Abstract
Purpose
A key goal in asthma treatment is improvement in quality of life (QoL), but existing measures often confound QoL with symptoms and functional impairment. The current study addresses these limitations and the need for valid patient-reported outcome measures by using state-of-the-art methods to develop an item bank assessing QoL in adults with asthma. This article describes the process for developing an initial item pool for field testing.
Methods
Five focus group interviews were conducted with a total of 50 asthmatic adults. We used “pile sorting/binning” and “winnowing” methods to identify key QoL dimensions and develop a pool of items based on statements made in the focus group interviews. We then conducted a literature review and consulted with an expert panel to ensure that no key concepts were omitted. Finally, we conducted individual cognitive interviews to ensure that items were well understood and inform final item refinement.
Results
661 QoL statements were identified from focus group interview transcripts and subsequently used to generate a pool of 112 items in 16 different content areas.
Conclusions
Items covering a broad range of content were developed that can serve as a valid gauge of individuals’ perceptions of the effects of asthma and its treatment on their lives. These items do not directly measure symptoms or functional impairment, yet they include a broader range of content than most existent measures of asthma-specific QoL.
Keywords: Asthma, Quality of Life, Patient-reported outcomes, Instrument Development
INTRODUCTION
Asthma is a common chronic disease that takes a serious toll on those who suffer from it. While more prevalent among children, asthma affects as many as 7.3% of U.S. adults [1]. Annually, asthma results in an average of 12.3 million physician visits, 1.8 million emergency department visits, and approximately half a million hospitalizations, over half of which are for adults [2]. It accounts for approximately 14.2 million lost work days annually.
Given how pervasive and impairing asthma is, there has been increased attention to the development and use of asthma patient-reported outcomes that may be more meaningful to patients than traditional clinical markers of asthma severity. One key outcome is disease-specific quality of life (QoL), which is defined as the patient’s subjective perception of the impact of a disease (e.g., asthma) and its treatment on his or her well-being (in contrast to general health-related QoL, a broader measure of total physical, mental and social well-being). Asthma-specific QoL can be distinguished from asthma symptoms—for example, frequency of wheezing—and functional impairment—for example, difficulty climbing stairs—in that asthma-specific QoL is a gauge of how much symptoms and impairments bother or matter to a patient in different areas of life. Bother is a function of the level of distress from asthma symptoms and associated functional impairments. Reduced QoL is often a key reason a patient seeks treatment, thus QoL measures can assess whether treatment is improving the issues most important to the patient.
A number of asthma-specific QoL measures have been developed, including Juniper’s Asthma Quality of Life Questionnaires (AQLQ) [3-5], Mark’s Asthma Quality of Life Questionnaire (AQLQ-Marks) [6-8], the Integrated Therapeutics Group Asthma Short-Form (ITG-SF) [9], the Asthma Impact Survey (AIS) [10], the Living With Asthma Questionnaire (LWAQ) [11], the Airways Questionnaire (AQ20/AQ30) [12], the St. George’s Respiratory Questionnaire (SGRQ) [13], and the Asthma Bother Profile (ABP) [14]. These instruments have contributed significantly to our knowledge of asthma, but they have some key limitations, described below.
According to the expert panel report (EPR-3) released by the National Heart, Lung, and Blood Institute’s (NHLBI) National Asthma Education and Prevention Program, the goals of asthma treatment include improving QoL for people with asthma, controlling symptoms, reducing the risk of exacerbations, and preventing asthma-related death [15]. Because these targeted aspects of asthma may respond differently to treatment, they should be monitored separately. Yet available asthma-specific QoL instruments can confound QoL with asthma control, defined as the extent to which manifestations of asthma (symptoms, functional impairments, and risks of negative events) are minimized and goals for treatment are met [15]. Similarly, QoL items are commonly combined with items measuring asthma symptoms (e.g., shortness of breath, wheezing) and functional impairment (e.g., limitations in daily activities, such as housework and walking up hills) in creating total scores.
Thus, no existing instrument provides unconfounded information on the impact of asthma on functional status and well-being. This key limitation was emphasized by leaders in asthma research and practice at the Asthma Outcomes Workshop convened by the NHLBI in March 2010. At this workshop and in their subsequent article describing their systematic review of asthma QoL measures [16], the Asthma Related QoL Subcommittee declined to recommend any existing instrument as a core outcome measure of asthma-specific QoL, and instead strongly recommended that new instruments be developed that measure the impact of asthma on QoL as a construct distinct from asthma control.
Another limitation of existent measures is that they may not reflect all the QoL domains that are important to individuals with asthma [17]. Further, most existent asthma-specific measures do not apply modern test development methods such as Item Response Theory (IRT; for exceptions, see [18,19]). Most existent measures were developed using clinician recommendation and/or classical test theory, and are administered to respondents as a fixed-length set in which the same questions are asked for everyone regardless of their underlying asthma-related health status or QoL. As such, item administration does not take into account the respondent’s level of QoL (i.e., the latent trait) and will either: 1) require administration of many more items; 2) cover only a small range of QoL; or 3) cover a broader range of QoL with less precision. Thus, current measures may not reliably cover the full impact of asthma on QoL experienced across the entire range of asthma severity.
Given these limitations of existent measures, the current study sought to use state-of-the-art methods in developing a new measure of asthma-specific QoL that: 1) avoids confounding QoL with asthma symptomatology, control, or functional impairment; and 2) aims to be more comprehensive in scope. The purpose of this article is to describe the first phase in the development of the item bank; namely, developing an initial item pool for field testing. Later studies will report on the psychometric characteristics of the resultant item bank that aims to be an improvement over current alternatives [20,21].
