Abstract
The reversible post-translational modification (PTM) of eukaryotic proteins by ubiquitin (Ub) regulates key cellular processes including protein degradation and gene transcription. Studies of the mechanistic roles for protein ubiquitylation require quantities of homogenously modified substrates that are typically inaccessible from natural sources or by enzymatic ubiquitylation in vitro. Therefore, we developed a facile and scalable methodology for site-specific chemical ubiquitylation. Our semisynthetic strategy utilized a temporary ligation auxiliary, 2-(aminooxy)ethanethiol, to direct ubiquitylation at specific lysines in peptide substrates. Mild reductive removal of the auxiliary after ligation yielded ubiquitylated peptides with the native isopeptide linkage. Alternatively, retention of the ligation auxiliary yielded protease-resistant analogues of ubiquitylated peptides. Importantly, our strategy was fully compatible with protein sulfhydryl groups, as demonstrated by the synthesis of peptides modified by the human small ubiquitin-related modifier 3 (SUMO-3) protein.
Keywords: Conjugation, peptides, ubiquitin, SUMO, protein modifications
The 76-amino acid long protein ubiquitin (Ub) is highly conserved in eukaryotes,[1] and regulates almost every aspect of cellular function.[2] Ubiquitin and the family of structurally related small ubiquitin-like modifier proteins (SUMOs) play vital roles in cellular processes ranging from histone-mediated gene activation[3] or silencing[4] to 26S proteasome-mediated protein degradation.[5] Elucidating the functional consequences of ubiquitylation and SUMOylation of the many hundreds of known protein substrates in human cells is a major challenge for modern cell biology.[6] Such studies require the ability to follow the dynamic regulation and sub-cellular localization of ubiquitylated and SUMOylated proteins in vivo,[7] as well as the ability to investigate the direct biochemical and biophysical consequences of these post-translational modifications in vitro.[8]
Protein modification by Ub is a multi-step process involving a family of E1, E2 and E3 ligase enzymes that utilize the hydrolysis of ATP to activate the C-terminus of Ub, then conjugate it with specific Lys ε-amines in protein substrates by means of an isopeptide linkage.[2] More than 600 different E3 ligases, a majority of which remain uncharacterized, are involved in site-specific protein ubiquitylation in humans.[9] This poses a significant challenge toward generating useful quantities of site-specifically ubiquitylated proteins for in vitro investigations. Hence, multiple chemical strategies have been developed to generate native isopeptide-linked ubiquitylated peptides[10] as well as disulfide,[11] triazole,[12] and hydroxamate-linked analogues of ubiquitylated proteins.[13] Until now, methods to conjugate Ub with its targets by a native linkage have employed ligation auxiliaries such as γ- and δ-thiolysine,[10b, 14] or a photolytically removable auxiliary based on the o-nitrobenzyl scaffold.[10a] When incorporated at the desired Lys ubiquitylation site these auxiliaries permit native chemical ligation[15] with a Ub C-terminal α-thioester to generate an isopeptide linkage, after which the auxiliaries can be chemically or photolytically removed. However, the complex multi-step synthesis of these ligation auxiliaries, the requisite desulfurization of thialysine analogues post-ligation, and the slow kinetics of ligation with the photocleavable auxiliary ultimately limit their broad applicability. Therefore, a readily achievable ligation auxiliary that exhibits good ligation kinetics and is removable under conditions that do not affect native Cys residues would greatly expand the scope of ubiquitylated and SUMOylated proteins accessible for mechanistic studies.
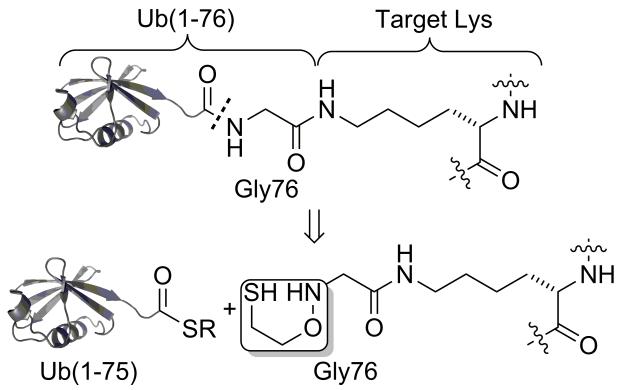
With this goal in mind, we turned our attention to a derivative of the 2-(aminooxy)ethanethiol auxiliary first reported by Kent and co-workers.[16] The 2-(aminooxy)ethanethiol group was shown to facilitate native chemical ligation at sterically unhindered Gly-Gly and Gly-Ala sites in short peptide sequences. Since Ub and indeed most ubiquitin-like proteins terminate in a C-terminal Gly-Gly sequence (Figure S1),[17] we envisioned that the auxiliary could be applied for peptide ubiquitylation as depicted in Scheme 1.
