Abstract
Background
We evaluated the effects of local flax seed oil and glycerol application for reducing adhesion formation after thyroidectomy.
Material/Method
We randomly assigned 18 female Wistar albino rats (median weight: 275 g, median age: 4.5 mth) to 3 groups: 0.1 ml 0.9% NaCl, glycerol, and flax seed oil were sprayed in a perithyroidal area after thyroidectomy operation on all animals as anti-adhesive barriers. Rats were sacrificed on the postoperative 14th day and adhesions were evaluated macroscopically and microscopically.
Results
The median macroscopic adhesion score was 3.0±0.0 in the 0.9% NaCl group, 1.33±0.52 in the glycerol group, and 1.67±0.53 in the flax seed oil group. The median histopathological fibrosis scores were 2.33±0.82, 0.67±0.52, and 0.83±0.75, respectively. Both glycerol and flaxseed oil group macroscopic and microscopic scores were significantly lower than the 0.9% NaCl group (p<0.05). There was no significant difference among the groups (p>0.05).
Conclusions
Glycerol and flax seed oil both decrease the incidence of post-thyroidectomy adhesion in rats, but glycerol is more effective.
MeSH Keywords: Glycerol - therapeutic use, Linseed Oil - therapeutic use, Thyroidectomy - adverse effects, Tissue Adhesions - complications, Tissue Adhesions - prevention & control
Background
Postoperative adhesions (PA) cause serious problems for all surgical operations. PAs consist of residual collagen fibers that appear during the fibrosis process activated after surgical trauma [1,2]. PAs are most important in abdominal surgical procedures [3–5]. PAs are formed due to thyroidectomies performed for benign thyroid disorders, and in the recurrences after thyroid cancer surgeries, which are higher in recurrent thyroid cancer operations [6,7]. No real success has been achieved, although minimally invasive techniques, pharmacological agents targeting the formation of fibrin and/or inflammatory response, and liquids and gels that create mechanical barriers have been tried [3,8,9].
The glycerol we used in this study is one of the most common molecules in living organisms. Glycerol is the building block of lipids. Due to its high bio-adaptability, glycerol is used as an additive in many pharmaceutical and cosmetic products [10–12]. Because of its absorbability and low incidence of irritant and toxic effects, some of the meshes used in surgical treatment of hernias are either made of or later covered with glycerol-containing composites [13,14]. Flax seed and flax seed oil have anti-proliferative, anti-inflammatory, anti-edema, and anti-oxidant features, as they are rich in the fatty acid omega-3 [15–18]. Flax and flax products are uses in kidney disorders, certain types of cancer, and atherosclerosis/coronary artery disorders [19].
In this study, we examined the efficiency of glycerol and flax seed oil in preventing PAs after thyroidectomy, neither of which had been used for this purpose previously.
Material and Methods
The protocol of the study was approved by Bezmialem Vakif University Animal Ethics Committee, and the research was conducted in the Bezmialem Vakif University Experimental Animal Research Laboratory. Throughout the experiment, the animals were kept alive in standard cages produced for mice and rats, the bases and sides of which were made of plastic, while the tops were covered with metal wire netting. The animals were fed with pellet-type fabricated feed produced especially for small experimental animals, and the room was on a 12:12 light cycle. A total of 18 female Wistar albino rats (median weight: 275 g, median age: 4.5 months) were divided into 3 groups. General anesthesia was delivered through intramuscular injection of 90 mg/kg ketamine (Ketalar®, Pfizer, USA) and 10 mg/kg xylazine HCl (Rompun®, Bayer, Germany).
The anterior neck was shaved and antisepsis used a povidone/iodine solution (Batticon 10 g Pvp-iyot, Adeka Ilac, Istanbul, Turkey). Following a 2-cm longitudinal incision in the anterior neck area, the submaxillary tissues and muscles were retracted laterally, and the trachea and the bilobular thyroid tissues on both sides were visualized. Bilateral subtotal thyroidectomy was made. Group 1 received 0.1 ml 0.9% NaCl (Serum Fizyolojik, 500 ml Eczacibasi Co, Istanbul, Turkey), group 2 received 0.1 ml glycerol (Gliserin®, Arifoglu Co. Istanbul, Turkey) and group 3 received 0.1ml flax seed oil (Keten Yagi®, Arifoglu Co. Istanbul, Turkey) sprayed on the thyroidectomy areas. The muscles were brought back to their initial position, and the skin was closed using the continuous stitching method with 5.0 polypropylene (Prolene®, Bicakcilar Co. Istanbul, Turkey). The rats were sacrificed on the 14th postoperative day. The operation area was re-explored and the adhesions in the operation area were scored based on the Evans [20] model (Table 1). Then, the thyroidectomy area was totally resected with trachea for histopathologic evaluation according to fibrosis grading (Table 2) [21].
