Summary
Translational control of gene expression plays a key role in many biological processes. Consequently, the activity of the translation apparatus is under tight homeostatic control. eIF4E, the mRNA 5′ cap-binding protein, facilitates cap-dependent translation and is a major target for translational control. eIF4E activity is controlled by a family of repressor proteins, termed 4E-binding proteins (4E-BPs). Here, we describe the surprising finding that despite the importance of eIF4E for translation, a drastic knockdown of eIF4E caused only minor reduction in translation. This conundrum can be explained by the finding that 4E-BP1 is degraded in eIF4E-knockdown cells. Hypophosphorylated 4E-BP1, which binds to eIF4E, is degraded, whereas hyperphosphorylated 4E-BP1 is refractory to degradation. We identified the KLHL25-CUL3 complex as the E3 ubiquitin ligase, which targets hypophosphorylated 4E-BP1. Thus, the activity of eIF4E is under homeostatic control via the regulation of the levels of its repressor protein 4E-BP1 through ubiquitination.
Introduction
Translational control plays an important role in cell physiology, including cell growth, proliferation, differentiation, and metabolism. Translation is chiefly controlled at the initiation step in which the 40S ribosome is recruited to the mRNA. This process generally requires the presence of a cap structure (m7GpppN, where N is any nucleotide) at mRNA 5′ end and is facilitated by eukaryotic initiation factors (eIFs). Translation initiation commences with the binding of the eIF4F complex to the cap structure. eIF4F is comprised of the cap-binding protein, eIF4E, an ATP-dependent helicase, eIF4A, and a scaffolding protein, eIF4G. eIF4E abundance is much lower than other translation factors (Duncan et al., 1987 and Hiremath et al., 1985), consistent with its being a rate-limiting factor in translation initiation. Formation of the eIF4F complex is inhibited by members of a family of proteins termed eIF4E-binding proteins (4E-BPs) (Pause et al., 1994). There are three isoforms of 4E-BPs in mammals (4E-BP1, 4E-BP2, and 4E-BP3), whereas only one in flies (Lavoie et al., 1996). 4E-BP1 is the most abundant form in the majority tissues, with some exceptions, notably brain (Tsukiyama-Kohara et al., 2001). 4E-BPs are phosphoproteins, whose phosphorylation status changes in response to a bevy of inputs (Gingras et al., 1998). The hypophosphorylated 4E-BPs, but not the hyperphosphorylated forms, strongly bind to eIF4E, and consequently repress translation (Gingras et al., 1999). Under favorable growth conditions, which promote translation, cell growth, and proliferation, 4E-BPs become phosphorylated mainly as a consequence of the activation of mammalian target of rapamycin (mTOR) (Gingras et al., 1998 and Gingras et al., 2001b).
The stability of 4E-BPs was reported to be controlled in a few instances in mammals (Lin et al., 1995 and Elia et al., 2008) and sea urchins (Cormier et al., 2001), but the mechanisms have not been elucidated. The ubiquitin-proteasome pathway is the major player in the control of protein turnover. Ubiquitination is involved in major cellular processes, such as cell cycle, DNA repair, and transcriptional control. Here, we show that non-eIF4E-bound hypophosphorylated 4E-BP1 is degraded. We describe a molecular mechanism to explain this degradation via ubiquitination and the proteasome pathway, by demonstrating that the KLHL25-CUL3 ubiquitin ligase targets the hypophosphorylated 4E-BP1.
Results
Translation Is Marginally Impaired in eIF4E-Knockdown Cells
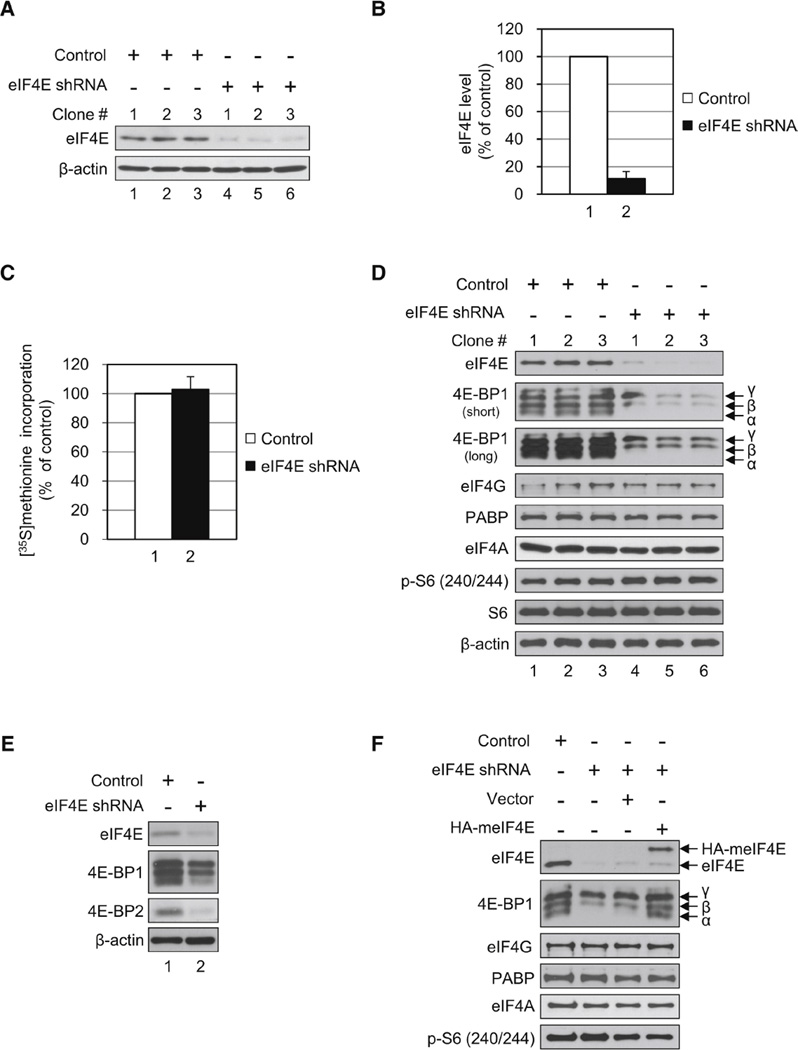
To study the physiological role of eIF4E in vivo, it was depleted from HeLa S3 cells using small hairpin RNA (shRNA) targeting eIF4E. Levels of eIF4E decreased to ~10% of control cells treated with scrambled shRNA in three different experiments (Figures 1A and 1B). To determine the effect of eIF4E decrease on protein synthesis, [35S]methionine metabolic labeling was performed. It was stunning that there was no change in protein synthesis in eIF4E-knockdown (KD) cells as compared to control cells (Figure 1C). The most appealing explanation for this unexpected finding is based on the precedence of a homeostatic compensatory mechanism of translation, which was reported for the poly(A)-binding protein (PABP) (Yoshida et al., 2006). In this case depletion of PABP in cells failed to cause inhibition of translation because the PABP repressor, PABP-interacting protein 2A (PAIP2A), was concomitantly degraded by the proteasome system. To investigate whether a similar mechanism applies to eIF4E-KD cells, we examined the levels of the eIF4E repressor proteins, 4E-BP1 and 4E-BP2, which constitute the bulk of 4E-BP family (Poulin et al., 1998). In addition we examined other translation factors, such as eIF4G, eIF4A, and PABP. The phosphorylation of ribosomal protein S6 (rpS6) was monitored as a readout of mTOR activity. Although there was no change in protein levels of eIF4G, eIF4A, and PABP, a dramatic decrease in 4E-BP1 was evident. In particular the hypophosphorylated 4E-BP1 (α form) was barely detectable in eIF4E-KD cells (Figure 1D). Furthermore, 4E-BP2 was also decreased in eIF4E-KD cells (Figure 1E). 4E-BP2 might have a smaller effect on translation because 4E-BP1 is the major isoform in HeLa S3 cells (data not shown). These results are consistent with the notion that translation is not decreased despite the strong reduction in eIF4E levels because the eIF4E repressors are also dramatically reduced. rpS6 phosphorylation was not affected by the eIF4E decrease, demonstrating that the disappearance of the hypophosphorylated 4E-BP1 was not due to activation of mTOR activity. To further support these conclusions, we rescued 4E-BP1 by expressing HA-tagged mouse eIF4E (meIF4E) that is resistant to KD by eIF4E shRNA. Expression of HA-tagged meIF4E mitigated the decrease in 4E-BP1, and completely averted the elimination of the hypophosphorylated 4E-BP1 (Figure 1F, compare lane 4 to lanes 1–3) without any change in the phosphorylation of rpS6. These results demonstrate that the decrease of eIF4E is the cause of the disappearance of the hypophosphorylated 4E-BP1.
Figure 1.