Scheme 1.
Site-specific peptide ubiquitylation.
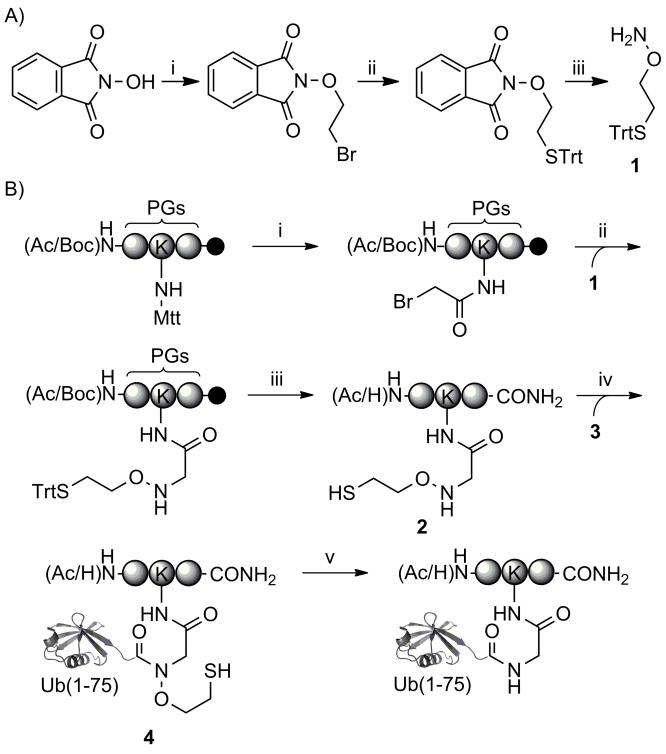
A facile 3-step synthetic scheme afforded the suitably protected auxiliary, 1, in multi-gram quantities and 46% overall yield (Scheme 2A and Figures S2-S4) for application in fluorenylmethoxycarbonyl (Fmoc) based solid-phase peptide synthesis. In order to test the utility of the auxiliary for peptide ubiquitylation, we first considered an internal peptide sequence from Ub itself. There exist seven Lys residues at positions 6, 11, 27, 29, 33, 48 and 63 in Ub, and all seven are found to be modified by ubiquitin in vivo.[18] Therefore, we first chose a short N-terminally acetylated and C-terminally amidated tripeptide, QKE, containing Lys63 of Ub, as a test substrate for auxiliary-mediated ubiquitylation. The peptide was assembled on rink amide resin using an orthogonal 4-methyltrityl (Mtt) protecting group at the Lys ε-amine targeted for ubiquitylation (Scheme 2B).[19] Following chain assembly, the Lys side-chain was selectively deprotected by 1% TFA in dichloromethane and the free ε-amine was subsequently coupled with bromoacetic acid. The auxiliary was readily introduced via direct nucleophilic displacement of bromine with a 1 M solution of 1 in DMSO overnight at room temperature. Importantly, the unreacted auxiliary could be filtered away from the solid-phase and reused at least four times without appreciable losses in incorporation. Finally, TFA-mediated cleavage from the resin afforded the auxiliary containing peptide, 2, in 38% purified yield based on the initial resin loading (Figure S5).
Scheme 2. Synthesis and application of the ligation auxiliary.