Table 1.
The macroscopic adhesion grading of Evans.
| Grade 0 | No adhesion |
| Grade 1 | Spontaneously seceding adhesions |
| Grade 2 | Adhesions seceding through traction |
| Grade 3 | Adhesions seceding through dissection |
Table 2.
Histopathologic fibrosis grading.
| Grade 0 | No fibrosis (no fibroblast and/or collagen fibers) |
| Grade 1 | Low-level fibrosis (low number of fibroblast and/or collagen fibers) |
| Grade 2 | Medium-level fibrosis (more fibroblast and/or collagen fibers) |
| Grade 3 | Advanced fibrosis (high number of fibroblast and/or collagen fibers) |
Histopathologic evaluation
Resected tissues were fixated in formol. Five-mm cross-sections were taken, dyed first with hematoxylin eosin and then with collagen paint. The whole evaluation was conducted with a light microscope at 100× magnification. The evaluation was made by a pathologist who was not informed about the group (blind evaluation), using “histopathological fibrosis grading” (Table 2).
Statistical method
The statistical analyses were conducted using the SPSS 11.00 package software. A Wilcoxon test evaluation was made for each of the 3 groups. The comparison of the macroscopic and histopathologic inter-group scores was made using a Kruskal-Wallis test. To see which 2 groups caused the inter-group difference, the post hoc Dunn test was used. The results were evaluated at the p<0.05 significance level.
Results
In both the glycerol and the flax seed oil groups, there was 1 rat that developed granuloma with 3 mm and 2 mm diameter, respectively, in to the thyroidectomy area. No statistically meaningful differences were found in the macroscopic and microscopic scores for in-group analyses with the Wilcoxon test of the groups (p=0.102) (Table 3).
Table 3.
The median adhesion scores and in-group statistical evaluation results.
| Group | Macroscopic adhesions ccore | Microscopic adhesion score | Wilcoxon test |
|---|---|---|---|
| Glycerol | 1.33±0.52 | 0.67±0.52 | p: 0.102 |
| Flax seed oil | 1.67±0.52 | 0.83±0.75 | p: 0.102 |
| 0.9% NaCl | 3.00±0.0 | 2.33±0.82 | p: 0.102 |
When inter-group analysis were performed according to median adhesion scores, glycerol and flax seed oil groups were statistically significantly different than that control group both macroscopically and microscopically. The glycerol group median adhesion score was lower than in the flax seed oil group, but statistically significant differences were not obtained (Table 4).
Table 4.
Statistical analyses of the three groups according to median adhesion scores.
| Compared groups | Macroscopic adhesion score | Microscopic adhesion score |
|---|---|---|
| Control vs. glycerol | p<0.05 | p<0.05 |
| Control vs. flax seed oil | p<0.05 | p<0.05 |
| Glycerol vs. flax seed oil | p>0.05 | p>0.05 |
Histopathological view of minimal fibrosis at grade-1 level adhesion (example from glycerol group) (Figure 1), modarate level fibrosis at grade 2 level adhesion (example from flax-oil group) (Figure 2) and advanced fibrosis at grade 3 level adhesion (example from control group) (Figure 3) are shown in the figures.
Figure 1.