Translational Homeostasis in eIF4E-KD Cells Occurs Concomitantly with the Disappearance of the Hypophosphorylated 4E-BP1. (A) Control HeLa S3 cells expressing a scrambled shRNA sequence of eIF4E and cells expressing shRNA targeting eIF4E (eIF4E-KD) were generated. eIF4E and β -actin levels were determined by western blotting. (B) Quantification of KD levels of eIF4E from (A). eIF4E band intensities were measured using NIH ImageJ and normalized against β-actin. The value in control was set as 100%, and data are mean ± SD. (C) Effect of eIF4E KD on protein synthesis. Cells were incubated with [35S]methionine (10 µCi/ml) for 30 min. Radioactivity incorporated into 5% trichloroacetic acid-precipitated material was measured in a scintillation counter. The value in control was set as 100%, and data are mean ± SD of three separate experiments. (D) Protein levels of translation factors in eIF4E-KD as compared to control cells. Protein levels of the indicated proteins were analyzed along with phosphorylated rpS6 by western blotting. Short and long refer to exposure time against an X-ray film. (E) 4E-BP2 level is decreased in eIF4E-KD cells. 4E-BP2 was analyzed in control and eIF4E-KD cells as described above with an antibody specific for 4E-BP2. (F) Levels of the hypophosphorylated 4E-BP1 were maintained by expression of HA-tagged mouse eIF4E (meIF4E). A stable eIF4E-KD cell expressing HA-tagged meIF4E was generated, and protein levels of translation factors were analyzed by western blotting. See also Table S1.
Hypophosphorylated 4E-BP1 Is Degraded Rapidly in eIF4E-KD Cells
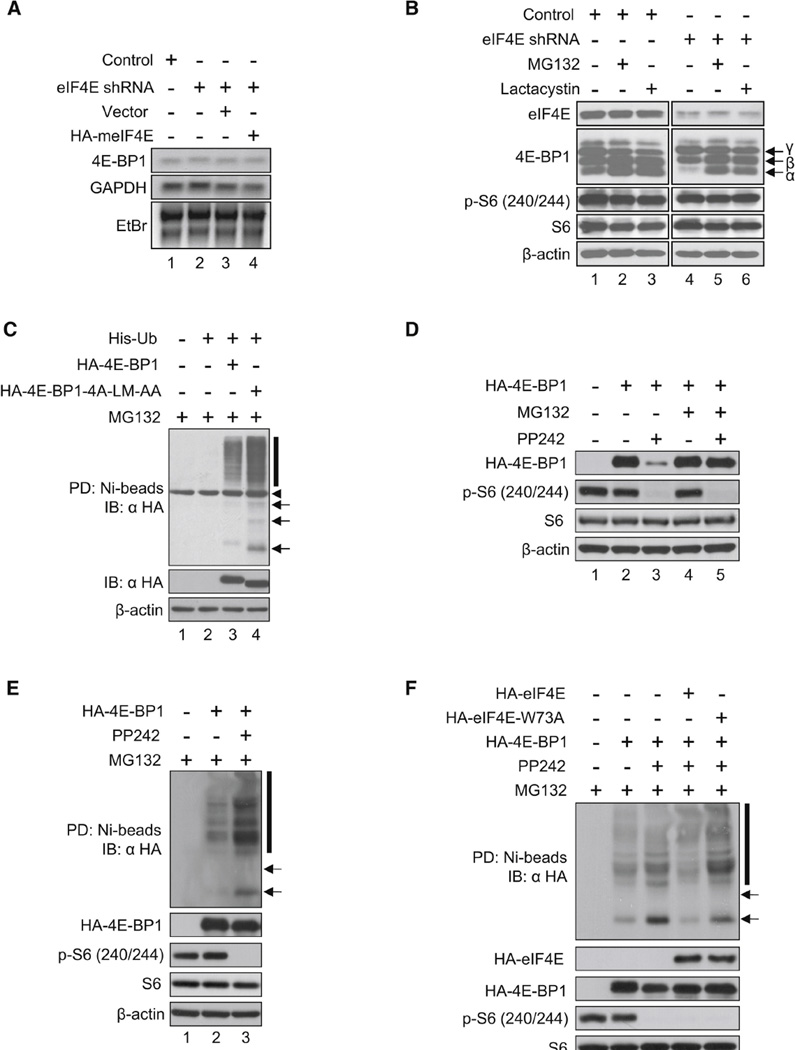
Next, it was pertinent to elucidate the mechanism by which a decrease in eIF4E leads to reduction in 4E-BP1. There was no change in 4E-BP1 mRNA levels in eIF4E-KD cells as determined by northern blot analysis ( Figure 2A), indicating that the effect on 4E-BP1 must occur at the protein level. Because the signal was relatively weak, it was confirmed by quantitative reverse-transcription PCR (see Figure S1A available online). To explore the possibility that the dramatic reduction in the hypophosphorylated 4E-BP1 is due to degradation, cells were treated with proteasome inhibitors. Treatment with MG132 ( Figure 2B, lanes 2 and 5) or Lactacystin (lanes 3 and 6) completely protected the hypophosphorylated 4E-BP1 (4EBP1α) from degradation (compare lanes 5 and 6 to 4). It is thus highly likely that the hypophosphorylated 4E-BP1 is degraded in eIF4E-KD cells by the ubiquitin-proteasome pathway. To support this conclusion, HA-tagged 4E-BP1 and a nonphosphorylatable form of 4E-BP1 were transfected into HEK293 cells; the mutant (4E-BP1-4A-LM-AA) contained four threonine and serine residues (Thr37, Thr46, Ser65, and Thr70, which are targets for phosphorylation) that were replaced by alanines (Rong et al., 2008). In addition the mutant protein contained a change (LM to AA) that inactivates the eIF4E-binding site (Mader et al., 1995). Thus, this mutant protein cannot bind to eIF4E and, in addition, cannot become phosphorylated. Ubiquitination of HA-4E-BP1 was detected by a pull-down experiment using His-ubiquitin (His-Ub) on a nickel column followed by western analysis with anti-HA antibody. Highmolecular ubiquitinated species of 4E-BP1 were detected with 4E-BP1 ( Figure 2C, lane 3). Importantly, a strong increase in ubiquitinated species was detected with 4E-BP1-4A-LM-AA (indicated by arrows and a vertical bar in Figure 2C, lane 4). These results demonstrate that the free form of the hypophosphorylated 4E-BP1 is unstable. To show that the phosphorylation status of 4E-BP1 is an important determinant of its stability, HA-4E-BP1 was expressed in eIF4E-KD cells, and cells were treated with an active site inhibitor of mTOR kinase, PP242 (Feldman et al., 2009 and García-Martínez et al., 2009). Hypophosphorylated 4E-BP1 levels were decreased in the presence of PP242 as compared to PP242-untreated cells ( Figure 2D, compare lane 3 to 2), and this decrease was rescued by MG132 treatment (compare lane 5 to 3). These results show that the hypophosphorylated 4E-BP1 generated by treatment with PP242 was degraded efficiently. To support these conclusions, raptor-KD cells were generated using eIF4E-KD cells, and HA-4E-BP1 was transiently expressed. Depletion of raptor diminished the phosphorylation of HA-4E-BP1 (Dowling et al., 2010), which resulted in a dramatic decrease of HA-4E-BP1 level in raptor-KD cells (Figure S1B, compare lane 5 to 2). HA-4E-BP1 was barely seen in the absence or presence of PP242 in raptor-KD cells (Figure S1B, lanes 5 and 6). Taken together, these results show that the hypophosphorylated 4E-BP1 is highly unstable when eIF4E amounts are reduced.
Figure 2.