METHODS
Our methodology for developing asthma-specific QoL items followed the model set forth by PROMIS® (Patient-Reported Outcomes Measurement Information System, http://www.nihpromis.org/default.aspx) [20], part of the National Institute of Health’s Roadmap initiative. PROMIS® uses modern measurement theory and advances in computer technology to create item banks for standardized assessment of patient-reported outcomes (e.g., [22])
Focus Group Interview Procedures and Participants
We conducted a series of focus groups with a diverse sample of adults to get input which could be used to generate specific items regarding the impact of asthma on QoL. The focus group interviews started by querying the general impact of asthma on participants’ lives, and then, following our theoretical framework, went on to query impact on physical functioning, emotional status, social and role functioning (family, work, school, etc.), relationships with others, and spiritual life. We also queried fears related to asthma and positive coping mechanisms.
We conducted five focus groups: three in Santa Monica, CA and two in Boston, MA. One group included only participants who self-identified as Hispanic; another included patients from a University asthma clinic. For all but the clinic-based group, participants were recruited through local vendors. Participants met the following eligibility criteria: age 18-65, speak English, and had been told by a doctor that they had asthma. In addition, they had to meet at least one of the following: hospitalization in the past 12 months, at least 2 emergency room/urgent care visits in the past 12 months, or at least 5 days with asthma symptoms within the past 14 days. Finally, to capture the impact of mild asthma on QoL, we allowed in each focus group 3-4 participants who self-rated their asthma as very mild or mild. A total of 50 individuals participated, of varied age, race, ethnicity, and asthma severity (see Table 1).
Table 1. Characteristics of Focus Group Interview Participants (N=50).
| Demographic | Count | Percent |
|---|---|---|
| Age | ||
| 18-30 | 17 | 34% |
| 31-45 | 19 | 38% |
| 46-65 | 13 | 26% |
| Missing | 1 | 2% |
| Gender | ||
| Male | 25 | 50% |
| Female | 25 | 50% |
| Education | ||
| No high school diploma or GED | 3 | 6% |
| High school diploma or GED | 10 | 20% |
| Some college but no degree | 5 | 10% |
| 2-year college degree | 7 | 14% |
| 4-year college degree | 20 | 40% |
| More than 4-year college degree | 5 | 10% |
| Race/Ethnicity | ||
| American Indian or Alaskan | 2 | 4% |
| Asian | 3 | 6% |
| Native Hawaiian or other Pacific Islander | 0 | 0% |
| Black or African American | 9 | 18% |
| Hispanic/Latino | 16 | 32% |
| White/Caucasian, not of Hispanic/Latino descent | 19 | 38% |
| Missing | 1 | 2% |
| Work Status | ||
| Working full- or part-time at a regular job | 30 | 60% |
| Student | 4 | 8% |
| Homemaker | 9 | 18% |
| Unemployed, but looking for work | 4 | 8% |
| Unemployed, and not looking for work | 1 | 2% |
| Retired | 1 | 2% |
| Missing | 1 | 2% |
| # times visited emergency room in past 12 monthsa | ||
| Never | 30 | 60% |
| One time | 11 | 22% |
| Two or three times | 7 | 14% |
| Four or more times | 1 | 2% |
| Missing | 1 | 2% |
| # times stayed overnight in hospital in past 12 monthsa | ||
| Never | 42 | 84% |
| One time | 3 | 6% |
| Two or more times | 4 | 8% |
| Missing | 1 | 2% |
| # days with asthma symptoms in past 14 daysa | ||
| No days | 0 | 0% |
| 1-4 days | 28 | 56% |
| 5-13 days | 14 | 28% |
| 14 days | 7 | 14% |
| Missing | 1 | 2% |
| # nights woke up with asthma symptoms in past 14a nights |
||
| No nights | 12 | 24% |
| 1 night waking up | 7 | 14% |
| 2-7 nights | 27 | 54% |
| 8-14 nights | 4 | 8% |
| Self-reported asthma severity b | ||
| Very mild | 4 | 8% |
| Mild | 13 | 26% |
| Moderate | 23 | 46% |
| Severe | 9 | 18% |
| Very Severe | 1 | 2% |
Focus Group Interview Items
All focus group interviews were audio-recorded and professionally transcribed. Two researchers read the transcripts and extracted statements regarding asthma-specific QoL.
Participants’ statements were classified, using a “binning” (or “pile sorting”) process that enabled us to identify key themes. “Binning”— a procedure used by PROMIS® [23]—is the process of systematically grouping together items or statements that have similar content and meaning. For example, statements about not being able to engage in valued activities such as swimming, hiking, and running would be in the same “impact on activities” bin. We had hypothesized that asthma would impact the major domains of health-related QoL which include physical, psychological/emotional, social/interpersonal, role, spiritual and economic. Thus we used these major domain groupings to guide the sorting process. However, we did not restrict the size or number of piles and allowed for unhypothesized domains (e.g., dependence on medications) that might be unique to persons with asthma. As a result some of the initial piles contained closely related constructs that later were combined.
Groups consisting of at least three of the five core study team members met together in person over a series of several days to pile sort QoL statements from the focus group discussions into bins of similar content. As part of this process, team members discussed statements that were unclear, resolved discrepancies in sorting to produce consensus, and agreed on labels to characterize the piles.