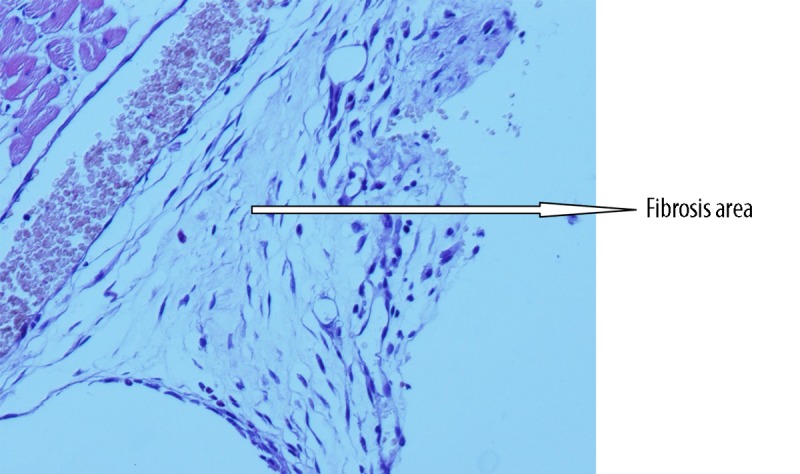
Histopathological view of minimal fibrosis at grade-1 level adhesion (example from glycerol group).
Figure 2.
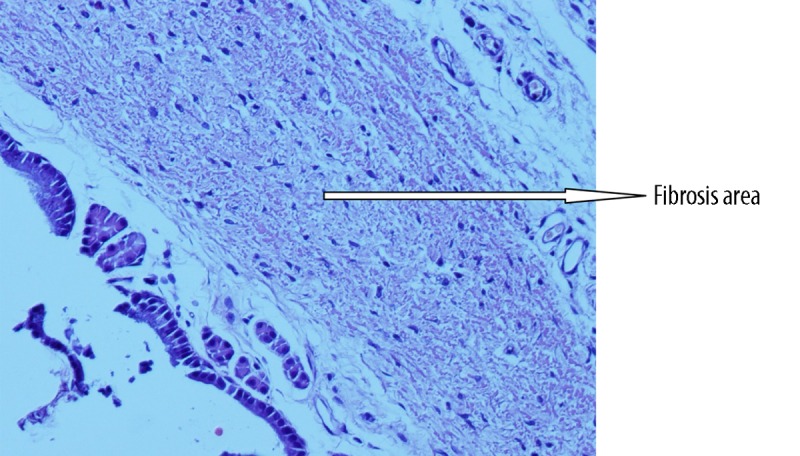
Histopathological view of modarate level fibrosis at grade 2 level adhesion (example from flax-oil group).
Figure 3.
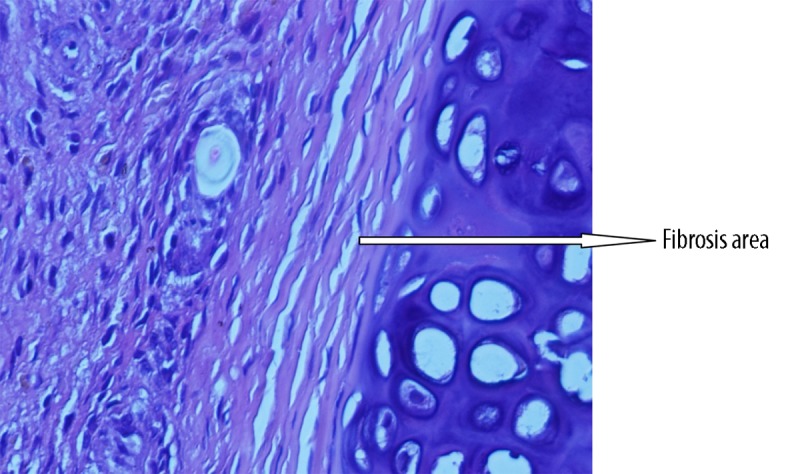
Histopathological view of advanced fibrosis at grade 3 level adhesion (example from control group).
Discussion
Thyroidectomy is the most common endocrine surgical operation [22–25]. The complications monitored after thyroidectomy are more frequent after secondary attempts. The primary reason for this is the PAs [26–31]. Complications monitored after thyroidectomy in a series of studies are reported to be between 4.8% and 7.1% in temporary hypoparathyroidy, 1.6% and 2.5% in permanent hypoparathyroidy, 1.2% and 2% in temporary RLS paralysis, 0.5% and 1.5% in permanent RLS paralysis, 0.9% and 2.5% in postoperative hematoma formation, and 0.2% and 0.5% in postoperative wound infection [7,30,32]. Osmólski et al. studied 847 thyroidectomies, scanned retrospectively, and the temporary and permanent recurrent nerve injuries found in the secondary-operation thyroidectomies were significantly higher than those that were primary operations. Moreover, temporary and permanent hypoparathyroidy frequencies were compared, and both were found significantly higher after the secondary operations [33].