Hypophosphorylated 4E-BP1 Is Degraded by the Ubiquitin-Proteasome Pathway. (A) 4E-BP1 mRNA levels in control and eIF4E-KD cells are shown. Total RNA was extracted from control, eIF4E-KD, and eIF4E-KD expressing the empty vector or HA-tagged meIF4E. 4E-BP1 mRNA levels were analyzed by northern blotting. GAPDH mRNA, 18S and 28S ribosomal RNAs detected by ethidium bromide (EtBr) staining served as loading controls. (B) Degradation of the hypophosphorylated 4E-BP1 is blocked by MG132 and Lactacystin. Control and eIF4E-KD cells were treated with DMSO, MG132 (20 µM), or Lactacystin (20 µM) for 3 hr. eIF4E, 4E-BP1 levels, and phosphorylated rpS6 were determined by western blotting. (C) eIF4E-unbound hypophosphorylated 4E-BP1 is highly ubiquitinated. 4E-BP1 and the eIF4E-unbound hypophosphorylated 4E-BP1 mimic mutant (4E-BP1-4A-LM-AA) were expressed in HEK293 cells together with His-Ub, and cells were treated with 20 µM MG132 for 6 hr. His-Ub proteins were pulled down with Ni-NTA agarose and analyzed by western blotting. Expression of exogenous proteins and β-actin in the lysates is shown in the panels below. Arrows and a bar indicate mono-ubiquitinated and polyubiquitinated 4E-BP1. An arrowhead indicates a nonspecific band. PD, pull-down; IB, immunoblot. (D) Hypophosphorylated 4E-BP1 levels are restored by MG132 treatment in cells treated with PP242. HA-4E-BP1 was transiently expressed in eIF4E-KD HEK293 cells, which were treated with 2.5 µM PP242 for 24 hr. Cells were cultured for the last 3 hr in the absence or presence of 20 µM MG132. 4E-BP1, rpS6, and β-actin levels were analyzed by western blotting. (E) Hypophosphorylated 4E-BP1 in PP242-treated cells is more ubiquitinated than in PP242-untreated cells. HA-4E-BP1 was expressed in 4E-BP DKO MEFs along with His-Ub, and cells were cultured in the absence or presence of PP242 for 24 hr, followed by in vitro ubiquitination assay. Pull-downed proteins were analyzed by western blotting. HA-4E-BP1, phosphorylated rpS6, and β-actin levels in cell lysates are shown below. Arrows and a bar indicate mono-ubiquitinated and polyubiquitinated 4E-BP1. (F) The interaction between 4E-BP1 and eIF4E prevents the hypophosphorylated 4E-BP1 from becoming ubiquitinated. HA-4E-BP1, HA-eIF4E, and HA-eIF4EW73A were expressed in 4E-BP DKO MEFs along with His-Ub, and an in vitro ubiquitination assay was performed as described in (C). See also Figure S1 and Table S2.
To investigate the role of 4E-BP1 phosphorylation in its ubiquitination, HA-4E-BP1 was expressed in 4E-BP double-knockout (DKO) mouse embryonic fibroblasts (MEFs), and its ubiquitination was monitored in the presence or absence of PP242. The hypophosphorylated 4E-BP1 was more ubiquitinated in the presence of PP242 than in nontreated cells (Figure 2E, compare lane 3 to 2). The ubiquitination of the free form of the hypophosphorylated 4E-BP1 and eIF4E-binding hypophosphorylated 4E-BP1 was also studied using an in vitro ubiquitination assay (Figure 2F). HA-4E-BP1 was expressed together with eIF4E (Figure 2F, lane 4) or eIF4E-W73A, which cannot bind to 4E-BP1 (Figure 2F, lane 5). 4E-BP1 ubiquitination was enhanced in the presence of PP242 (Figure 2F, compare lane 3 to 2), but this enhancement was reduced by HA-eIF4E expression (Figure 2F, compare lane 4 to 3). The expression of HA-eIF4E-W73A did not affect 4E-BP1 ubiquitination (Figure 2F, compare lane 5 to 3), indicating that the formation of the eIF4E/4E-BP1 complex prevents 4E-BP1 ubiquitination.
The Half-Life of the eIF4E-Unbound Hypophosphorylated 4E-BP1 Is Shortened
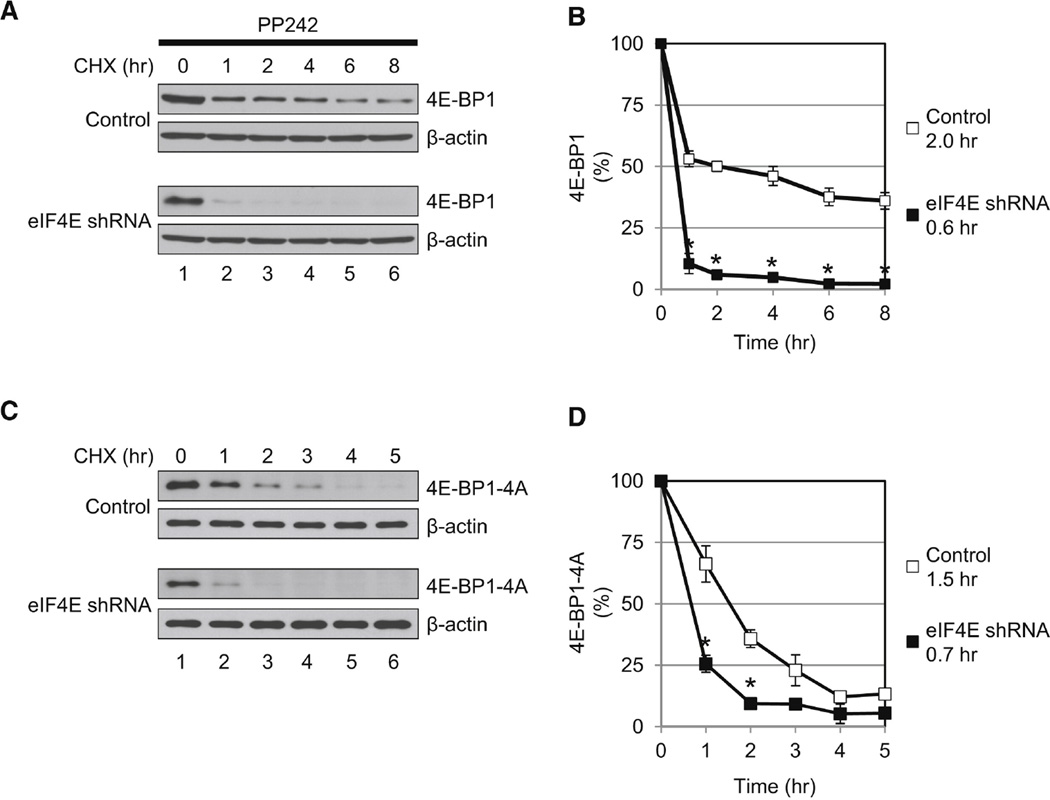
It is predicted that the half-life of the hypophosphorylated 4E-BP1 would be reduced in the absence of eIF4E. To investigate this, control and eIF4E-KD cells were treated with the mTOR inhibitor, PP242, and 4E-BP1 half-life was measured. The hypophosphorylated 4E-BP1 was degraded faster in eIF4E-KD as compared to control cells (a decrease from 2.0 to 0.6 hr) (Figures 3A and 3B), demonstrating that the decrease in eIF4E results in enhanced degradation of the endogenous hypophosphorylated 4E-BP1 because of reduced ability to form the eIF4E/4E-BP1 complex. Interestingly, the degradation of the hypophosphorylated 4E-BP1 in control cells followed a two-phase kinetics. This implies that there are two pools of hypophosphorylated 4E-BP1 in cells: one, which is unstable and is not bound to eIF4E; and a second, which is relatively stable and is eIF4E bound. In contrast the majority of the hypophosphorylated 4E-BP1 in eIF4E-KD cells is unstable eIF4E-unbound hypophosphorylated 4E-BP1. Next, control and eIF4E-KD cells expressing 4E-BP1-4A were generated to determine whether 4E-BP1-4A, as an exogenous nonphosphorylated form of 4E-BP1, also exhibits stability properties similar to endogenous hypophosphorylated 4E-BP1. 4E-BP1-4A exhibited a shorter half-life in eIF4E-KD as compared to control cells (a decrease from 1.5 to 0.7 hr) (Figures 3C and 3D). These data demonstrate that the free form of the hypophosphorylated 4E-BP1 is less stable than eIF4E-bound hypophosphorylated 4E-BP1.
Figure 3.
Half-Life of the Hypophosphorylated 4E-BP1 Is Reduced in eIF4E-KD Cells. (A) Half-life of the hypophosphorylated 4E-BP1 is shown. Control and eIF4E-KD cells were treated with 2.5 µM PP242 for 24 hr and then treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. 4E-BP1 and β-actin levels were analyzed by western blotting. (B) Quantitative analysis of the hypophosphorylated 4E-BP1 amounts in (A). The intensities of the bands were measured using NIH ImageJ and normalized against β-actin. The intensity of the band at 0 hr (lane 1) is set as 100%. The half-life of the hypophosphorylated 4E-BP1 in control (2.0 hr) and in eIF4E-KD cells (0.6 hr) is indicated. (C) Half-life of the nonphosphorylatable 4E-BP1 mutant, 4E-BP1-4A, is shortened in the absence of eIF4E. Control and eIF4E-KD cells expressing 4E-BP1-4A were generated. Cells were treated with CHX (100 µg/ml) and harvested at the indicated times. 4E-BP1-4A and β-actin levels were determined by western blotting. (D) Quantitative analysis of 4E-BP1-4A in (C). The intensities of the bands were normalized against β-actin. The intensity of the band at 0 hr is set as 100%. The half-life of 4E-BP1-4A in control (1.5 hr) and eIF4E-KD cells (0.7 hr) is indicated. The data shown in (A) and (C) are representative of three experiments. Quantitative data with mean ± SD are shown in (B) and (D). *p < 0.05 using an unpaired Student’s t test.