A) i) BrCH2CH2Br, Et3N, DMF, 18 h, 25 °C, 64%; ii) TrtSH, NaH, DMF, 2 h, 25 °C, 79%; iii) H 2NNH2, CHCl3, 2.5 h, 25 °C, 90%. B) i) 1. 1% TFA, 1% TIS in CH2Cl2; 2. BrCH2COOH, DIC, DMF; ii) 1, 1 M in DMSO; iii) Reagent K; iv) Ub(1-75)-α-thioester (3), 6 M Gn-HCl, 100 mM Na2HPO4, 10 mM TCEP, pH 7.5; v) 6 M Gn-HCl, Zn, pH 3.0, 37 °C, 24 h. PG = protecting group, Trt = trityl, Mtt = 4-methyltrityl, Ac = acetyl, Boc = tert-butyloxycarbonyl, Fmoc = 9-Fluorenylmethoxycarbonyl, TFA = trifluoroacetic acid, TIS = triisopropylsilane, DIC = N,N′-Diisopropylcarbodiimide, Reagent K = 82.5:5:5:5:2.5, TFA: thioanisole: H2O: phenol: 1,2-ethanedithiol (v/v), Gn-HCl = guanidinium hydrochloride, TCEP = Tris(2-carboxyethyl)phosphine.
A heterologously expressed human Ub(1-75)-α-thioester, 3, was obtained by thiolysis of the corresponding GyrA intein fusion with mercaptoethanesulfonic acid (MES) (Figure S6).[10a] Ligation was initiated by mixing 2.5 mM of 2 and 0.5 mM of 3 in 6 M Gn-HCl, 100 mM Na2HPO4, 10 mM TCEP, pH 7.5 at room temperature. Although auxiliary-mediated ligation onto a secondary amine was previously shown to be slower than ligation to the primary amine in Cys,[20] the enhanced nucleophilicity of the aminooxy group at pH 7.5 led to complete acyl transfer within 12 h (Figure S7).[21] The ligation product was then purified by reversed phase high performance liquid chromatography (RP-HPLC) to obtain the auxiliary-containing branched protein QKUb(aux)E, 4 (Figure S8). Surprisingly, and contradictory to previous results with short peptides,[16] our initial attempts to reductively cleave the ligation auxiliary from 4 with activated Zn in acidic HPLC buffers or water proved largely unsuccessful (Table 1, entries 1, 2). The inclusion of 6 M Gn-HCl during the reduction step overcame this issue and led to greater removal of the β-mercaptoethanol group resulting in the native isopeptide linkage at Lys (Figure 1 and Table 1, entry 3). The requirement for a denaturant to facilitate efficient electron transfer from Zn to the low-energy N-O bond in 4 is consistent with previous biophysical studies which showed that Ub retains its tertiary structure in solution at pH ~1 (Figure S9).[22] However, this may be a limitation for some Ub targets.
Table 1.
Reductive removal of the ligation auxiliary.
| No. | Product | Reagent | pH | temp (°C) | time (h) | Yield (%)[a] |
|---|---|---|---|---|---|---|
| 1 | QKubE | Zn, 1:1 AcN:H2O | 1 | 22 | 24 | 5 |
| 2 | QKubE | Zn, H2O | 1 | 22 | 24 | 12 |
| 3 | QKubE | Zn, 6M Gn-HCl | 1 | 22 | 24 | 28 |
| 4 | QKubE | Zn, 6M Gn-HCl | 1 | 37 | 24 | 54 |
| 5 | QKubE | Raney-Nickel[b] | 7 | 37 | 17 | 5 |
| 6 | QKubE | In, 6 M Gn-HCl | 3 | 37 | 24 | 31 |
| 7 | QKubE | Zn, 6 M Gn-HCl | 3 | 37 | 24 | 80 |
| 8 | QKSuE | Zn, 6 M Gn-HCl | 3 | 37 | 24 | 75 |
| 9 | KAKubI | Zn, 6 M Gn-HCl | 3 | 37 | 24 | 80 |
| 10 | KAKSuI | Zn, 6 M Gn-HCl | 3 | 37 | 24 | 60 |
Yields were calculated from purified product weights and relative signal intensities in ESI-MS.