Studies on prevention of PAs are usually for postoperative peritoneal adhesions. In the literature, there are very few studies done to prevent adhesions forming after thyroidectomy [30,34,35]. Yiğit et al. evaluated the efficiency of the anti-adhesive barrier Seprafilm® (Genzyme Corporation, USA) and Interceed® (Johnson & Johnson, USA). Both materials meaningfully decreased the postoperative adhesion incidence and prevalence under parameters used in fibrosis formation histopathologically (chronic inflammation, histiocyte, fibroblast, fibrosis, collagen, vascularization, granuloma, giant cell, and fat necrosis), except histiocyte. A decrease of 69.5% was observed in the Seprafilm group, and 84.7% in the Intercede group [34]. Park et al. applied 5 ml of hyaluronic acid-carboxymethyl cellulose (HA-CMC) solution in the operation area of 74 patients after operations for benign or malignant ipsilateral lobectomy, subtotal lobectomy, total thyroidectomy, ipsilateral lobectomy and modified neck dissection, and total thyroidectomy and modified neck dissection. Patents were evaluated postoperative 2nd week, 2nd month and 6th month. In this examination, attention was paid to 7 criteria, and each criterion was evaluated with a scale of 1–10: difficulty in swallowing saliva, water, and solid food; unusual feeling of pulling in the neck; wrinkles; symmetry; surgical inflammatory reaction; and scar formation in the neck. As a result of the objective and subjective evaluations, HA-CMC solution was not shown to be effective in preventing adhesions [36]. In the experimental study conducted on 48 rats by Çipe et al., hyaluronic acid-carboxymethyl cellulose (HA-CMC, Seprafilm®) and polylactic acid barrier film (PLA, Surgiwrap™) covered the operation area to decrease PAs. The subjects were divided into 3 groups to examine the effectiveness of these substances. Eight subjects from each group were sacrificed on the 7th day; the rest of the subjects were sacrificed on the 28th day. During reoperations, 2 surgeons separately scored the adhesions. Afterwards, the thyroids were taken out along with the surrounding tissues, and histopathologic examination was conducted. The surgical adhesion score of the HA-CMC group on the 7th and 8th days was significantly lower when compared to the control group. No meaningful difference was found between the control and the PLA groups [35].
In this study, we investigated the effectiveness of glycerol and flax seed oil in preventing post-thyroidectomy adhesions, neither of which had been used for this purpose before. Aysan et al. evaluated flax seed oil and glycerol for effectiveness in preventing postoperative peritoneal adhesions in 2 separate studies, both of which found that flax seed oil and glycerol significantly decreased peritoneal adhesion formation [37,38]. Building on these studies, we evaluated the effectiveness of flax seed oil and glycerol in preventing post-thyroidectomy adhesions. We observed no adverse effects except for 2 subjects that developed granuloma. In both groups, we observed significant decreases in adhesions after thyroidectomy, both macroscopically and histopathologically.
Conclusions
Both glycerol and flax seed oil applied on the thyroidectomy area are effective in preventing post-thyroidectomy adhesions. Although not significant statistically, the effectiveness of glycerol was higher. Further studies in larger series are needed.