Proteins Involved in Degradation of the Hypophosphorylated 4E-BP1 by the Ubiquitin-Proteasome Pathway
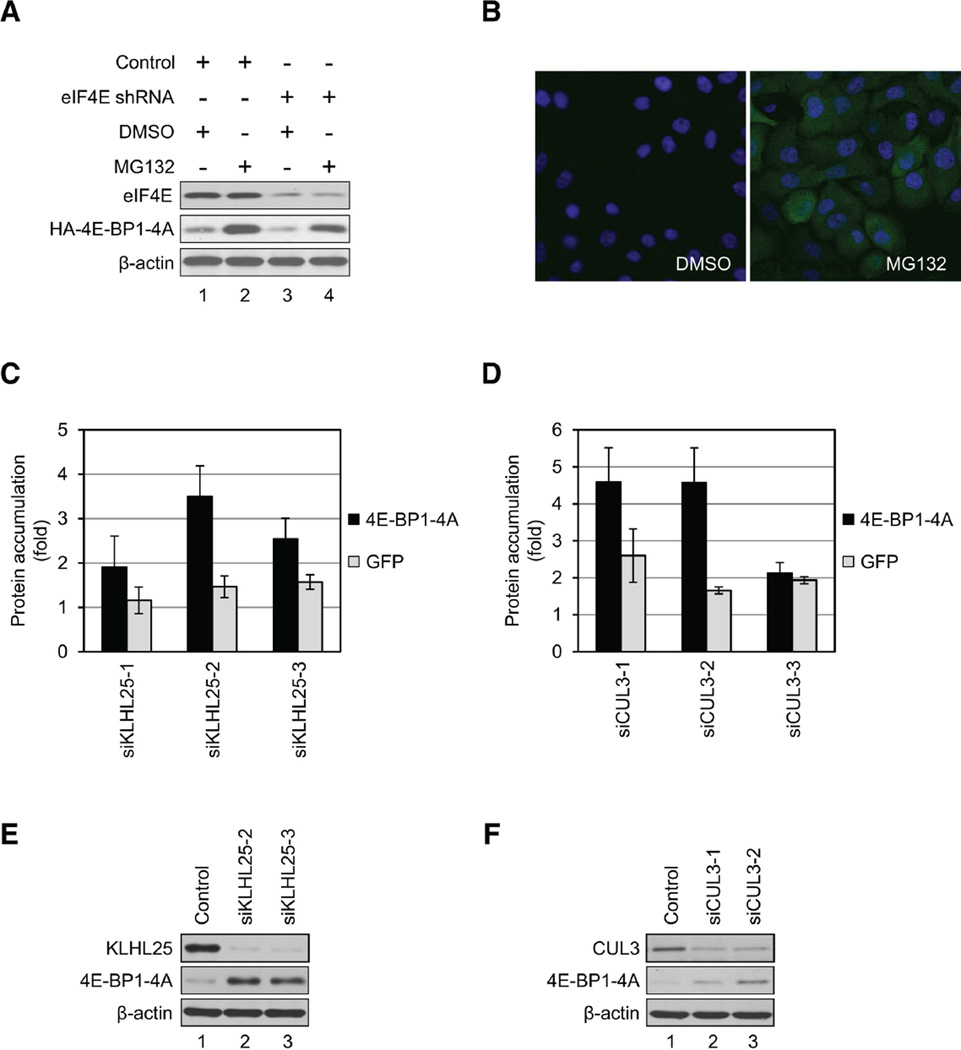
An siRNA library was used to screen 1,171 siRNAs directed against components of E3 ubiquitin ligase complexes, to identify the E3 ubiquitin ligase that targets the hypophosphorylated 4E-BP1. Control and eIF4E-KD cells expressing 4E-BP1-4A were treated with DMSO or 5 µM MG132 for 3 hr, followed by western blotting (Figure 4A) and immunocytochemistry (Figure 4B) for 4E-BP1-4A. Stabilization of 4E-BP1-4A by MG132 was demonstrated by western blotting in both control and eIF4E-KD cells (Figure 4A, compare lanes 2 and 4 to 1 and 3), and by immunofluorescence in eIF4E-KD cells (Figure 4B). For the high-content screening, 4E-BP1-4A accumulation was quantified by measuring the fluorescent signal, and the average fluorescence intensity was calculated and used to score for siRNA activity. MG132 treatment caused an approximately 4-fold increase in accumulation of 4E-BP1-4A, whereas a nonspecific siRNA had no effect on 4E-BP1-4A expression (Figure S2A). Three different siRNAs for every gene were used. Two library sets were screened in parallel to allow duplicate measurements per siRNA, and the average fold of 4E-BP1-4A accumulation was used for further data analysis (Figure S2B). siRNAs, which engendered more than 2-fold 4E-BP1-4A accumulation, were selected as positive hits, and genes that were scored positive by two separate siRNAs were selected for further analysis (Table S3). Genes with only one positive siRNA were excluded because of their possible nonspecific and/or off-target effects (Pei and Tuschl, 2006). Eight genes encoding proteasome subunits served as positive controls. We therefore repeated the experiments using siRNAs targeting 28 positive genes, of which 16 genes were confirmed to elicit more than 2-fold 4E-BP1-4A accumulation as shown in Table 1.
Figure 4.
KLHL25-CUL3 Ubiquitin Ligase Targets the Hypophosphorylated Form of 4E-BP1 for Degradation. (A) Generation of stable cell lines expressing 4E-BP1-4A using control or eIF4E-KD cells is shown. Cells were treated with DMSO or 5 µM MG132 for 3 hr. eIF4E, 4EBP1-4A, and β-actin levels were analyzed by western blotting. (B) 4E-BP1-4A was stabilized in eIF4E-KD cells by MG132 treatment. eIF4E-KD cells expressing 4E-BP1-4A were treated with either DMSO (left panel) or 5 mM MG132 (right panel) for 3 hr. Accumulation of 4E-BP1-4A was analyzed by immunofluorescence. Cells were fixed, and HA-4E-BP1-4A was determined with anti-HA antibody and Alexa Fluor 488 anti-rabbit IgG (green). Nuclei were stained with DAPI (blue). (C and D) eIF4E-KD cells expressing either 4E-BP1-4A or GFP were transfected with siRNAs targeting KLHL25 (C) or CUL3 (D). Accumulation of 4E-BP1-4A (black bars) and GFP (gray bars) was determined by microscopy. Each sample was normalized against samples treated with the nonspecific siRNAs. Data are mean ± SD of two separate experiments. (E and F) 4E-BP1-4A levels are restored by siRNAs targeting KLHL25 (E) and CUL3 (F). To confirm the results obtained by the high-content screening, the KD efficiencies of both KLHL25 (E) and CUL3 (F) and 4E-BP1-4A levels were determined by western blotting. See also Figure S2 and Tables S3 and S4.
Table 1.
Positive Genes Confirmed by siRNA Screening
| NCBI Gene Symbol | Entrez Gene ID | siRNA 1 | siRNA 2 | siRNA 3 |
|---|---|---|---|---|
| BTBD14A | 138151 | 1.1 | 2.4 | 2.3 |
| C4orf30 | 54876 | 2.8 | 0.5 | 2.1 |
| COPS5 | 10987 | 0.8 | 2.2 | 2.6 |
| CUL3 | 8452 | 4.6 | 4.6 | 2.1 |
| FBXO11 | 80204 | 2.6 | 2.0 | 2.9 |
| FBXO28 | 23219 | 2.7 | 2.4 | 1.3 |
| GNB2 | 2783 | 2.4 | 3.3 | 0.4 |
| KLHL25 | 64410 | 1.9 | 3.5 | 2.6 |
| PPP1R7 | 5510 | 2.0 | 3.8 | 1.9 |
| RCHY1 | 25898 | 3.2 | 2.2 | 1.6 |
| RNF25 | 64320 | 2.1 | 2.5 | 3.1 |
| TRIM61 | 391712 | 2.4 | 2.4 | 4.8 |
| TRIML1 | 339976 | 1.6 | 2.5 | 3.0 |
| UBAC2 | 337867 | 1.2 | 3.0 | 2.6 |
| UBR2 | 23304 | 2.3 | 3.1 | 1.1 |
| WSB1 | 26118 | 0.7 | 2.3 | 3.3 |
A total of 16 positive genes confirmed by siRNA screening is shown. 4E-BP1-4A accumulation (fold) by siRNAs targeting each gene are indicated.
