Abstract
Background
Nasal saline irrigation is a safe treatment for chronic rhinosinusitis; however, its effect on olfaction is unclear. Cyclic adenosine monophosphate (cAMP) is a key second messenger in the mechanism of olfaction and has been shown to be associated with smell function. In animal studies, olfactory cilia may be harvested by simple saline preparations. This study aimed to characterize the effect of nasal saline irrigation on smell function.
Methods
Volunteers with normal olfaction were randomized into a control or irrigation cohort. In the initial appointment, subjects completed a University of Pennsylvania Smell Identification Test (UPSIT) and nasal samples were obtained by 2 methods, the nasal curette and cytobrush. The irrigation cohort performed daily nasal saline irrigations. Both cohorts then returned in 1 week. The UPSIT and nasal cell collection were repeated, and each subject completed a subjective olfactory transition scale. Nasal samples were processed for cAMP levels using a commercial assay.
Results
32 subjects were enrolled and randomized into each cohort. Control and post-irrigation mean UPSIT scores were 36.8 and 36.7 (P=0.48). No subjects reported a subjective smell loss. Ten pairs of nasal samples were assayed. Using the curette, control and post-irrigation cAMP levels were 509 and 490 fmol/(mg/ml), respectively (p=0.94). Using the cytobrush, respective cAMP levels were 424 and 449 fmol/(mg/ml), respectively (p=0.94).
Conclusion
Nasal saline irrigation has no subjective or objective effect on olfaction. It also does not appear to affect cAMP levels, a potential marker of smell function.
Keywords: irrigations, olfaction, UPSIT
Introduction
Nasal saline irrigation plays an important role in the adjuvant management of chronic rhinosinusitis and allergic rhinitis. Research studies show that nasal saline irrigations can actually improve the symptoms of these two diseases.1,2,3 Nasal saline irrigations are well tolerated, with reports of only infrequent mild side effects and extremely rare severe adverse events.3,4 Despite these infrequent side effects, there are no known clinical studies on the effect of nasal saline irrigation on olfaction. Our interest stemmed from animal studies on olfactory cilia, which are critical to our understanding of human and animal olfactory function. In animal models, cilia may be harvested by hypertonic saline or calcium chloride preparations5,6. This brought into question the potential effects of nasal saline irrigation on human olfactory cilia and hence, olfactory function.
Animal studies examining olfactory cilia show that cyclic adenosine monophosphate (cAMP) is an important second messenger in the mechanism of olfaction7. Clinical studies show that cAMP levels relate to olfactory function and may therefore serve as a potential objective marker of olfaction8.
The purpose of this study is to evaluate the effect of nasal saline irrigation on human olfactory function using the University of Pennsylvania Smell Identification Test (UPSIT) and nasal cAMP levels9.
Methods
This was a prospective, randomized controlled trial approved by the University of Washington Institutional Review Board. Thirty-two healthy volunteers with self-reported normal olfaction were recruited. The inclusion criteria were as follows: 1) Healthy individuals with self-reported normal smell function; and 2) Age greater than 18. Volunteers were excluded from enrollment for any of the following reasons: 1) unable to give informed consent or complete self-administered questionnaires written in English because of cognitive impairment, language barriers, or severe medical conditions; 2) allergy to Lidocaine; 3) active sino-nasal disease; 4) previous nasal or sinus surgery; 5) currently smoking or using other smoked or inhaled drugs; and 6) pregnant or planning to become pregnant.
We aimed to recruit a total of 32 subjects, 16 in each cohort. This was derived from a power calculation based on the UPSIT using the following parameters: an effect size of 4, standard deviation of 4.1, power of 80%, and α = 0.05%10,11.
Two instruments were used to measure olfactory function: the UPSIT and a transition scale. The UPSIT is a validated 40-item scratch-and-sniff test. The transition scale asks subjects if their smell has improved, worsened, or remained the same.