Footnotes
Source of support: Departmental sources
References
- 1.Herrick EH, Mutsaers SE, Ozua P, et al. Human peritoneal adhesions are highly cellular, innervated and vascularized. J Pathol. 2000;192:67–72. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH678>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Hellebrekers BWJ, Trimbos-Kemper TCM, Trimbos JBMZ, et al. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74:203–12. doi: 10.1016/s0015-0282(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 3.Senthilkumar MP, Dreyer JS. Peritoneal adhesions: pathogenesis, assessment and effects. Trop Gastroenterol. 2006;27:11–18. [PubMed] [Google Scholar]
- 4.Hershalg A, Diamond MP, DeCherney AH. Adhesiolysis. Clin Obstet Gynecol. 1991;34:395–98. doi: 10.1097/00003081-199106000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Canbaz MA, Ustun C, Kocak D, Yanık FF. The comparsion of gonadotropinreleasing hormone agonist therapy and intraperitoneal Ringer’s lactate solution in prevention of postopertive adhesion formation in rat medels. Obstet Gynecol. 1999;82:219–22. doi: 10.1016/s0301-2115(98)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Lefevre JH, Tresallet C, Leenhardt L, et al. Reoperative surgery for thyroid disease. Langenbecks Arch Surg. 2007;392:685–91. doi: 10.1007/s00423-007-0201-6. [DOI] [PubMed] [Google Scholar]
- 7.Menegaux F, Turpin G, Dahman M, et al. Secondary thyroidectomy in patients with prior thyroid surgery for benign disease: A study of 203 cases. Surgery. 1999;126:479–83. [PubMed] [Google Scholar]
- 8.Davey AK, Maher PJ. Surgical adhesions: a timely update, a great challenge for the future. J Minim Invasive Gynecol. 2007;14:15–22. doi: 10.1016/j.jmig.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Liakakos T, Thomakos N, Fine PM, et al. Peritoneal adhesions: etiology, pathophysiology, and clinicalsignificance. Recent advances in prevention and management. Dig Surg. 2001;18:260–73. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe M. Acrolein synthesis from glycerol in hot-compressed water. Bioresour Technol. 2007;98:1285–90. doi: 10.1016/j.biortech.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Yazdani SS, Gonzalez R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol. 2007;18:213–19. doi: 10.1016/j.copbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Melero JA, VanGrieken R, Morales G, Paniagua M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel. Energy Fuels. 2007;21:1782–91. [Google Scholar]
- 13.Bellon JM, Serrano N, Rodriguez M, et al. Composite prostheses used to repair abdominal wall defects: physical or chemical adhesion barriers? J Biomed Mater Res B Appl Biomater. 2005;74:718–24. doi: 10.1002/jbm.b.30248. [DOI] [PubMed] [Google Scholar]
- 14.Bellon JM, Rodriguez M, Garcia-Honduvilla N, et al. Peritoneal effects of prosthetic meshes used to repair abdominal wall defects: monitoring adhesions by sequential laparoscopy. J Laparoendosc Adv Surg Tech A. 2007;17:160–66. doi: 10.1089/lap.2006.0028. [DOI] [PubMed] [Google Scholar]
- 15.Pasieka JL. Anaplastic thyroid cancer. Curr Opin Oncol. 2003;15:78–83. doi: 10.1097/00001622-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Dupasquier CM, Dibrov E, Kneesh AL, et al. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am J Physiol Heart Circ Physiol. 2007;293:2394–402. doi: 10.1152/ajpheart.01104.2006. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Nair V, Jain S, Gupta YK. Evaluation of anti-inflammatory activity of plant lipids containing alpha-linolenic acid. Indian J Exp Biol. 2008;46:453–56. [PubMed] [Google Scholar]
- 18.Sankaran D, Bankovic-Calic N, Cahill L, et al. Late dietary intervention limits benefits of soy protein or flax oil in experimental polycystic kidney disease. Nephron Exp Nephrol. 2007;106:122–28. doi: 10.1159/000104836. [DOI] [PubMed] [Google Scholar]