Item Writing
The focus group statements varied in style and were not phrased as typical survey items. Thus, they were transformed to be more succinct, clear, and consistent in style with one another. In addition, many of the original comments were overly specific, and thus were rewritten to be more broadly applicable. For instance, if people gave examples of specific incidents that were troublesome (e.g., feeling bothered by not being able to keep up with others when going uphill), the comments were transformed into items that referred to more general problematic situations (e.g., feeling that it was hard to keep up with others when physically active). Similarly, comments that referred to specific relationships (mother, child, brother, spouse, etc.) were rewritten into items that applied to a wider variety of relationships and thus would be applicable to most individuals with asthma. Proposed items were reviewed by at least two group members to achieve consensus about wording.
The item writing process included a “winnowing” stage. “Winnowing” is the process of narrowing down a large item pool into a smaller, less redundant pool that is representative of the content of interest. Thus, in evaluating each item we considered the following questions: 1) Does this item reflect QoL and not symptoms or level of functioning? 2) Is this item redundant with other items? 3) Is this item relevant to most individuals with asthma?
Themes initially identified in the binning process were also occasionally reorganized or combined during this phase.
Literature Review
A literature review was used to supplement the focus group data, and fill in any gaps. First, we examined asthma-specific QoL measures identified by the QoL Subcommittee of the NHLBI Asthma Outcomes Workshop (see Table 2). Second, we conducted a comprehensive literature search to identify other English-language asthma-related QoL instruments published between May 1995 and May 2011, when the literature review was conducted. PubMed, Cochrane, and Web of Science databases were searched using the following key terms: asthma AND instrument or measurement AND quality of life, burden, bother, well-being, impact, impairment, worry, concerns, feelings, matter, anxious, or depressed. Google searches and citations from relevant articles were used to identify any measures that the other methods potentially missed.
Table 2. Commonly Used Asthma-specific QoL Measures, Recommended as Supplemental Outcomes by the NHLBI.
| Questionnaire Name | Relevant Citation | Domains |
|---|---|---|
|
aThe Asthma Quality of Life Questionnaire Standardized Asthma Quality of Life Questionnaire “Mini” Asthma Quality of Life Questionnaire |
AQLQ; Juniper, 1992 AQLQ-S; Juniper, 1999 Mini-AQLQ; Juniper, 1999 |
4 domains: symptoms, activity limitation, emotional function, environmental stimuli |
| The St. Georges Respiratory Questionnaire |
Barr, 2000; Jones, 1992 | 3 domains: symptoms, activity, impact |
| The Airways Questionnaire-20 | AQ-20; Barley, 1998 | No domains |
| The Integrated Therapeutics Group Asthma Short-Form |
ITG-SF; Bayliss, 2000 | 5 domains: symptom-free index, functioning with asthma, psychosocial impact of asthma, asthma: energy, asthma: confidence in health. |
| The Asthma Quality of Life Questionnaire - Marks Modified Asthma Quality of Life Questionnaire - Marks |
AQLQ-Marks; Marks, 1992; 1993 Adams et al., 2000 |
4 domains: breathlessness, mood, social, concerns |
| The Asthma Impact Survey | Schatz, 2007 | No domains |
| The Living With Asthma Questionnaire |
Hyland, 1991 | 11 domains: social/leisure, sport, holidays, sleep, work and other activities, colds, mobility, effects on others, medication usage, sex, dysphoric states and attitudes OR 2 constructs: problems, evaluation OR 4 constructs: activities, avoidance, distress, preoccupation |
| The Asthma Bother Profile | Hyland, 1995 | 2 domains: distress, asthma management |
Note that the items from the Juniper measures were not included in our item catalog and subsequent field test because permission to use them was not granted. If any item included in the other measures was included in our field test, we first asked permission from lead authors for their inclusion.
We created a catalog of all items identified, obtained permission to use items from several scales [18,12,24,11,14,8,25-27], and deleted items which were not publicly available and/or which we did not obtain permission to use. Two team members narrowed down the literature items by deleting symptoms and/or poorly worded or redundant items. The remaining items were also subjected to a “binning” and “winnowing” process. The items in each domain were compared to those generated from the focus groups, and only literature items with unique content or better wording of similar concepts were retained. Any items from the literature that were included in the final pool of 112 items were, in general, not modified except to conform to the tense and time frame of the focus group items.
Consultation with Expert Panel and Item Refinement
We next convened a panel of asthma experts to develop consensus on the breadth and scope of the item pool. Experts included three clinical and/or research experts, one member of an asthma advocacy organization, and one consumer representative. The panel provided feedback on the comprehensiveness of item content, the content areas that were more and less important, additional aspects of asthma QoL that should be assessed, and ideal time period to query.
Cognitive Interviews and Final Item Revisions
We conducted structured cognitive interviews of 12 adults with asthma to elicit feedback on the resultant item pool prior to field testing. The 12 respondents were evenly split by gender and included four each with mild, moderate and severe asthma. Seven reported they were White, two Black, two Asian, and one “other,” and three were also Hispanic. The interviews identified potentially problematic items and helped clarify items that were not easily understood. Respondents were asked to read and answer sets of items, and were encouraged to describe what they were thinking about as they answered. For some items, the interviewer asked probes about specific terms or phrases (e.g., understanding of the word “trigger”) or kinds of activities people were thinking about when answering a question. Respondents were asked whether they would have answered differently had the question referenced a 2-week period instead of a 4-week period. Applicability of response options was explored. Initially, protocols were broken into two versions each containing half of the items. However, after completing the first two interviews in less than an hour each, it was clear that respondents could complete all items in 90 minutes. So the rest of the respondents were asked all of the items. Respondents were paid $100 for each interview.