All subjects were scheduled for two appointments. Block randomization with random block sizes was used to generate assignments. The assignments were revealed to the primary author in an envelope during the initial visit. Half of the subjects was assigned to use nasal saline irrigation (240ml delivered with NeilMed Sinus Rinse kit) once daily for 1 week. All subjects randomized into the control cohort had no additional intervention. All subjects then returned for a follow-up appointment one week later. During each appointment, subjects completed an UPSIT and underwent nasal cell collection with 2 comfortable methods, the nasal curette and cytobrush. Both are established methods used to collect nasal specimens for cytologic studies12,13. At follow-up, subjects also completed a subjective transition scale regarding their smell function.
Nasal cell collections were performed by the same operator (JL) using the same protocol at both appointments. The procedure began with anterior rhinoscopy to rule out any nasal pathology. A mixture of Afrin and 4% topical lidocaine was sprayed into each nostril to decongest and anesthetize the nasal mucosa. A small cotton ball soaked with 4% lidocaine was then placed into the anterior nasal cavity for 5 minutes to further anesthetize the mucosa. Samples were obtained either from the middle or inferior turbinate depending on the extent of visualization on anterior rhinoscopy. Each sample was placed on dry ice immediately after collection.
Cytobrush and curette samples were resuspended in 500 μL of Phosphate Buffered Saline (PBS) with 0.5 mM isobutyl-methlyxanthine(IBMX), a general PDE inhibitor that inhibits most PDE’s. The cells were disrupted using a sonicator over two 30 second cycles, interrupted by incubation on ice. After sonication, a 50ul aliquot was removed and set aside for determination of protein concentration. Two volumes of cool 100% ethanol were added for protein precipitation. The samples were placed on ice for 10 minutes and then centrifuged at 2000g at 4°C for 10 minutes. The supernatant was transferred to a new Eppendorf tube and allowed to dry overnight at 55°C. The dried supernatant was resuspended in 320ul of an assay buffer and cAMP concentrations were determined using an Amersham cAMP Biotrak Enzymeimmunoassay (EIA) 96-well system (catalog number RPN2251).
Protein concentration was determined using the 50ul aliquot after sonication. This was determined using a spectrophotometric assay in a BioTek Epoch microplate reader.
All data were maintained in an Excel spreadsheet. All cAMP concentrations (fmol) were standardized with the total protein concentration (mg/ml) of each sample. Statistical analyses were performed using Stata software. A Fischer’s test was used to compare demographics. A Wilcoxon test was used to compare UPSIT scores and cAMP levels between cohorts and within the irrigation cohort.
Results
A total of 32 subjects with self-reported normal olfaction were enrolled into the study. Sixteen were randomized into the irrigation cohort and 16 into the control cohort. Due to technical and equipment issues during sample processing, cAMP results are reported for 10 pairs of curette and cytobrush samples. Three subjects were found to have olfactory dysfunction on the UPSIT, 1 in the control and 2 in the irrigation cohort. Demographic data for each cohort are listed in table 1.
Table 1.
Demographic information.
| Control (N=16) | Irrigation (N=16) | p-value | |
|---|---|---|---|
| Mean age | 34.6 (23–60) | 34.8 (23–57) | 0.94 |
| Gender (% female) | 6 (38%) | 10 (63%) | 0.29 |
| Ethnicity (% Caucasian) | 13 (81%) | 9 (56%) | 0.25 |
All subjects (32) returned for their follow-up appointment. All subjects had normal nasal exams and had no olfactory-related co-morbidities such as neurodegenerative diseases. There was 1 case of self-limited epistaxis after nasal cell collection. All other subjects tolerated the procedure well and had no complications.
All but one subject in the irrigation cohort performed saline irrigations for all 7 days. None of the subjects in the irrigation cohort reported a subjective smell loss on the transition scale. Two subjects in the irrigation cohort reported a subjective improvement in smell.
Baseline mean UPSIT scores for the control and irrigation cohorts were 36.3 and 36.4 (p=0.96), respectively (Table 2). Baseline curette mean cAMP levels were 509 and 536, respectively (p=0.88). Baseline cytobrush mean cAMP levels were 424 and 523, respectively (p=0.36).
Table 2.