As per the procedure reported by McGinty et al.[20]
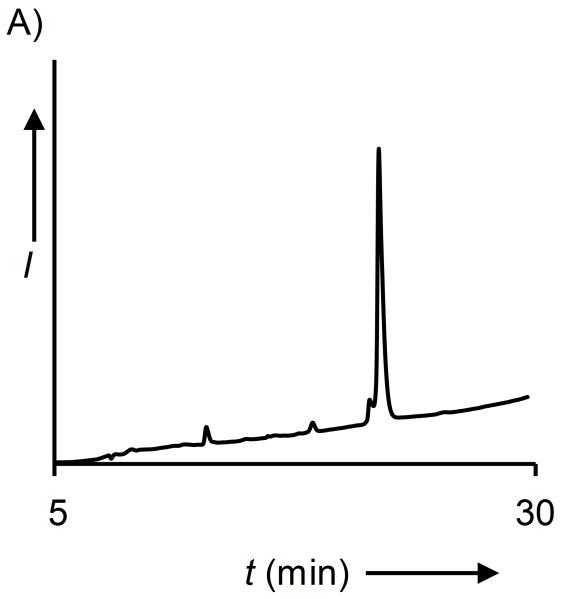
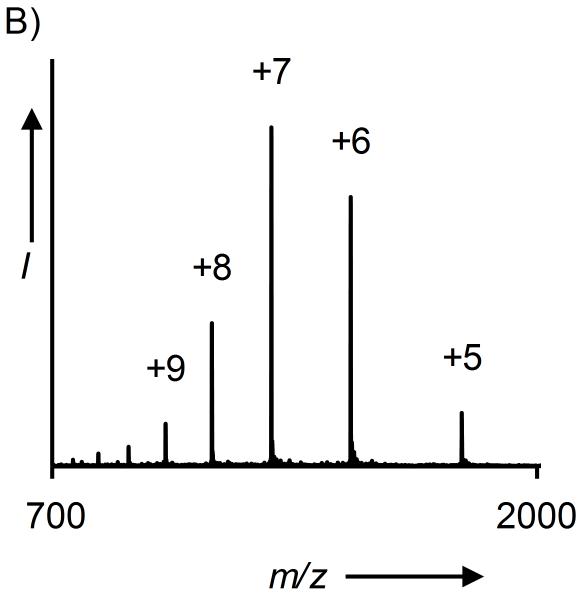
Figure 1. Synthesis of QKUbE.
A) C18 analytical RP-HPLC chromatogram of reduced and purified QKUbE. B) ESI-MS of reduced QKUbE. Calculated m/z [M+H]+ 8,992.0 Da, observed 8,992.2 ± 1.8 Da.
We next sought to identify suitable reagents and conditions that maximize the yields of reduced products (Table 1, entries 4-7 and Figure S10). Several methods have been reported for the conversion of N,N-disubstituted hydroxylamines to the corresponding amines, including reduction with Raney Ni[23] and Indium metal.[24] However, we observed that treatment of 4 with activated Zn in 6 M Gn-HCl at pH 3.0 and 37 °C for 24 h yielded the best results with minimal side-products (Table 1, entry 7). A small amount of hydrolyzed Ub(1-75) was the major side-product observed during auxiliary removal. An N-to-S acyl shift of the amide backbone that would yield a thioester susceptible to hydrolysis has previously been demonstrated at Gly-Cys junctions[25] and with N-methylated Cys at low pH and elevated temperatures.[26] However, for 4 the hydrolysis product is more likely a result of direct nucleophilic attack by water on the protonated tertiary amide. Evidence toward this comes from our observation that alkylation of the auxiliary thiol post-ligation, which precludes an N-to-S acyl shift, did not significantly affect the small degree of hydrolysis (Figure S11).
With a robust methodology for peptide ubiquitylation in hand we proceeded to test its scope for peptide SUMOylation with a recombinant human SUMO-3(2-91)-α-thioester and the peptide QK(aux)E (Figures S12-S13). Ligation proceeded with similar kinetics as for Ub and was complete in 12 h (Figures S14-S15). A previously reported ligation of SUMO to a secondary amine required 7 days,[10a] which highlights the advantage of employing the aminooxy group for acyl transfer. As expected, reductive removal of the pendant auxiliary group afforded the isopeptide-linked QKSuE in good yield while retaining Cys47 in SUMO (Table 1, entry 8 and Figure S16).