The KLHL25-CUL3 E3 Ubiquitin Ligase Targets the Hypophosphorylated 4E-BP1
KLHL25 and CUL3 were selected from the 16 positive genes for further analysis because CUL3 exhibited the highest score, and KLHL25 is a BTB domain-containing protein, which binds to CUL3. There were two BTB domain-containing proteins among the 16 positive genes: KLHL25 and BTBD14A. Because siRNAs targeting BTBD14A exhibited an off-target effect, as determined by western blotting (Figures S2C and S2D), we chose to study the KLHL25-CUL3 complex as a potential candidate for E3 ubiquitin ligase targeting the hypophosphorylated 4E-BP1. CUL3 associates with a BTB domain-containing protein to form the E3 ubiquitin ligase complex (Furukawa et al., 2003, Geyer et al., 2003, Pintard et al., 2003 and Xu et al., 2003). We used siRNAs targeting KLHL25 and CUL3 (hereafter siKLHL25 and siCUL3, respectively) to demonstrate their role in 4E-BP1 ubiquitination. eIF4E-KD cells expressing GFP were used in parallel with eIF4E-KD cells expressing 4E-BP1-4A as control to demonstrate the specific siRNA effect on 4E-BP1-4A. All three siKLHL25s caused increased 4E-BP1-4A accumulation (1.9-, 3.5-, and 2.6-fold), whereas GFP accumulation was not significantly increased (Figure 4C), confirming their specificity. Two out of the three siCUL3s caused an increase in 4E-BP1-4A accumulation (Figure 4D). To further confirm the KD of siKLHL25s and siCUL3s, their corresponding proteins and 4E-BP1-4A protein levels were examined by western blotting. Increased 4E-BP1-4A levels (3.6-fold by siKLHL25-2, 3.2-fold by siKLHL25-3) coincided with decreased KLHL25 (Figure 4E, compare lanes 2 and 3 to 1). siKLHL25-1 failed to silence KLHL25 (data not shown). 4E-BP1-4A levels were also increased upon reduction of CUL3 levels by siCUL3-1 (2-fold) and siCUL3-2 (3.5-fold) (Figure 4F, compare lanes 2 and 3 to 1). These results demonstrate that KD of KLHL25 and CUL3 results in 4E-BP1-4A accumulation, thus suggesting that the KLHL25-CUL3 complex plays a key role in ubiquitination of the hypophosphorylated 4E-BP1.
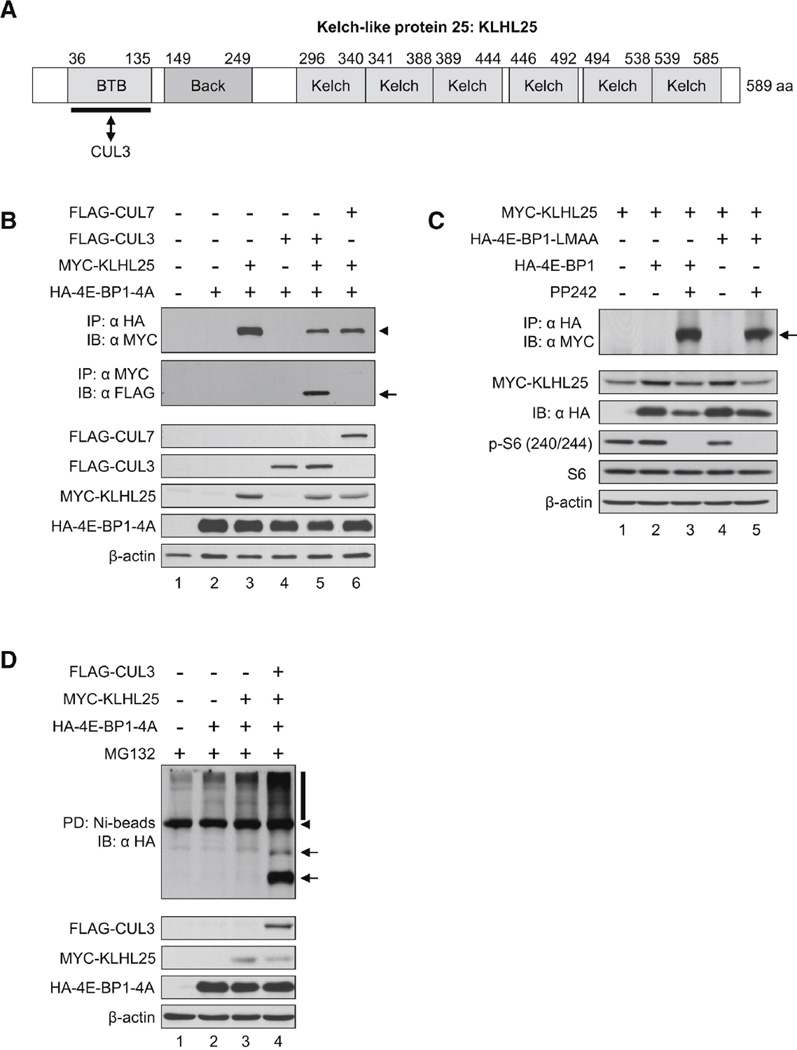
CUL3 is a scaffold protein that binds to both the BTB domain-containing proteins, such as KLHL25 and the RING finger protein (RNF). The BTB domain-containing proteins are substrate-specific adaptors and bind directly to CUL3 at the BTB domain (Figure 5A). To demonstrate the interactions between 4E-BP1, KLHL25 and CUL3 in vivo, HA-4E-BP1-4A, MYC-KLHL25, and FLAG-CUL3 were expressed in HEK293 cells. As a negative control, FLAG-CUL7, which does not bind to the BTB domain-containing protein, was also used. MYC-KLHL25 was immunoprecipitated using an anti-HA antibody, demonstrating an interaction between 4E-BP1-4A and KLHL25 (Figure 5B, lanes 3, 5, and 6, upper panel). FLAG-CUL3 was immunoprecipitated with KLHL25 (Figure 5B, middle panel, lane 5), demonstrating an interaction between KLHL25 and CUL3. To investigate the role of 4E-BP1 phosphorylation in the interaction between 4E-BP1 and KLHL25, HA-4E-BP1 was expressed along with MYC-KLHL25. 4E-BP1-LM-AA, which cannot bind to eIF4E, was also investigated to determine whether KLHL25 competes with eIF4E for binding to 4E-BP1 at the same site. The interaction between 4E-BP1 and KLHL25 was examined in the presence or absence of PP242. The hypophosphorylated 4E-BP1 strongly bound KLHL25 (Figure 5C, compare lane 3 to 2). 4E-BP1-LM-AA interacted with KLHL25, suggesting that KLHL25 does not bind to 4E-BP1 at the same site as eIF4E (Figure 5C, lane 5). Next, to investigate the role of KLHL25 and CUL3 in 4E-BP1 ubiquitination, HA-4E-BP1-4A, MYC-KLHL25, and FLAG-CUL3 were expressed in HEK293 cells, and an in vitro ubiquitination assay was performed. 4E-BP1-4A ubiquitination was enhanced by MYC-KLHL25 (Figure 5D, compare lane 3 to 2), and increased by coexpression of MYC-KLHL25 and FLAG-CUL3 (lane 4), clearly corroborating the role of the KLHL25-CUL3 E3 ubiquitin ligase in ubiquitination of hypophosphorylated 4E-BP1.
Figure 5.
KLHL25-CUL3 Complex Decreases the Stability of the Hypophosphorylated 4E-BP1. (A) A schematic diagram of KLHL25 is shown. The BTB domain, Back domain, and six repeats of the kelch motif are shown. The numbers above indicate amino acids. (B) KLHL25 interacts with 4E-BP1-4A and CUL3. HA-4EBP1-4A, MYC-KLHL25, FLAG-CUL3, or FLAG-CUL7 was expressed in HEK293 cells. Two days after transfection, cells were treated with 20 µM MG132 for 3 hr. Cell lysates (2 mg) were incubated with either anti-HA antibody (upper panel) or anti-MYC antibody (second panel from top). Immunoprecipitated proteins were analyzed by western blotting. Input proteins for immunoprecipitation (IP) are shown in the panels below. An arrowhead indicates immunoprecipitated protein by anti-HA antibody, and an arrow indicates immunoprecipitated protein by anti-MYC antibody. IB, immunoblot. (C) The hypophosphorylated 4E-BP1 in PP242-treated cells strongly interacts with KLHL25. HA-4E-BP1 and HA-4E-BP1-LM-AA were expressed together with MYCKLHL25 in HEK293 cells, which were cultured in the absence or presence of 2.5 mMPP242 for 24 hr. Cells were treated with 20 µM MG132 for 3 hr before sample collection. Cell lysate (4 mg) was incubated with anti-HA antibody, and immunoprecipitated proteins were analyzed by western blotting. Expression of HA-4E-BP1, HA-4E-BP1-LM-AA, MYC-KLHL25, β-actin, and the phosphorylation of rpS6 in cell lysates are shown below. An arrow indicates KLHL25 that interacts with the hypophosphorylated 4E-BP1. (D) KLHL25 and CUL3 promote ubiquitination of the hypophosphorylated 4E-BP1. HA-4E-BP1-4A, MYCKLHL25, and FLAG-CUL3 were expressed along with His-Ub, and an in vitro ubiquitination assay was performed as described above. Arrows and a bar indicate monoubiquitinated and polyubiquitinated HA-4E-BP1-4A. An arrowhead indicates a nonspecific band. Input HA-4EBP1-4A, MYC-KLHL25, FLAG-CUL3, and β-actin are shown in the panels below.