Baseline mean and standard error of University of Pennsylvania Smell Inventory Test (UPSIT) scores and cyclic adenosine monophosphate (cAMP) levels for the curette and cytobrush in each cohort.
| Control | Pre-Irrigation | p-value | |
|---|---|---|---|
| UPSIT score (N=16) | 36.3 ± 0.5 | 36.4 ± 0.5 | 0.96 |
| Curette cAMP (N=10) | 521 ± 33 | 536 ± 39 | 0.88 |
| Cytobrush cAMP (N=10) | 456 ± 42 | 523 ± 50 | 0.36 |
There was no significant difference in UPSIT scores (36.8 for control, 36.7 for post-irrigation) between the two cohorts at 1 week follow-up (Table 3). Mean cAMP levels were not significantly different between the two cohorts. Curette mean cAMP levels were 509 and 490 for the control and irrigation cohorts, respectively (p=0.94). Cytobrush mean cAMP levels were 424 and 449, respectively (p=0.94). These cAMP results are also illustrated in figure 1.
Table 3.
Mean UPSIT scores and cAMP levels with standard error for the curette and cytobrush at 1week follow-up stratified by each cohort.
| Control | Post-Irrigation | p-value | |
|---|---|---|---|
| UPSIT score (N=16) | 36.8 ± 0.7 | 36.7 ± 0.4 | 0.48 |
| Curette cAMP (N=10) | 509 ± 38 | 490 ± 32 | 0.94 |
| Cytobrush cAMP (N=10) | 424 ± 36 | 449 ± 63 | 0.94 |
FIGURE 1.
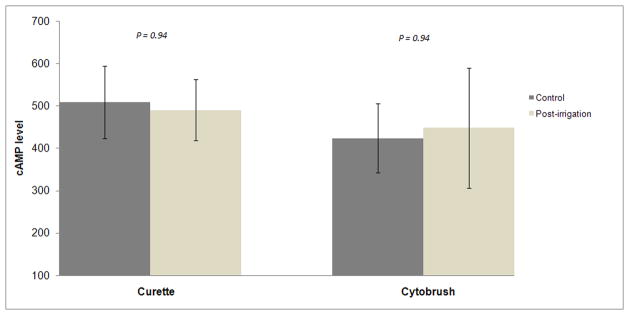
Bar graph comparing control and irrigation cohort cAMP levels stratified by curette and cytobrush collections. The error bars indicate 95% confidence intervals. cAMP = cyclic adenosine monophosphate.
When comparing pre-irrigation and post-irrigation values within the irrigation cohort (Table 4), mean UPSIT scores were 36.4 and 36.7, respectively (p=0.52). Using the curette, mean cAMP levels were 536 and 490, respectively (p=0.11). Using the cytobrush, mean cAMP levels were 523 and 449, respectively (p=0.09).
Table 4.
Mean UPSIT scores and cAMP levels with standard error using the curette and cytobrush within the irrigation cohort.
| Pre-irrigation | Post-irrigation | p-value | |
|---|---|---|---|
| UPSIT score (N=16) | 36.4 ± 0.5 | 36.7 ± 0.4 | 0.52 |
| Curette cAMP (N=10) | 536 ± 39 | 490 ± 32 | 0.11 |
| Cytobrush cAMP (N=10) | 523 ± 50 | 449 ± 63 | 0.09 |
Discussion
This is the first known prospective randomized trial showing that short-term nasal saline irrigation has no detrimental effects on olfaction. There was no subjective smell loss in subjects after daily irrigation. In fact, 2 subjects experienced a subjective improvement in smell function. The primary measure of smell function in this study, the UPSIT score, remained essentially unchanged after 1 week of daily nasal saline irrigation. There was no significant difference in cAMP levels between each cohort or within the irrigation cohort.
Randomization appeared to be adequate. The 2 cohorts had comparable demographics and there was no statistically significant difference in baseline UPSIT scores and cAMP levels.
Reported side effects of nasal saline irrigation in the literature include ear fullness, nasal discomfort, and epistaxis1,2,14. There have been no known links or reports of olfactory dysfunction and the results of this study confirm the safety of nasal saline irrigation.
The downward trend of cAMP levels within the irrigation cohort is likely insignificant. It has been shown that even partial resection of the superior turbinate does not reliably affect olfaction15. Perhaps even if nasal saline is able to displace olfactory cilia, it does not appear to cause a significant clinical change.