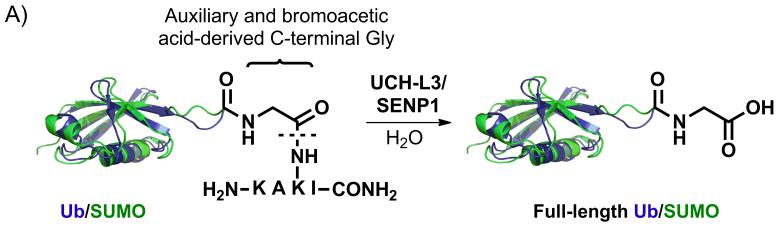
We further explored the substrate scope of ligation with a KAKI peptide sequence, which contains both Lys27 and Lys29 of Ub (Figure S17). Installation of the ligation auxiliary at the Lys29 position in KAKI led to similar yields for ubiquitylation and SUMOylation of this peptide (Table 1, entries 9 and 10 and Figures S18-S19). However, unlike the N-terminally acetylated QKE peptide, the reaction of Ub(1-75)-and SUMO-3(2-91)-α-thioesters with KAKI could formally proceed through nucleophilic attack by either the peptide N-terminus, the Lys27 ε-amine, or the auxiliary alkoxyamine. In order to test the precise site of ligation, KAKUbI and KAKSuI were assayed with the ubiquitin C-terminal hydrolase L3 (UCH-L3) and the Sentrin-specific protease 1 (SENP1), which are cysteine proteases that remove Ub and SUMO, respectively, from diverse cellular targets (Figure 2A).[27]
Figure 2. Testing the site of Ub and SUMO linkage.
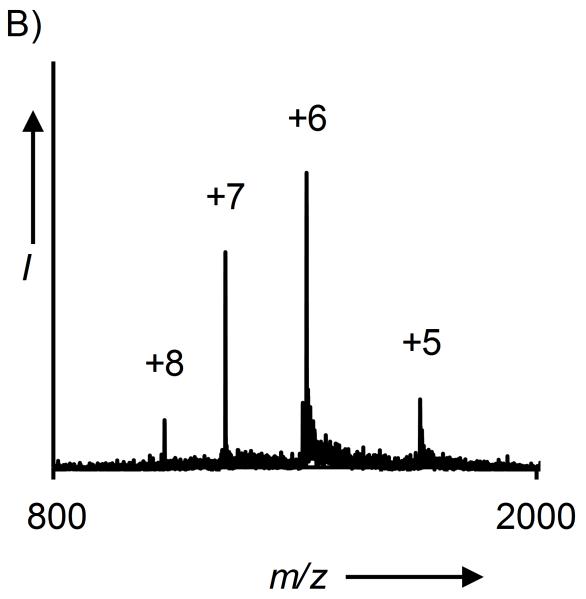
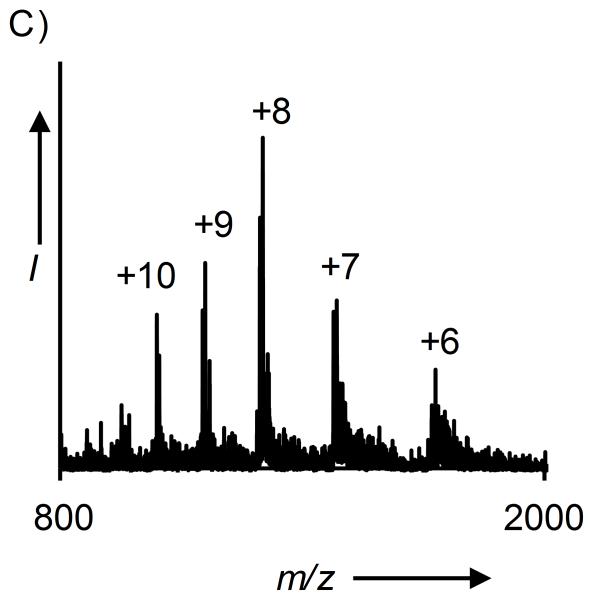
A) Hydrolysis of KAKUbI and KAKSuI by UCH-L3 and SENP1, respectively. B) ESI-MS of KAKUbI assayed with UCH-L3. Calculated m/z [M+H]+ 8,565.8 Da, observed 8,566.4 ± 0.8 Da. C) ESI-MS of KAKSuI assayed with SENP1. Calculated m/z [M+H]+ 13,394.6 Da, observed 13,394.6 ± 2.1 Da.