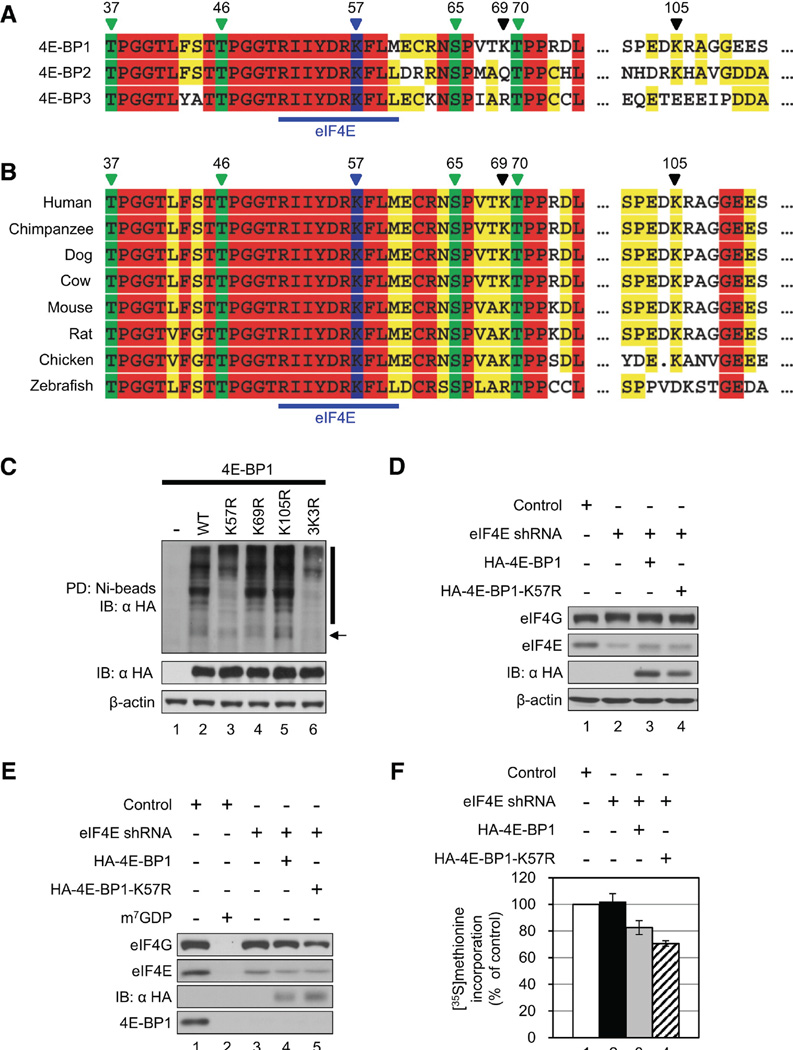
Lys57 Is a Potential Ubiquitination Site in 4E-BP1
There are three lysine residues in 4E-BP1: Lys57, Lys69, and Lys105 (Figure 6A). Only Lys57 is conserved among all three human 4E-BP isoforms. Importantly, Lys57 is located in the eIF4E-binding site. Lys57 is also highly conserved among vertebrates (Figure 6B), whereas the other two lysines are not, consistent with a role of Lys57 in ubiquitination of hypophosphorylated 4E-BP1. To examine which lysine residue is ubiquitinated, 4E-BP1 mutants in which each of three lysine residues was replaced with arginine were generated (K57R, K69R, and K105R), and tested in an in vitro ubiquitination assay. Also, a 4E-BP1 mutant whose all three lysine residues were replaced with arginine (3K3R) was used. The ubiquitination of 4E-BP1-K57R and 4E-BP1-3K3R, but not 4E-BP1-K69R and 4E-BP1-K105R, was reduced, demonstrating that Lys57 is the most likely ubiquitination site on 4E-BP1 (Figure 6C). To investigate the effect of nonubiquitinatable 4E-BP1 mutant in eIF4F complex formation and global protein synthesis, 4E-BP1 and 4E-BP1-K57R were overexpressed in control (Figure S3) and eIF4E-KD cells (Figure 6D) and assessed by m7GDP pull-down and [35S]methionine incorporation. In control cells, eIF4G bound to eIF4E less well following 4E-BP1-K57R overexpression than after 4E-BP1 overexpression (Figure S3B, compare lane 4 to 3). The overexpression of 4E-BP1-K57R in control cells inhibited global translation by ~19%, which is greater than the inhibition caused by overexpression of 4E-BP1 (~11%) (Figure S3C, compare bar 3 to 2). Consistently, 4E-BP1-K57R reduced cell proliferation by ~30% after 4 days; more than 4E-BP1 (~20%) in control cells (Figure S3D). The residual eIF4E in eIF4E-KD cells bound the same amount of eIF4G as eIF4E in control cells because of the lack of hypophosphorylated 4E-BP1 (Figure 6E, compare lane 3 to 1). 4E-BP1-K57R bound somewhat better to eIF4E than 4E-BP1, presumably because of the stabilized hypophosphorylated 4E-BP1, which resulted in stronger inhibition of the interaction between eIF4E and eIF4G than 4E-BP1 (Figure 6E, compare lane 5 to 4). In accordance with this result, translation was inhibited by overexpression of 4E-BP1-K57R to 70.6% ± 2.0% (Figure 6F, bar 4), which was greater than control cells (81.4% ± 4.7%) (Figure S3C, bar 3). 4E-BP1-K57R also impeded cell proliferation by ~49% after 4 days in eIF4E-KD cells (Figure S3E), which was greater than control cells (~30%) (Figure S3D). A similar result was observed using a bicistronic luciferase reporter construct containing the hepatitis C virus (HCV) internal ribosome entry site (IRES) (Figure S3F), where cap-dependent translation normalized to HCV IRES-mediated translation was inhibited by overexpression of 4E-BP1-K57R to 78.2% ± 0.4%. These data demonstrate the role of Lys57 in the stability of hypophosphorylated 4E-BP1 and the subsequent eIF4F complex formation and translational homeostasis.
Figure 6.
Lys57 Is the Most Probable Ubiquitination Site of 4E-BP1. (A) Amino acid sequence alignment of human 4E-BPs is shown. Four phosphorylation sites (Thr37, Thr46, Ser65, and Thr70) are marked in green, and the putative ubiquitination site (Lys57) is marked in blue. A blue bar indicates the eIF4E-binding site. Numbers above indicate amino acids. (B) Phylogenetic amino acid sequence alignment of 4E-BP1. (C) Lys57 is the likely ubiquitination site of 4E-BP1. HAtagged 4E-BP1, 4E-BP1-K57R, 4E-BP1-K69R, 4E-BP1-K105R, or 4E-BP1-3K3R was expressed in 4E-BP DKO MEFs along with His-Ub. An in vitro ubiquitination assay was performed as described before. Expression levels of HA-4E-BP1s and β-actin in cell lysates are shown below. An arrow and a bar indicate mono-ubiquitinated and polyubiquitinated 4E-BP1, respectively. (D) HA-4E-BP1 or HA-4E-BP1-K57R was transiently expressed in eIF4E-KD cells. eIF4G, eIF4E, HA-4E-BP1s, and b-actin levels were determined by western blotting. (E) eIF4F complex formation in cells expressing the nonubiquitinatable 4E-BP1. Cell lysates were subjected to m7GDP pull-down. One millimolar m7GDP was added as a competitor (lane 2). m7GDP-associated proteins were assessed by western blotting. (F) The effect of the nonubiquitinatable 4E-BP1 on protein synthesis is shown. Control (white), eIF4E-KD (black), eIF4E-KD cells expressing HA-4E-BP1 (gray) or HA-4EBP1-K57R (hatched) were subjected to [35S]methionine metabolic labeling. The value in control was set as 100%, and data are mean ± SD of three separate experiments. IB, immunoblot; PD, pull-down. See also Figure S3.