At this time, there are no validated methods to collect nasal samples to determine cAMP levels. The methods used in this study determine intracellular cAMP levels and are undergoing further investigation in a separate study. Olfactory cilia have been identified as inferior as the anterior middle turbinate, but their presence on the inferior turbinate is still unclear16. The clinical significance and quantification of cAMP levels in olfactory function is still an emerging concept and requires more investigation.
The main strength of this study is its prospective, randomized design. Other strengths include a zero drop-out rate and an adequate age range in each cohort. The primary weaknesses of this study are small sample size, limited power to detect a small difference in UPSIT scores, and limited duration of nasal saline irrigation. Other potential short-comings include lack of blinding and single institution design.
In conclusion, although olfactory cilia can be harvested by salt preparations in animal models, it does not appear to have any subjective or clinically significant effect on human olfaction with short-term use.
Acknowledgments
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 RR025015 (GED). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial disclosures: This project was supported by National Institutes of Health grants from several authors: KL2 RR025015 (GED), RO1 DC004156 (DRS), T32 DC000018-27 (JJL), and TL1 RR025016 (ASH)
References
- 1.Robago D, Zgierska A, Mundt M, et al. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. J Fam Pract. 2002;51:1049–1055. [PubMed] [Google Scholar]
- 2.Pynnonen MA, Mukerji SS, Kim HM, et al. Nasal saline for chronic sinonasal symptoms. Arch Otolaryngol Head Neck Surg. 2007;133:1115–1120. doi: 10.1001/archotol.133.11.1115. [DOI] [PubMed] [Google Scholar]
- 3.Khianey R, Oppenheimer J. Is nasal saline irrigation all it is cracked up to be? Ann Allergy Asthma Immunol. 2012;109:20–28. doi: 10.1016/j.anai.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 4.U.S. food and drug administration. [Accessed 3/12/2013];Is rinsing your sinuses safe? Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm316375.htm.
- 5.Linck RW. Comparative isolation of cilia and flagella from the Lamellibranch Mollusc, Aequipecten Irradians. J Cell Sci. 1973;12:345–367. doi: 10.1242/jcs.12.2.345. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Lancet D. Membrane proteins unique to vertebrate olfactory cilia: Candidates for sensory receptor molecules. Proc Natl Acad Sci. 1984;81:1859–1863. doi: 10.1073/pnas.81.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- 8.Henkin RI, Velicu I. cAMP and cGMP in nasal mucus related to severity of smell loss in patients with smell dysfunction. Clin Invest Med. 2008;31:E78–E84. doi: 10.25011/cim.v31i2.3367. [DOI] [PubMed] [Google Scholar]
- 9.Doty R, Shaman P, Kimmelman C, et al. University of Pennsylvania smell identification test: A rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 10.London B, Nabet B, Fisher AR, et al. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63:159–166. doi: 10.1002/ana.21293. [DOI] [PubMed] [Google Scholar]
- 11.Doty . Olfactory dysfunction in neurodegenerative diseases. In: Calhoun KH, Eibling DE, editors. Geriatric otolaryngology. 1. New York, NY: Taylor and Francis group; 2006. pp. 181–193. [Google Scholar]
- 12.Lin RY, Clarin E, Lee M, et al. Patterns of nasal eosinophilia in allergy clinic patients as determined by swab and curette sampling. Allergy Asthma Proc. 1997;18:221–226. doi: 10.2500/108854197778594089. [DOI] [PubMed] [Google Scholar]
- 13.Lin RY, Nahal A, Lee, Menikoff H. Cytologic distinctions between clinical groups using curette-probe compared to cytology brush. Ann Allergy Asthma Immunol. 2001;86:226–231. doi: 10.1016/S1081-1206(10)62696-8. [DOI] [PubMed] [Google Scholar]
- 14.Tamooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope. 2000;110:1189–1193. doi: 10.1097/00005537-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Say P, Leopold D, Cochran MT, et al. Resection of the inferior superior turbinate: Does it affect olfactory ability or contain olfactory neuronal tissue? Am J Rhino. 2004;18:157–160. [PubMed] [Google Scholar]
- 16.Leopold D, Hummel T, Schwob JE, et al. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–421. doi: 10.1097/00005537-200003000-00016. [DOI] [PubMed] [Google Scholar]