Complete hydrolysis of the isopeptide linkage was observed in both cases and the full-length Ub(1-76) and SUMO-3(2-92) proteins were observed after 3.5 and 8 h, respectively (Figures 2B-2C and S20). As the C-terminal Gly in the full-length proteins could only be derived from conjugation at Lys29 (Scheme 1), these results indicated that auxiliary-mediated ligation of Ub and SUMO to the KAKI peptide occurred at the desired Lys29 side-chain. Complete hydrolysis of KAKUbI by UCH-L3 suggests that our chemical strategy does not interfere with the correct folding of Ub in the final ubiquitylated product. Furthermore, X-ray crystal structures of Ub bound to UCH-L3 reveal extensive protein-protein interactions that may only exist in the native folded form of Ub (Figure S21).[28] Thus, our methodology yields site-specifically ubiquitylated and SUMOylated peptides that adopt a native fold and are suitable for in vitro biochemical studies.
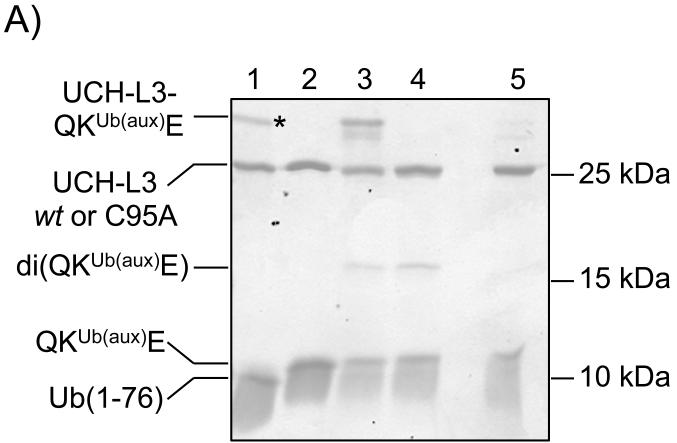
When subjecting semisynthetic ubiquitylated proteins to assays in complex mixtures, such as cell lysates, one must contend with the presence of ~100 deubiquitylating enzymes (DUBs) that may hydrolyze the isopeptide linkage.[29] In thinking of this problem, we noted that retention of the ligation auxiliary in the final ubiquitylated peptide yields an N-alkylated Gly76. The enhanced proteolytic stability of polymeric N-substituted glycines, also known as peptoids, and peptoid-peptide hybrid molecules has previously been demonstrated.[30] Therefore, we wondered if simply retaining the ligation auxiliary in the final ubiquitylated product would inhibit proteolysis of the isopeptide bond. To our delight, incubation of the unreduced ligation product containing the auxiliary group, QKUb(aux)E, with UCH-L3 for 3.5 h at 37 °C led to no observable hydrolysis of the isopeptide linkage (Figure 3A, lane 3). In comparison, the reduced ligation product, QKUbE, was completely hydrolyzed to produce Ub(1-76) under identical conditions (Figure 3A, lane 1). To test the scope of DUB resistance, we also assayed KAKUb(aux)I with the ubiquitin specific proteases Usp2 and Usp5, which are structurally vastly different from UCH-L3.[31] We observed that the ligation auxiliary also inhibited the removal of Ub from peptide targets by these enzymes (Figure S22).
Figure 3. Protease-resistant ubiquitylated peptide.
A) Coomassie stained 18% SDS-PAGE gel of DUB activity towards QKUbE and QKUb(aux)E. Lane 1 = UCH-L3 + QKUbE; lane 2 = UCH-L3(C95A) + QKUbE; lane 3 = UCH-L3 + QKUb(aux)E; lane 4 = UCH-L3(C95A) + QKUb(aux)E; lane 5 = UCH-L3 + QKUb(aux)E + 50 mM DTT, 95 °C, 5 min. An asterisk indicates the Ub(1-76)-UCH-L3 acyl enzyme intermediate. B) ESI-MS of the observed UCH-L3-QKUb(aux)E adduct. Calculated m/z [M+H]+ 35,247.5 Da, observed 35,252.5 ± 5.6 Da.
Recently, N-methylation of the isopeptide bond was demonstrated to be a means to inhibit UCH-L3 activity.[32] This required the challenging chemical synthesis of an N-methylated branched polypeptide and a final desulfurization step to generate wild-type Ub. We demonstrate that N-alkylation of Gly76 offers an alternate and facile means to access both protease resistant and wild-type isopeptide-linked ubiquitylated peptides from a single synthetic route.