The study team reviewed the items again after the interviews to address issues raised, ensure consistency in item wording, and attempt to further reduce the item pool.
RESULTS
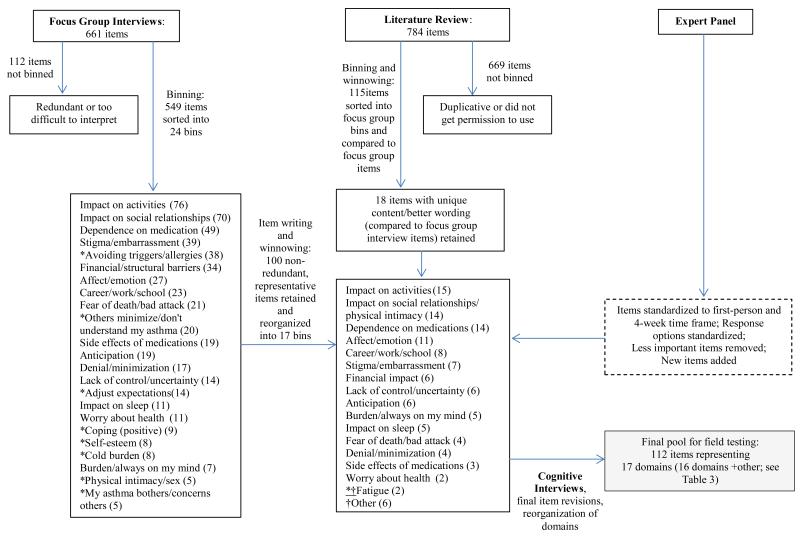
Figure 1 provides a flow chart of the results derived from the methods described above. Specifically, the focus groups initially yielded 661 statements, but 112 were either too redundant or ambiguous to be binned. Thus, 549 statements were included in the binning process, which initially yielded 24 bins. The winnowing and item writing process reduced the pool considerably, yielding 100 non-redundant statements in 17 bins. The lower number of bins was predominately the result of combining related categories (e.g., categories related to “impact on social relationships,” including “others minimize/don’t understand my asthma,” “physical intimacy/sex,” and “my asthma bothers/concerns others” were combined into the more general category of “impact on social relationships/physical intimacy”). In other instances, we deleted statements that were not measuring the negative impact of asthma on QoL (i.e., “coping (positive) and “spiritual/religious” categories). Expert panel feedback was also helpful in deciding which statements to add and remove at this phase.
Figure 1. Results of Item Development Process.
Note. *Indicates that the bin was not retained as a separate domain in the next step. †Indicates a new domain that was introduced in this 1 step.
The literature review searches yielded 2167 relevant abstracts, from which 23 asthma-related QoL instruments were obtained. Our review of these measures yielded 784 items. Of these, 669 were discarded because they were duplicative, poorly worded, or we were not able to obtain permission to use them, and 115 were sorted into the 17 bins we had created through the focus group statement sorting process. When we compared focus group and literature items we found a great deal of overlap (i.e., themes related to impact of asthma on activities, social relationships, work/school/home, affect/emotion, and finances were well-represented in both the focus group and literature items), and ultimately retained only 18 literature items that had unique content or better wording than the focus group items.
The expert panel recommended including items on impact for people with both mild and severe asthma and targeting adults of all ages (with no upper limit). They suggested including items on trade-offs related to medication use, and agreed that the content area of doctor-patient relationships was not relevant to the measurement of QoL.
All items were standardized to have a first-person orientation and a 4-week time frame. Four weeks was chosen because: 1) it was recommended by asthma experts, 2) it is a common time period for self-report measures of asthma control [28,29], 3) it is long enough to allow time for asthma-related events queried to occur and to have a measure that is responsive to change over time, and 4) it is short enough to have good recall. We also modified response options so most items would be rated on a 5-point response scale reflecting magnitude of impact (i.e., “not at all” to “very much”). For items measuring the impact of asthma on negative emotions, response choices reflected frequency (i.e., “never” to “almost always”).
Altogether, this process yielded 118 items which were tested in cognitive interviews. Cognitive interview results indicated that almost all items were well-understood and the few identified as potentially problematic were reworded (e.g., “problem in my relationships” was clarified to include relationships with family, friends, significant others, or co-workers). When probed whether they would have answered differently had the question referenced a 2-week period instead of 4 weeks, respondents all said “no.” Because respondents sometimes mentioned experiences outside the time frame, we decided to reiterate the phrase “in the past 4 weeks” before each item during administration.
Based on the cognitive interview results, we deleted some items and reorganized others, such that in the final step we had 112 items representing 17 content domains (16 specific domains, plus an “other” category) to bring forward to the field test. In this final step, we eliminated one domain (“fatigue”), refined one domain (“career/work/school” was changed to “impact on work/school/home,”), and added one new category (“general impact on life goals/enjoyment of life”).
Table 3 presents definitions of the final content areas, the number of items in each, and representative sample items (see Appendix for full item content). Briefly, the final item pool for the field test included items measuring the negative impact of asthma on: valued activities (15), relationships with others (13), ability to fulfill roles and obligations at work, school or home (8), negative emotions (11), fear of having a life-threatening asthma event (4), stigma and embarrassment associated with asthma and its treatment (7), worries about long term effects on health (2), he burden of having to plan ahead to avoid asthma problems (6), having to think about asthma all the time (3), minimizing the impact of asthma (4), feeling a lack of control (7), finances (5), the burden associated with dependence on asthma medication (13), bother from medication side effects (3), sleep (4), life goals and enjoyment of life (2), and other (5).