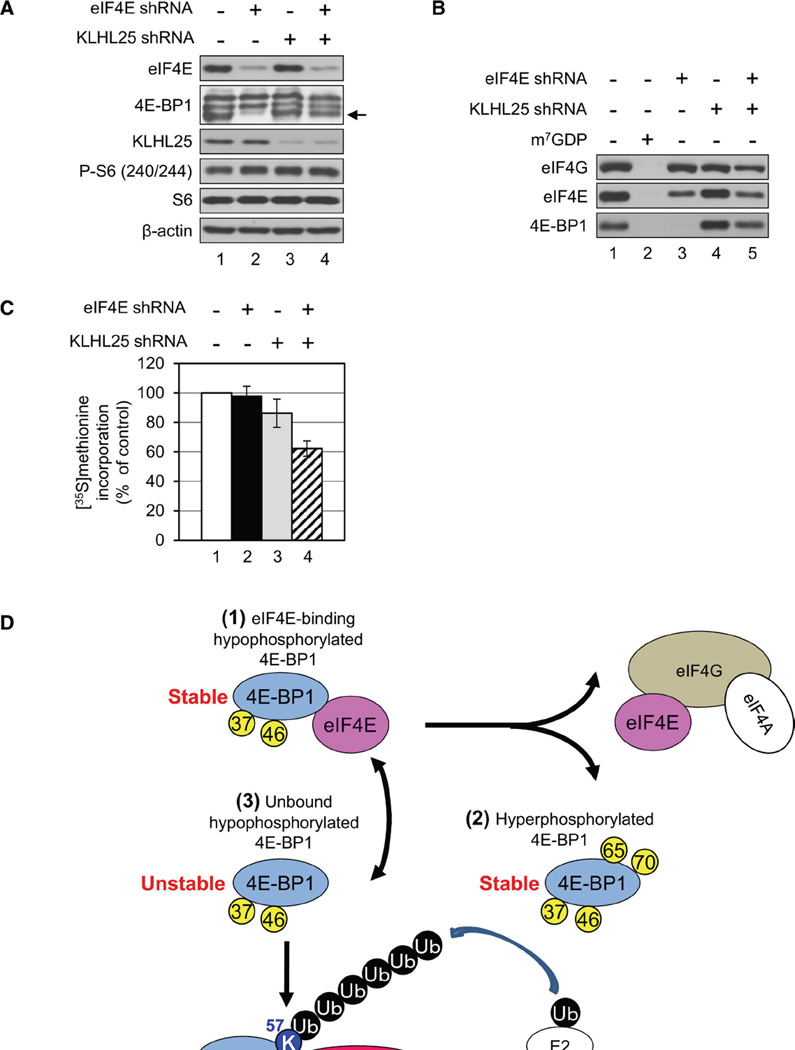
KLHL25 KD Results in Decreased Translation
To show that KLHL25 plays a role in translation, its amount was reduced in control and eF4E-KD cells using lentiviral shRNA. Hypophosphorylated 4E-BP1 levels were elevated in eIF4E-KLHL25-double-KD (DKD) cells (Figure 7A, lane 4, indicated by an arrow), which is consistent with the role of KLHL25 in the stability of hypophosphorylated 4E-BP1. Next, eIF4F complex formation was investigated by the m7GDP pull-down assay. eIF4G binding to eIF4E was slightly decreased (~12%) in KLHL25-KD cells as compared to control cells (Figure 7B, compare lane 4 to 1). However, eIF4G binding to eIF4E decreased significantly (~50%), whereas hypophosphorylated 4E-BP1 binding to eIF4E increased, in eIF4E-KLHL25-DKD cells as compared to eIF4E-KD cells (Figure 7B, compare lane 5 to 3). To investigate the effect of KLHL25 on translation, metabolic labeling of proteins with [35S]methionine was performed using control, eIF4E-KD, KLHL25-KD, and eIF4E-KLHL25-DKD cells. Protein synthesis in eIF4E-KLHL25-DKD cells was decreased to 62.3% ± 5.2% as compared to control cells, whereas a slight decrease in KLHL25-KD cells to 86.2% ± 9.6% was observed (Figure 7C). In accordance with this result, capdependent translation normalized against HCV IRES-mediated translation was also impeded in eIF4E-KLHL25-DKD cells to 71.8% ± 2.6% of control (Figure S4B). These results support the conclusion that the translational homeostasis in eIF4E-KD cells is due to the degradation of the hypophosphorylated 4E-BP1, which is mediated by KLHL25-CUL3 ubiquitin ligase.
Figure 7.
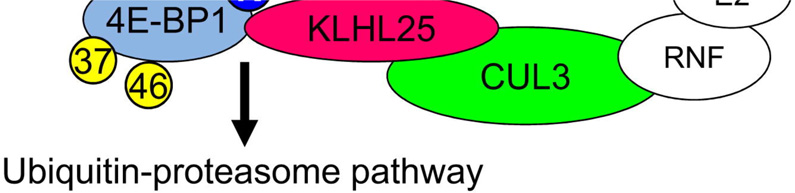
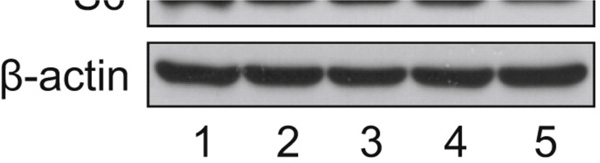
KLHL25 Silencing in eIF4E-KD Cells Inhibits Protein Synthesis, and the Molecular Mechanism of 4E-BP1 Stability. (A) Establishment of KLHL25-KD cells is shown. Control and eIF4E-KD cells were infected with lentivirus expressing control shRNA or shRNA against KLHL25. eIF4E, 4E-BP1, KLHL25, β-actin, and phosphorylated rpS6 were analyzed by western blotting. An arrow indicates the hypophosphorylated 4E-BP1. (B) eIF4F complex formation in control, eIF4E-KD, KLHL25-KD, and eIF4E-KLHL25-DKD cells. Cell lysates were subjected to m7GDP pull-down. One millimolar m7GDP was added as a competitor (lane 2). m7GDP-associated proteins were assessed by western blotting. (C) Total protein synthesis in KLHL25-KD cells is shown. Protein synthesis in control (white), eIF4E-KD (black), KLHL25-KD (gray), and eIF4E-KLHL25-DKD cells (hatched) was investigated by metabolic radiolabeling using [35S]methionine. Protein synthesis is shown as a percentage of the value in control cells. The average of the three different experiments with SD is shown. (D) Model of the molecular mechanism of 4E-BP1 stability is illustrated. eIF4E-bound hypophosphorylated 4E-BP1 (1) and the hyperphosphorylated 4E-BP1 (2) are stable. eIF4E-unbound hypophosphorylated 4E-BP1 (3) is unstable. It is degraded after ubiquitination by the KLHL25-CUL3 E3 ubiquitin ligase. See also Figure S4.
To investigate the possibility that there might be another compensatory mechanism in the absence of eIF4E, IRES-dependent translation, which does not require eIF4E, was analyzed in control and eIF4E-KD cells by cotransfection using monocistronic Firefly luciferase mRNA possessing HCV IRES along with Renilla luciferase mRNA (Figure S5A). HCV IRES-dependent translation, which is independent of eIF4F, was not affected in eIF4E-KD cells (Figure S5B). Control and eIF4E-KD cells were labeled with [35S]methionine, and cell lysates were analyzed by SDS-PAGE and autoradiography. No difference in the profiles of newly synthesized proteins was found (Figure S5C). It is therefore unlikely that IRES-mediated translation compensates for the deficit of cap-dependent translation in eIF4E-KD cells.
Discussion
In this study we demonstrate that hypophosphorylated 4E-BP1 is rapidly degraded by ubiquitination when eIF4E levels are reduced, thus serving as a homeostatic mechanism to maintain translation at levels commensurate with cell survival. Our findings have important implications for translational control under conditions in which eIF4E levels increase or decrease in physiological or pathological states in order to sustain cell survival. A striking example is cancer in which eIF4E amounts are elevated in a significant number of patients (30%), which is also often accompanied by an increase in 4E-BP1 (Braunstein et al., 2007, Martín et al., 2000 and Kremer et al., 2006). In prostate cancer, for example, eIF4E protein and in particular the hypophosphorylated 4E-BP1 form are increased (Kremer et al., 2006). These results are consistent with findings in NIH 3T3 cells in which we have shown earlier that 4E-BP1 becomes hypophosphorylated by overexpression of eIF4E (Khaleghpour et al., 1999).
It is significant that only the hypophosphorylated form of 4E-BP1 that cannot bind to eIF4E is a target for degradation. This indicates that eIF4E-bound hypophosphorylated 4E-BP1 (Figure 7D, state 1) and hyperphosphorylated 4E-BP1 (state 2) are stable, whereas the eIF4E-unbound hypophosphorylated 4E-BP1 (state 3) is unstable. The eIF4E/4E-BP1 complex is stable, suggesting that the KLHL25-CUL3 ubiquitin ligase cannot access the eIF4E-bound hypophosphorylated 4E-BP1 because of steric hindrance. Because the 4E-BP1-4A-LM-AA mutant, whose eIF4E-binding site is mutated, was ubiquitinated, the KLHL25-CUL3 ubiquitin ligase does not compete with eIF4E for binding to 4E-BP1 at the same site.