Interestingly, non-reducing denaturing polyacrylamide gel electrophoresis of the products obtained from incubating QKUb(aux)E with UCH-L3 in the presence of ~6 mM dithiothreitol (DTT) revealed a new higher molecular weight band (Figure 3A, lane 3). Reverse phase liquid chromatography of the assay products followed by electrospray ionization mass spectrometry (LC-ESI-MS) revealed a species corresponding to disulfide-linked QKUb(aux)E and UCH-L3 (Figure 3B). This higher molecular-weight species was also observed upon incubation of KAKUb(aux)I with UCH-L3 (Figure S23). In keeping with a disulfide-linkage, the adduct was not observed when assay products were boiled with 50 mM DTT prior to gel electrophoresis (Figure 3A, lane 5), or when the active site mutant UCH-L3(C95A) was employed in assays (Figure 3A, lane 4 and Figure S24). Disulfide formation is however not the sole mechanism for UCH-L3 resistance as unlinked QKUb(aux)E remained intact while QKUbE was effectively hydrolyzed (Figure S25). A higher molecular weight band was also observed during the deubiquitylation of QKUbE by UCH-L3, however, Ub has no intrinsic thiol groups and the mass of this species corresponded to an enzyme-bound Ub(1-76) acyl-intermediate (Figure S26).
In conclusion, we have demonstrated the successful application of a synthetically facile and readily removable ligation auxiliary toward the ubiquitylation and SUMOylation of various peptide targets. Our methodology significantly advances current ubiquitylation strategies, as it is orthogonal to Cys residues in proteins. When employed in combination with native chemical ligation at Cys this will greatly expand the substrate scope of protein targets. Furthermore, post-ligation the auxiliary mimics an N-substituted Gly76 and inhibits several deubiquitylating enzymes. Our preliminary results also indicate that the auxiliary group has utility as a probe in pull-down assays to identify ubiquitin C-terminal hydrolases. Finally, the mild conditions for removal of the auxiliary after ligation do not inhibit protein recognition by specific proteases such as UCH-L3 and SENP1, which is promising for studies of the biochemical effects of ubiquitylation and SUMOylation.
Experimental Section
See the Supporting Information
Supplementary Material
Acknowledgements
This work was supported by the Department of Chemistry and the Royalty Research Fund at the University of Washington, Seattle. C.E.W. gratefully acknowledges an ARCS Foundation Fellowship and an NSF Graduate Research Fellowship (grant number DGH-1256082).
Footnotes
This paper is dedicated to Professor Niels H. Andersen on the occasion of his 70th birthday
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cbic.20xxxxxxx
References
- [1].Sharp PM, Li WH. Trends Ecol. Evol. 1987;2:328–332. doi: 10.1016/0169-5347(87)90108-X. [DOI] [PubMed] [Google Scholar]
- [2].Hershko A, Ciechanover A. Ann Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- [3].Weake VM, Workman JL. Mol. Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- [4].Shiio Y, Eisenman RN. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Finley D. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xu G, Paige JS, Jaffrey SR. Nat. Biotechnol. 2010;28:868–873. doi: 10.1038/nbt.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]; Becker J, Barysch SV, Karaca S, Dittner C, Hsiao HH, Berriel Diaz M, Herzig S, Urlaub H, Melchior F. Nat. Struct. Mol. Biol. 2013;20:525–531. doi: 10.1038/nsmb.2526. [DOI] [PubMed] [Google Scholar]