Table 3. Content Areas Identified (Number of Items), and Sample Items.
| Content Areas | Operational Definition | Sample Item |
|---|---|---|
| Impact on activities (15) | Impact on valued activities, or activities the person wants to do. (This is different from functional impairment in that the activities must be valued.) |
I was unable to do all the things I wanted to do. |
| Impact on social relationships/physical intimacy (13) |
Impact of asthma on interpersonal relationships. |
Asthma placed stress on my relationships with family, friends, significant others, or co-workers. |
| Impact on work/school/home (8) |
Impact on ability to fulfill social roles and obligations, whether at work, school, or home. |
I was kept from doing things I needed to do at work, school, or home. |
| Affect/emotion (11) | Impact on various kinds of negative affect (sad, angry, stressed, tired, worried, annoyed, etc.) |
I felt irritable. |
| Fear of death/bad attack (4) |
Fear of having a severe asthma attack and having one’s life threatened. |
I worried about dying from an asthma attack. |
| Stigma/embarrassment (7) |
Feeling stigmatized by others due to asthma, or embarrassed by asthma and its treatment. |
I was embarrassed by using my inhaler in front of other people. |
| Worry about future health (2) |
Worry about the long-term effects of asthma on health. |
I worried about the long-term effects of asthma on my health. |
| Anticipation (6) | Having to plan ahead to anticipate asthma triggers and avoid potentially problematic situations. |
It bothered me that I have to plan ahead. |
| Burden/always on my mind (3) |
Having to constantly think about asthma; asthma as a burden that one carries around with them all the time. |
My asthma was on my mind. |
| Denial/minimization (4) | Denying or minimizing the effect that asthma has on one’s life. |
It was hard for me to admit that I have asthma. |
| Lack of control/ | Being unable to control asthma, | I felt frustrated that I can’t control |
| uncertainty (7) | things that trigger asthma, or one’s own life due to asthma. |
the things around me that trigger my asthma. |
| Financial impact (5) | Financial/economic effect of asthma and its treatment. |
The cost of treating my asthma was a burden to me. |
| Dependence on medications (13) |
Burden associated with dependence on asthma medications. |
I found it annoying having to carry my inhaler with me. |
| Side effects of medications (3) |
Bother by side effects of asthma medications on quality of life. |
I was bothered by side effects from asthma medication. |
| Impact on sleep (4) | Impact of asthma on quantity or quality of sleep. |
It was hard to get a good night’s sleep because of my asthma. |
|
†General impact on life goals/enjoyment of life (2) |
Impact of asthma on ability to achieve life goals or have an enjoyable and fulfilling life. |
I have felt that asthma is preventing me from achieving what I want from life. |
| Other (5) | Items that did not fit well in other categories (e.g., burden of common colds). |
I felt like I wasn’t normal. I worried about getting a cold with my asthma. |
Note: All items begin with “In the past 4 weeks,” and many items begin with “In the past 4 weeks, because of my asthma.” fIndicates a new domain that was introduced in this step.
DISCUSSION
The goal of our ongoing study is to develop item banks measuring asthma-specific QoL, with a focus on using state-of-the art test development methods, and including a comprehensive range of items that avoid confounding QoL with asthma symptomatology, control, or functional impairment. This article describes the first stage in development of the item banks; namely, how we used focus group interviews of asthma patients, literature review, and expert feedback in developing an initial item pool for field testing.
The initial steps described here resulted in a pool of QoL items based on words of people with asthma themselves. We identified many more content areas than are typically assessed by asthma-specific QoL measures. For instance, the AQLQ-Juniper yields scores for symptoms, activity limitation, emotional function, and environmental stimuli (symptomatology in response to triggers). The AQLQ-Marks has scores for breathlessness (symptoms and health status), mood, social, and concerns (worries related to asthma). The item pool we developed also measures activity limitation, although our items focus exclusively on assessing the extent to which such limitations bother or matter to the individual. Also like both widely used questionnaires, our item pool includes content related to affect and emotion. Like the Marks measure, we also have social items and items assessing worries or fears related to asthma.
There were some content areas that other measures assess with single items that our item pool covers with greater breadth. Specifically, our pool includes multiple items measuring impact on work/school/home, fear of a life threatening attack, worry about the effects of asthma on future health, impact on sleep, burden associated with dependence on medication, and general impact of asthma on ability to have a fulfilling life. In addition, we included content that appears only sporadically in other QoL measures, including stigma related to asthma, anticipation of asthma triggers and problematic situations, the burden of always having asthma on one’s mind, denial/minimization of asthma’s effects on one’s life, lack of control associated with having asthma, and impact of medication side effects on QoL. Thus, while the items we developed have some overlapping content with widely used measures, they have greater breadth in assessing the impact of asthma on QoL.
We believe that our focus group methodology allowed us to identify a broad range of content that is important to individuals with asthma. Specifically, we included individuals with varied severity, asked open-ended questions that spanned a wide range of life domains, and, to the extent possible, used people’s own words in writing the items. Although our approach focused first and foremost on patient perspectives, the resultant items also had face validity to experts.
Our methodological approach was based on the standards for measurement development set forth by PROMIS® but deviated in some key ways, particularly in our emphasis on developing items based on focus group content rather than prioritizing items in the existent research literature. This shift in emphasis was necessary given the limitations of existing measures identified by the NHLBI QoL Subcommittee.
