and asymptomatic Plasmodium falciparum infection following curative therapy in adult residents of northern Ghana. Am J Trop Med Hyg 2001;65:197-203. [DOI] [PubMed]
- 22.Paxton LA, Slutsker L, Schultz LJ, Luby SP, Meriwether R, Matson P, et al. Imported malaria in Montagnard refugees settling in North Carolina: implications for prevention and control. Am J Trop Med Hyg 1996;54:54-7. [DOI] [PubMed]
- 23.Fernando SD, Wickremasinghe AR. The clinical and epidemiological features of childhood malaria in a moderately endemic area of Sri Lanka. Southeast Asian J Trop Med Public Health 2002;33:671-7. [PubMed]
- 24.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000;94(Suppl 1):1-90. [PubMed]
- 25.Taylor WR, White NJ. Malaria and the lung. Clin Chest Med 2002;23:457-68. [DOI] [PubMed]
- 26.Murphy GS, Oldfield EC III. Falciparum malaria. Infect Dis Clin North Am 1996;10:747-75. [DOI] [PubMed]
- 27.Taylor TE, Strickland GT. Malaria. In: Strickland GT, editor. Hunter's tropical medicine and emerging infectious diseases. 8th ed. Philadelphia: WB Saunders; 2000. p. 614-43.
- 28.Bates I, Bedu-Addo G. Chronic malaria and splenic lymphoma: clues to understanding lymphoma evolution. Leukemia 1997;11:2162-7. [DOI] [PubMed]
- 29.Milne LM, Kyi MS, Chiodini PL, Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol 1994;47:740-2. [DOI] [PMC free article] [PubMed]
- 30.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 2002;15:66-78. [DOI] [PMC free article] [PubMed]
- 31.Humar A, Ohrt C, Harrington MA, Pillai D, Kain KC. ParaSight-F® test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg 1997; 56: 44-8. [DOI] [PubMed]
- 32.Hanscheid T. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin Lab Haematol 1999;21:235-45. [DOI] [PubMed]
- 33.Wongsrichanalai C. Rapid diagnostic techniques for malaria control. Trends Parasitol 2001;17:307-9. [DOI] [PubMed]
- 34.Pieroni P, Mills CD, Ohrt C, Harrington MA, Kain KC. Comparison of the ParaSight-F® test and the ICT Malaria PF® test with the polymerase-chain-reaction for the diagnosis of Plasmodium falciparum malaria in travelers. Trans R Soc Trop Med Hyg 1998;92:166-9. [DOI] [PubMed]
- 35.John SM, Sudarsanam A, Sitaram U, Moody AH. Evaluation of OptiMAL®, a dipstick test for the diagnosis of malaria. Ann Trop Med Parasitol 1998; 92: 621-2. [DOI] [PubMed]
- 36.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malarial species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 1993;58:283-92. [DOI] [PubMed]
- 37.Farcas GA, Zhong KJ, Mazzulli T, Kain KC. Evaluation of the RealArt Malaria LC real-time PCR assay for malaria diagnosis. J Clin Microbiol 2004; 42:636-8. [DOI] [PMC free article] [PubMed]
- 38.Hanscheid T, Valadas E, Grobusch MP. Automated malaria diagnosis using pigment detection. Parasitol Today 2000;16:549-51. [DOI] [PubMed]
- 39.Van Vianen PH, van Engen A, Thaithong S, van der Keur M, Tanke HJ, van der Kaay HJ, et al. Flow cytometric screening of blood samples for malaria parasites. Cytometry 1993;14:276-80. [DOI] [PubMed]
- 40.Maguire JD, Sumawinata IW, Masbar S, Laksana B, Prodjodipuro P, Susanti I, et al. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 2002;360:58-60. [DOI] [PubMed]
- 41.Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium falciparum malaria: a meta-analysis. Clin Infect Dis 2002;34:1192-8. [DOI] [PubMed]
- 42.Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. The effectiveness of insecticide-impregnated bednets in reducing cases of malaria infection: meta-analysis of published results. Am J Trop Med Hyg 1995; 52: 377-82. [DOI] [PubMed]
- 43.Koren G, Matsui D, Bailey B. DEET-based insect repellents: safety implications for children and pregnant and lactating women. CMAJ 2003(3);169: 209-12. [PMC free article] [PubMed]
- 44.Schlagenhauf P. Standby emergency treatment by travelers. In: Schagenhauf P, editor. Travelers' malaria. Hamilton (ON): BC Decker; 2001. p 446-62.
- 45.Geerligs PDP, Brabin BJ, Eggelte TA. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bull World Health Organ 2003;81:205-16. [PMC free article] [PubMed]
- 46.Cot M, Le Hesran JY, Miailhes P, Esveld M, Etya'ale D, Breart G. Increase of birth weight following chloroquine chemoprophylaxis during the first pregnancy: results of a randomized trial in Cameroon. Am J Trop Med Hyg 1995; 53: 581-5. [DOI] [PubMed]
- 47.Greenwood AM, Armstrong JR, Byass P, Snow RW, Greenwood BM. Malaria chemoprophylaxis, birth weight and child survival. Trans R Soc Trop Med Hyg 1992;86:483-5. [DOI] [PubMed]
- 48.Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community-wide use of insecticide-treated nets for 3-4 years on malarial morbidity in Tanzania. Trop Med Int Health 2002;7:1003-8. [DOI] [PubMed]
- 49.Howard SC, Omumbo J, Nevill C, Some ES, Donnelly CA, Snow RW. Evidence for a mass community effect of insecticide-treated bednets on the incidence of malaria on the Kenyan coast. Trans R Soc Trop Med Hyg 2000;94:357-60. [DOI] [PubMed]
- 50.Mutambu S, Shiff C. Implementing and sustaining community-based mosquito net interventions in Africa. Parasitol Today 1997;13:204-6.
- 51.Marchant T, Schellenberg JA, Edgar T, Nathan R, Abdulla S, Mukasa O, et al. Socially marketed insecticide-treated nets improve malaria and anaemia in pregnancy in southern Tanzania. Trop Med Int Health 2002;7:149-58. [DOI] [PubMed]
- 52.Fletcher C. The Plasmodium falciparum genome project. Parasitol Today 1998;14:342-4. [DOI] [PubMed]
Additional resources
- ·.Health Canada Travel Medicine Program: www.travelhealth.gc.ca; 24-hour malaria information by fax (613 941-3900)
- ·.US Centers for Disease Control and Prevention: www.cdc.gov/travel; 24-hour malaria information by phone (888 232-3228) and fax (888 232-3299)
- ·.Malaria Foundation International: www.malaria.org
- ·.World Health Organization: www.who.int/ith