- [7].Ayaydin F, Dasso M. Mol. Biol. Cell. 2004;15:5208–5218. doi: 10.1091/mbc.E04-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]; van Wijk SJ, Fiskin E, Putyrski M, Pampaloni F, Hou J, Wild P, Kensche T, Grecco HE, Bastiaens P, Dikic I. Mol. Cell. 2012;47:797–809. doi: 10.1016/j.molcel.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weller CE, Pilkerton ME, Chatterjee C. Biopolymers. 2014;101:144–155. doi: 10.1002/bip.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Metzger MB, Hristova VA, Weissman AM. J. Cell Sci. 2012;125:531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chatterjee C, McGinty RK, Pellois J-P, Muir TW. Angew. Chem. 2007;119:2872–2876. doi: 10.1002/anie.200605155. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. Eng. 2007;46:2814–2818. doi: 10.1002/anie.200605155. [DOI] [PubMed] [Google Scholar]; Kumar KSA, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew. Chem. 2009;121:8234–8238. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. Eng. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- [11].Chatterjee C, McGinty RK, Fierz B, Muir TW. Nat. Chem. Biol. 2010;6:267–269. doi: 10.1038/nchembio.315. [DOI] [PubMed] [Google Scholar]
- [12].Weikart ND, Mootz HD. ChemBioChem. 2010;11:774–777. doi: 10.1002/cbic.200900738. [DOI] [PubMed] [Google Scholar]
- [13].Shanmugham A, Fish A, Luna-Vargas MPA, Faesen AC, El Oualid F, Sixma TK, Ovaa H. J. Am. Chem. Soc. 2010;132:8834–8835. doi: 10.1021/ja101803s. [DOI] [PubMed] [Google Scholar]
- [14].Merkx R, de Bruin G, Kruithof A, van den Bergh T, Snip E, Lutz M, El Oualid F, Ovaa H. Chem. Sci. 2013;4:4494–4498. [Google Scholar]
- [15].Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–778. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- [16].Canne LE, Bark SJ, Kent SBH. J. Am. Chem. Soc. 1996;118:5891–5896. [Google Scholar]
- [17].Welchman RL, Gordon C, Mayer RJ. Nat. Rev. Mol. Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- [18].Komander D, Rape M. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- [19].Sax B, Dick F, Tanner R, Gosteli J. Pept. Res. 1992;5:245–246. [PubMed] [Google Scholar]
- [20].McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW. Nature. 2008;453:812–816. doi: 10.1038/nature06906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Castro EA. Chem. Rev. 1999;99:3505–3524. doi: 10.1021/cr990001d. [DOI] [PubMed] [Google Scholar]
- [22].Cary PD, King DS, Crane-Robinson C, Bradbury EM, Rabbani A, Goodwin GH, Johns EW. Eur. J. Biochem. 1980;112:577–580. doi: 10.1111/j.1432-1033.1980.tb06123.x. [DOI] [PubMed] [Google Scholar]
- [23].Denmark SE, Stolle A, Dixon JA, Guagnano V. J. Am. Chem. Soc. 1995;117:2100–2101. [Google Scholar]
- [24].Cicchi S, Bonanni M, Cardona F, Revuelta J, Goti A. Org. Lett. 2003;5:1773–1776. doi: 10.1021/ol034434l. [DOI] [PubMed] [Google Scholar]
- [25].Kang J, Reynolds NL, Tyrrell C, Dorin JR, Macmillan D. Org. Biomol. Chem. 2009;7:4918–4923. doi: 10.1039/b913886b. [DOI] [PubMed] [Google Scholar]
- [26].Erlich LA, Kumar KS, Haj-Yahya M, Dawson PE, Brik A. Org. Biomol. Chem. 2010;8:2392–2396. doi: 10.1039/c000332h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP. EMBO J. 1997;16:3787–3796. doi: 10.1093/emboj/16.13.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shen LN, Dong C, Liu H, Naismith JH, Hay RT. Biochem. J. 2006;397:279–288. doi: 10.1042/BJ20052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maiti TK, Permaul M, Boudreaux DA, Mahanic C, Mauney S, Das C. FEBS J. 2011;278:4917–4926. doi: 10.1111/j.1742-4658.2011.08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reyes-Turcu FE, Ventii KH, Wilkinson KD. Annu. Rev. Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stawikowski M, Stawikowska R, Jaskiewicz A, Zablotna E, Rolka K. ChemBioChem. 2005;6:1057–1061. doi: 10.1002/cbic.200400412. [DOI] [PubMed] [Google Scholar]
- [31].Komander D. Subcell. Biochem. 2010;54:69–87. doi: 10.1007/978-1-4419-6676-6_6. [DOI] [PubMed] [Google Scholar]
- [32].Haj-Yahya M, Eltarteer N, Ohayon S, Shema E, Kotler E, Oren M, Brik A. Angew. Chem. 2012;124:11703–11707. doi: 10.1002/anie.201205771. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. Eng. 2012;51 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.