One challenge related to our emphasis on focus group interviews is that some respondents expressed that there was little impact of asthma on their QoL. In some cases there was probably truly little impact, and in other cases individuals may have been reluctant to acknowledge the impact (captured by our denial/minimization domain). However, it seemed that many had simply adapted to their asthma and had habituated to a lower QoL. For instance, they might mention spending less time with friends or engaging in valued activities, but state that they were used to this and accepted it. It is unclear how we might adapt QoL measurement to address adaptation to asthma, but this may be an important direction for future research.
Our study was limited in that it may not be generalizable to all individuals with asthma. Although our focus group respondents were diverse in race, ethnicity, gender, severity, and somewhat diverse in geographical location (i.e., from two cities spanning the East and West coasts), all respondents were English speaking, and our sample was more highly educated than the greater population of individuals with asthma. Thus we may be missing some items relevant to less educated individuals. However, the focus groups included individuals with low levels of education, so that the perspectives of individuals from all types of backgrounds could be captured. We also had one group consisting only of those who self-identified as Hispanic in order to get their viewpoint about the impact of asthma on their lives. Future work, including translations, can confirm the applicability of our items to other cultures. Our field test will include a large, diverse sample which will allow us to evaluate whether our items perform differently in people with different educational levels, as well as in individuals who differ in other demographic characteristics.
Our next step is to conduct a field test of the 112 items and perform psychometric analyses using that data. Factor analyses will be used to determine whether the item pool is best characterized as multiple distinct item banks or as one overall unidimensional measure of asthma-specific QoL. Specifically, the content areas we identified in Table 3 may serve as the basis for specific item banks (e.g., banks assessing impact on activities, impact on social relationships, etc.) if factor analyses of field test data provide evidence that they are empirically distinct constructs. If the data support a unidimensional model, there will be a single item bank but the content areas will be used to describe the aspects of asthma QoL encompassed by the bank. IRT analysis will be used to generate information about item quality and to refine the component items in the bank(s). IRT analysis will also be used to create short form scales and Computer Adaptive Test (CAT) simulations. Preliminary validity tests will be reported. The short forms and item bank(s) will be free and publically available.
In sum, we developed a comprehensive item pool to assess health-related QoL in adults with asthma, using focus groups, literature review, consultation with experts, and cognitive interviews. We took care to avoid confounding QoL with symptoms, and identified original content areas—including anticipation, denial, lack of control, and financial concerns—that are not often assessed by existing instruments. Our hope is that ultimately this instrument will serve as a valid, comprehensive gauge of an individual’s perceptions of asthma-related QoL that can be used by health care organizations, clinicians, researchers and others interested in the effects of asthma and its treatment.
Acknowledgements
We would like to thank our expert panel which includes Eric Kleerup, MD (Clinical Professor, David Geffen School of Medicine, Division of Pulmonary and Critical Care Medicine), Steve Erickson, Ph.D. (Associate Professor at the University of Michigan College of Pharmacy), Cynthia Rand, PhD. (Professor of Medicine at the Johns Hopkins School of Medicine), Felita Jones (Asthma and Allergy Foundation of America), and Chris Draft (community activist and founder of the Chris Draft Family Foundation).
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL107312. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by grant R01HL10732 from the National Heart, Lung & Blood Institute.
Abbreviations
- NHLBI
National Heart Lung and Blood Institute
- QoL
Quality of Life
Appendix. Final Item Pool for Field Test
Responses for the following items indicated on the following 5 point scale:
1=Not at all
2=A little bit
3=Somewhat
4=Quite a bit
5=Very much
In the past 4 weeks, because of my asthma…
It was hard to keep up with others when we were physically active.
I had to do things for shorter amounts of time than I would have liked.
I was unable to do some kinds of work or other regular daily activities I would like to do.
I was unable to do all the things I wanted to do.
It was hard to do the things I enjoy doing.
I was unable to get as much done as I would’ve liked at work, school, or home.
I was afraid to be physically active.
I had to do my work, school, or other regular daily activities for shorter periods of time.
I cut back on things I enjoy.
I felt frustrated at not being fully able to participate in physical activities.
I was kept from doing things I needed to do at work, school, or home.
I felt generally limited.
Everyday activities were a struggle.
I felt bothered by limitations in what I could do.
I got tired easily.
I had to be careful what I did.
My work, school, or other regular daily tasks suffered.
I did worse at work, school, or other regular daily activities.
I cut back on going to places I like.
I was bothered at work, school, or home.
I worried that I would have an asthma attack while visiting a new place.
It bothered me that I always have to be aware of possible asthma triggers.
I had to be cautious when going to a new place.
I had to do a lot of planning to make sure I always had an inhaler ready.
It bothered me that I have to plan ahead.
I felt bothered by having to avoid situations or places.
I took time off from work or school.
I felt frustrated that I have to do things differently than people who don’t have asthma.
I felt like I wasn’t normal.
In the past 4 weeks…
It was hard for me to admit that I have asthma.
I tried to hide my asthma from others.
I was embarrassed by using my inhaler in front of other people.
Having to avoid asthma triggers created some problems in my relationships with family, friends, significant others, or coworkers.
I struggled with accepting that I have asthma.
Asthma placed stress on my relationships with family, friends, significant others, or co-workers.
I found myself making excuses to others because of my asthma.
I worried about what will happen to me when I get older because of my asthma.
I worried about the long-term effects of asthma on my health.
I worried that asthma medications will make my health worse in the future.