- 19.Basch E, Bent S, Collins J, et al. Flax and flaxseed oil (Flax usitatissimum): a review by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2007;5:92–105. doi: 10.2310/7200.2007.005. [DOI] [PubMed] [Google Scholar]
- 20.Duran HE, Kuscu E, Zeyneloglu HB, et al. Lipiodol versus methylene blue for prevention of postsurgical adhesion formation in a rat model. Eur J Obstet Gynecol Reprod Biol. 2002;102:80–82. doi: 10.1016/s0301-2115(01)00571-1. [DOI] [PubMed] [Google Scholar]
- 21.Pata O, Yazici G, Apa DD, et al. The effect of inducible nitric oxide synthase on postoperative adhesion formation in rats. Eur J Obstet Gynecol Reprod Biol. 2004;117:64–69. doi: 10.1016/j.ejogrb.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Bender Ö, Yüney E, Çapar H, et al. Total tiroidektomi deneyimlerimiz. Endokrin Diyalog. 2004;1:15–18. [Google Scholar]
- 23.Müller PE, Kabus S, Robens E, Spelsberg F. Indications, risks and acceptance oftotal thyroidectomy for multinodular benign goiter. Surg Today. 2001;31:958–62. doi: 10.1007/s005950170002. [DOI] [PubMed] [Google Scholar]
- 24.Gough IR, Wilkinson D. Total thyroidectomy for management of thyroid disease. World J Surg. 2000;24:962–65. doi: 10.1007/s002680010158. [DOI] [PubMed] [Google Scholar]
- 25.Zambudio AR, Rodriguez J, Riquelme J, et al. Prospective study of postoperative complications after total thyroidectomy formultinodular goiters by surgeons with experience in endocrine surgery. Ann Surg. 2004;240:18–25. doi: 10.1097/01.sla.0000129357.58265.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson DB, Staren ED, Prinz RA. Thyroid reoperations: Indications and risks. Am Surg. 1998;64:674–78. [PubMed] [Google Scholar]
- 27.Dener C. Complication rates after operations for benign thyroid disease. Acta Otolaryngol. 2002;122:679–83. doi: 10.1080/000164802320396394. [DOI] [PubMed] [Google Scholar]
- 28.Diaconescu MR, Glod M, Costea I, et al. Reoperations of the thyroid gland. Chirurgia. 2007;102:297–302. [PubMed] [Google Scholar]
- 29.Giles Y, Boztepe H, Terzioglu T, Tezelman S. The advantage of total thyroidectomy to avoid reoperation for incidental thyroid cancer in multinodular goiter. Arch Surg. 2004;139:179–82. doi: 10.1001/archsurg.139.2.179. [DOI] [PubMed] [Google Scholar]
- 30.Makay O, Unalp O, Icoz G, et al. Completion thyroidectomy for thyroid cancer. Acta Chir Belg. 2006;106:528–31. doi: 10.1080/00015458.2006.11679945. [DOI] [PubMed] [Google Scholar]
- 31.Vaiman M, Nagibin A, Olevson J. Complications in primary and completed thyroidectomy. Surg Today. 2010;40:114–18. doi: 10.1007/s00595-008-4027-9. [DOI] [PubMed] [Google Scholar]
- 32.Swain CT. The Heritage of the Thyroid. In: Brawerman LE, Utiger RD, editors. The Thyroid. 7th ed. New York: Lippincott-Raven; 1996. pp. 2–5. [Google Scholar]
- 33.Osmólski A, Frenkiel Z, Osmólski R. Complications in surgical treatment of thyroid diseases. Otolaryngol Pol. 2006;60:165–70. [PubMed] [Google Scholar]
- 34.Yigit O, Uslu Coskun B, et al. Efficacy of anti-adhesive barriers in secondary thyroidectomy: an experimental study. Laryngoscope. 2004;114:1668–73. doi: 10.1097/00005537-200409000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Cipe G, Köksal HM, Yıldırım S, et al. Efficacy of hyaluronic acid – carboxymethyl cellulose membrane (Seprafilm®) and polylactic acid barrier film (Surgiwrap™) for the prevention of adhesions after thyroid surgery: an experimental model. Turk J Med Sci. 2011;41:73–79. [Google Scholar]
- 35.Park WS, Chung YS, Lee KE, et al. Anti-adhesive effect and safety of sodium hyaluronate and sodium carboxymethyl cellulose solution in thyroid surgery. Asian J Surg. 2010;33:25–30. doi: 10.1016/S1015-9584(10)60005-X. [DOI] [PubMed] [Google Scholar]
- 37.Aysan E, Bektaş H, Kaygusuz A, Huq GE. Eficacy of flax oil in peventing peritoneal adhesions. Eur Surg. 2009;41:66–71. [Google Scholar]
- 38.Aysan E, Bektas H, Kaygusuz A. Efficacy of glycerol in preventing postoperative peritoneal adhesions. J Obstet Gynaecol Res. 2010;36:639–45. doi: 10.1111/j.1447-0756.2010.01168.x. [DOI] [PubMed] [Google Scholar]