I worried that asthma will shorten my life.
I worried about using too much asthma medication.
I worried about becoming immune to my asthma medication over time.
I felt frustrated that I can’t fix or get away from my asthma.
It bothered me that my asthma is always there.
I struggled with the pros and cons of taking asthma medication.
I was bothered by side effects from asthma medication.
It bothered me that I don’t know when my asthma will get worse.
The cost of my asthma medications bothered me.
The cost of treating my asthma was a burden to me.
I had to budget carefully because of expenses related to my asthma.
I had to make compromises because of the cost of treating my asthma.
I felt frustrated having to deal with insurance issues related to my asthma.
I worried about not having my inhaler when I need it.
I felt anxious when my inhaler was not nearby.
It was stressful when I couldn’t find my inhaler.
I worried about not getting to my inhaler quickly enough.
I felt frustrated when I woke up during the night because of my asthma.
It was hard to get a good night’s sleep because of my asthma.
I was frustrated by not being able to breathe at night.
I felt frustrated by not being able to fall asleep because of my asthma.
I was afraid of having an asthma attack in my sleep.
I felt frustrated that I couldn’t make plans in advance because of my asthma.
I felt like I couldn’t enjoy life because of my asthma.
Asthma interfered with my social life.
I felt like I missed out on doing things with others because of my asthma.
I felt that asthma is preventing me from achieving what I want in life.
I felt that asthma was controlling my life.
I enjoyed the time I spent with others less because of my asthma.
It bothered me that I cannot do something without first thinking about how it might affect my asthma.
Managing my asthma took a lot of effort.
Asthma affected my life more than I want to admit.
I felt different than other people.
I felt frustrated that other people didn’t understand my asthma.
Using an inhaler got in the way of what I was doing.
I felt that I could not control my asthma.
I worried about having an asthma attack in front of others.
I felt frustrated that my asthma got in the way of physical intimacy.
I was bothered by the unwanted attention I got because of my asthma.
Asthma interfered with my romantic relationships.
I worried about dying from an asthma attack.
I felt frustrated that I can’t control the things around me that trigger my asthma.
It bothered me that I can’t visit friends or family because of asthma triggers in their home.
I felt frustrated that I can’t control things in my home that trigger my asthma.
I avoided situations where my asthma might embarrass me.
I felt frustrated that people didn’t take my asthma seriously.
It was hard having to speak up when others did things that could trigger my asthma.
I felt scared when an asthma attack came on.
It was annoying having to make sure I had enough asthma medication on hand.
My asthma was on my mind.
My asthma bothered people I care about.
I worried about taking daily medications for my asthma.
I had to worry about asthma triggers.
It took a lot out of me when I had an asthma attack.
I was bothered by the way my asthma medication made me feel.
It bothered me that people thought my asthma symptoms were cold symptoms.
I worried about becoming addicted to my asthma medication.
My asthma kept me from having things I wanted to have (e.g., pets, carpet, perfume, etc.).
I found it annoying having to carry my inhaler with me.
I felt dependent on my asthma medication.
I worried about getting a cold with my asthma.
I avoided thinking about my asthma.
Responses for the following items indicated on the following 5 point frequency scale:
1=Never
2=Almost Never
3=Sometimes
4=Often
5=Almost always
In the past 4 weeks, because of my asthma…
I felt annoyed.
I felt frustrated.
I felt angry.
I felt fed up.
I felt stressed.
I felt irritable.
I felt sad.
I felt anxious.
I felt helpless.
I felt overwhelmed.
I felt tired.
Note. The items above will not all be in the final item bank(s), which will be informed by field test results. Final item bank(s) will be available online and from the lead author.
References
- 1.CDC . America Breathing Easier 2010: CDC’s National Asthma Control Program at a Glance. National Center for Environmental Health (NCEH): Division of Environmental Hazards and Health Effects; 2010. p. 4. [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980-2004. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2007. [Google Scholar]
- 3.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 4.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams RJ, Ruffin RE, Smith BJ. Validity of a modified version of the Marks Asthma Quality of Life Questionnaire. J Asthma. 2000;37(2):131–143. doi: 10.3109/02770900009055436. [DOI] [PubMed] [Google Scholar]
- 7.Marks GB, Dunn SM, Woolcock AJ. An evaluation of an asthma quality of life questionnaire as a measure of change in adults with asthma. J Clin Epidemiol. 1993;46(10):1103–1111. doi: 10.1016/0895-4356(93)90109-e. doi:0895-4356(93)90109-E [pii] [DOI] [PubMed] [Google Scholar]
- 8.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992;45(5):461–472. doi: 10.1016/0895-4356(92)90095-5. doi:0895-4356(92)90095-5 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Bayliss MS, Espindle DM, Buchner D, Blaiss MS, Ware JE. A new tool for monitoring asthma outcomes: the ITG Asthma Short Form. Qual Life Res. 2000;9(4):451–466. doi: 10.1023/a:1008939328060. [DOI] [PubMed] [Google Scholar]
- 10.Schatz M, Mosen D, Kosinski M, Vollmer WM, O’Connor E, Cook EF, et al. Validation of the asthma impact survey, a brief asthma-specific quality of life tool. Qual Life Res. 2007;16(2):345–355. doi: 10.1007/s11136-006-9103-2. doi:10.1007/s11136-006-9103-2. [DOI] [PubMed] [Google Scholar]
- 11.Hyland ME. The Living with Asthma Questionnaire. Respir Med. 1991;85(Suppl B):13–16. doi: 10.1016/s0954-6111(06)80163-0. discussion 33-17. [DOI] [PubMed] [Google Scholar]
- 12.Barley EA, Quirk FH, Jones PW. Asthma health status measurement in clinical practice: validity of a new short and simple instrument. Respir Med. 1998;92(10):1207–1214. doi: 10.1016/s0954-6111(98)90423-1. doi:S0954-6111(98)90423-1 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 14.Hyland ME, Ley A, Fisher DW, Woodward V. Measurement of psychological distress in asthma and asthma management programmes. Br J Clin Psychol. 1995;34(Pt 4):601–611. doi: 10.1111/j.2044-8260.1995.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 15.National Asthma Education and Prevention Program. Third Expert Panel on the Diagnosis and Management of Asthma . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute (US); Bethesda (MD): 2007. [Google Scholar]
- 16.Wilson SR, Rand CS, Cabana MD, Foggs MB, Halterman JS, Olson L, et al. Asthma outcomes: quality of life. J Allergy Clin Immunol. 2012;129(3 Suppl):S88–123. doi: 10.1016/j.jaci.2011.12.988. doi: 10.1016/j.jaci.2011.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijkers MP. Individualization in quality of life measurement: instruments and approaches. Arch Phys Med Rehabil. 2003;84(4 Suppl 2):S3–14. doi: 10.1053/apmr.2003.50241. doi:10.1053/apmr.2003.50241 S0003999303001904 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Adams R, Rosier M, Campbell D, Ruffin R. Assessment of an asthma quality of life scale using item-response theory. Respirology. 2005;10(5):587–593. doi: 10.1111/j.1440-1843.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 19.Turner-Bowker DM, Saris-Baglama RN, Anatchkova M, Mosen DM. A Computerized Asthma Outcomes Measure Is Feasible for Disease Management. Am J Pharm Benefits. 2010;2(2):119–124. [PMC free article] [PubMed] [Google Scholar]
- 20.Embretson SE, Reise S. Psychometric methods: item response theory for psychologists. Lawrence Erlbaum Associates, Publishers; Mahwah, N.J.: 2000. [Google Scholar]
- 21.Wainer H, Mislevy RJ. Item response theory, item calibration, and proficiency estimation. In: Wainer H, Dorans NJ, Eignor D, Flaugher R, Green BF, Mislevy RJ, et al., editors. Computerized adaptive testing: a primer. 2nd ed. Lawrence Erlbaum Associates; Mahwah, N.J.: 2000. p. xxiii.p. 335. [Google Scholar]
- 22.Reeve B, Hays R, Bjorner J, Cook K, Crane P, Teresi J, et al. Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Medical Care. 2007;45(1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 23.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5 Suppl 1):S12–21. doi: 10.1097/01.mlr.0000254567.79743.e2. doi:10.1097/01.mlr.0000254567.79743.e2 00005650-200705001-00003 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123(11):1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 25.Sibbald B, Collier J, D’Souza M. Questionnaire assessment of patients’ attitudes and beliefs about asthma. Fam Pract. 1986;3(1):37–41. doi: 10.1093/fampra/3.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Tu SP, McDonell MB, Spertus JA, Steele BG, Fihn SD. A new self-administered questionnaire to monitor health-related quality of life in patients with COPD. Ambulatory Care Quality Improvement Project (ACQUIP) Investigators. Chest. 1997;112(3):614–622. doi: 10.1378/chest.112.3.614. [DOI] [PubMed] [Google Scholar]
- 27.Yeatts KB, Stucky B, Thissen D, Irwin D, Varni JW, DeWitt EM, et al. Construction of the Pediatric Asthma Impact Scale (PAIS) for the Patient-Reported Outcomes Measurement Information System (PROMIS) J Asthma. 2010;47(3):295–302. doi: 10.3109/02770900903426997. doi:10.3109/02770900903426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. doi:10.1016/j.jaci.2003.09.008 S009167490302270X [pii] [DOI] [PubMed] [Google Scholar]
- 29.Vollmer WM, Markson LE, O’Connor E, Sanocki LL, Fitterman L, Berger M, et al. Association of Asthma Control with Health Care Utilization and Quality of Life. American Journal of Respiratory and Critical Care Medicine. 1999;160(51):6. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- 30.Lara M, Ramos G, Gonzales J, al e. Reducing Quality of Care Disparities for Childhood Asthma: La Red de Asma Infantil Intervention in San Juan, Puerto Rico. Pediatrics. doi: 10.1542/peds.2012-1427d. In Press. [DOI] [PubMed] [Google Scholar]
- 31.Cabana MD, Slish KK, Nan B, Clark NM. Limits of the HEDIS criteria in determining asthma severity for children. Pediatrics. 2004;114(4):1049–1055. doi: 10.1542/peds.2003-1162-L. doi:114/4/1049 [pii] 10.1542/peds.2003-1162-L. [DOI] [PubMed] [Google Scholar]
- 32.Harrington S, Glauber J. [Accessed October 4, 2012 2012];Improving Outcomes in Asthma: Advancing Quality Using NCQA HEDIS Measures. 2011 http://www.ncqa.org/Portals/0/Education/NCQA%20Asthma%20webinar%20Oct%202011.pdf.
- 33.Lara M, Sherbourne C, Duan N, Morales L, Gergen P, Brook RH. An English and Spanish Pediatric Asthma Symptom Scale. Med Care. 2000;38(3):342–350. doi: 10.1097/00005650-200003000-00011. [DOI] [PubMed] [Google Scholar]