An E3 ubiquitin ligase attaches ubiquitin molecules to a lysine residue in its substrate. There are three lysines in 4E-BP1: Lys57, Lys69, and Lys105. Lys57 is evolutionarily conserved and located in the eIF4E-binding site (Arg51-Ile52-Ile53-Tyr54-Asp55-Arg56-Lys57-Phe58-Leu59-Met60). Lys57 is expected to be sequestered in the eIF4E/4E-BP1 complex (Gross et al., 2003), which would result in the stabilization of the eIF4E-bound 4E-BP1, whereas eIF4E-unbound hypophosphorylated 4E-BP1 would be susceptible to ubiquitination. 4E-BP1 is phosphorylated hierarchically. Thr37/Thr46 phosphorylation serves to prime Thr70 phosphorylation, and then Ser65 phosphorylation (Gingras et al., 2001a). Because the phosphorylation at Thr70 and Ser65 prevents 4E-BP1 from binding to eIF4E, a similar scenario might occur for the interaction between 4E-BP1 and KLHL25, which would result in stabilization of the hyperphosphorylated 4E-BP1.
Previous studies have reported contradicting findings concerning the stability of the phosphorylated versus unphosphorylated 4E-BP1. In sea urchin eggs, phosphorylation of 4E-BP occurs after fertilization resulting in its degradation (Cormier et al., 2001). In contrast, 4E-BP1 in rats becomes more stable when it is phosphorylated in response to insulin in adipocytes (Lin et al., 1995). However, Elia et al. reported that the hyperphosphorylated, rather than the hypophosphorylated, form of 4E-BP1 is ubiquitinated (Elia et al., 2008). There are a number of possible reasons why our study and that of Elia et al. reach seemingly different conclusions.Elia et al. (2008) observed an increase in the accumulation of the hypophosphorylated 4E-BP1 by MG132 treatment but concluded that MG132 treatment caused dephosphorylation of 4E-BP1. Indeed, the phosphorylation of rpS6 was inhibited by long incubation (16 hr) using MG132 (Elia et al., 2008), but such inhibition was not observed in our shorter incubation (<3 hr) (A.Y., unpublished data).
There is heightened interest in using antisense eIF4E in clinical trials in patients with cancer (Graff et al., 2007 and Graff et al., 2008). It is conceivable, based on our findings, that the effects of this drug are attenuated because of the degradation of the hypophosphorylated 4E-BP1. Consequently, a combination of antisense eIF4E and Velcade (bortezomib, a proteasome inhibitor), which is an FDA-approved anticancer drug, should become a potent regimen to treat cancer. Indeed, the levels of the hypophosphorylated 4E-BP1 were rescued by treatment with Velcade (Figure S6A, lane 4), and the proliferation of eIF4E-KD cells was more inhibited by Velcade than control cells (Figure S6B).
Ubiquitination is known to regulate translation. For example, many ribosomal proteins and eIF4E are ubiquitinated (Spence et al., 2000, Hitchcock et al., 2003 and Murata and Shimotohno, 2006). Moreover, the E3 ubiquitin ligase, EDD, mediates the turnover of PAIP2A in response to PABP depletion. This mechanism functions as a homeostatic feedback to control PABP-dependent translation activity in cells (Yoshida et al., 2006). Thus, the results of this paper together with earlier reports establish a paradigm of homeostatic control of translation, whereby ubiquitination controls the levels of translation repressors to maintain cellular homeostasis.
Experimental Procedures
Establishment of a Stable eIF4E-KD Cell Line
HeLa S3 and HEK293 cells were transfected with either pTER-shSceIF4E (scrambled) or pTER-sheIF4E to generate control or eIF4E-KD cells, respectively, followed by 100 µg/ml Zeocin (Invitrogen) selection 2 days after transfection. Three cell clones were selected by single-colony isolation for each line. eIF4E levels were analyzed by western blotting.
Establishment of a Stable eIF4E-KD Cell Line Expressing Mouse eIF4E
Phoenix-Ampho cells (Pear et al., 1993) were transfected with pMV7-HA-mouse eIF4E (Lazaris-Karatzas et al., 1990) to generate retrovirus expressing mouse eIF4E, and the supernatant containing retrovirus was collected. eIF4E-KD cells were infected with the filtered supernatant. Geneticin (400 µg/ml) (GIBCO) was used for selection 2 days after infection.
Protein Half-Life
Cells were treated with 100 µg/ml CHX for the indicated times in Figures 3A and 3C. Protein was analyzed by western blotting.
In Vivo Ubiquitination Assay
HEK293 cells or 4E-BP DKO MEFs were cotransfected with plasmids expressing 4E-BP1, KLHL25, or CUL3 along with pHis-Ub by Lipofectamine (Invitrogen) according to the manufacturer's instructions. At 48 hr post-transfection, cells were treated with 20 µM MG132 for 6 hr. Cells were lysed in urea buffer (8 M Urea, 0.1 M NaH2PO4, 0.1 M Tris-HCl [pH 8.0], 0.05% Tween 20, and 10 mM imidazole [pH 8.0]). A total of 5 mg of protein was incubated with Ni-NTA agarose beads (QIAGEN) overnight. Beads were extensively washed with denaturing wash buffer twice (8 M Urea, 0.1 M NaH2PO4, 0.1 M Tris-HCl [pH 8.0], 0.05% Tween 20, 20 mM imidazole [pH 8.0]) and then with native wash buffer (0.1 M NaH2PO4, 0.1 M Tris-HCl [pH 8.0], 0.05% Tween 20, 20 mM imidazole [pH 8.0]). Protein was dissolved in Laemmli buffer and resolved by SDS-PAGE. Monoclonal antibody HA.11 (Covance) was used to detect ubiquitinated 4E-BP1.
Statistics
Statistical significance was analyzed by a two-tailed Student's t test. Data represent the mean ± SD.
Supplementary Material
Highlights.
► Silencing of the cap-binding protein, eIF4E, causes no reduction in translation ► The hypophosphorylated form of 4E-BP1 is degraded rapidly in eIF4E-knockdown cells ► KLHL25-CUL3 complex is the E3 ubiquitin ligase targeting hypophosphorylated 4E-BP1 ► eIF4E activity is under homeostatic control by the ubiquitin-proteasome pathway
Acknowledgments
We thank Susanne Heynen for supporting the development of HCS assay, Chih-Cheng Yang for bioinformatics in building the gene list covered in the library, Colin Lister for exceptional technical assistance, Stephane Angers for pFLAG-CUL3, Reuven Agami for pTER vector, Zhen-Qiang Pan for pCR3.1-Flag-CUL7, Yi Liu for PP242, and Maritza Jaramillo, Lian Wee Ler, Tommy Alain, Takayuki Murata, and Michael Bidinosti for experimental assistance and suggestions. A.Y. was the recipient of a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad. Y.M. is the recipient of a postdoctoral fellowship from the Fondation pour la Recherche Medicale. This work was supported by a Canadian Institutes of Health Research (CIHR) grant (MOP 7214) to N.S., and NCI grant (CA51995) to Z.R.
References
- Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Cormier P, Pyronnet S, Morales J, Mulner-Lorillon O, Sonenberg N, Belle R. eIF4E association with 4E-BP decreases rapidly following fertilization in sea urchin. Dev. Biol. 2001;232:275–283. doi: 10.1006/dbio.2001.0206. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R, Milburn SC, Hershey JW. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J. Biol. Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- Elia A, Constantinou C, Clemens MJ. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene. 2008;27:811–822. doi: 10.1038/sj.onc.1210678. [DOI] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, He YJ, Borchers C, Xiong Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 2003;5:1001–1007. doi: 10.1038/ncb1056. [DOI] [PubMed] [Google Scholar]
- García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem. J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R, Wee S, Anderson S, Yates J, Wolf DA. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell. 2003;12:783–790. doi: 10.1016/s1097-2765(03)00341-1. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, Schwier P, Capen A, Goode RL, Dowless MS, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- Gross JD, Moerke NJ, von der Haar T, Lugovskoy AA, Sachs AB, McCarthy JE, Wagner G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. 2003;115:739–750. doi: 10.1016/s0092-8674(03)00975-9. [DOI] [PubMed] [Google Scholar]
- Hiremath LS, Webb NR, Rhoads RE. Immunological detection of the messenger RNA cap-binding protein. J. Biol. Chem. 1985;260:7843–7849. [PubMed] [Google Scholar]
- Hitchcock AL, Auld K, Gygi SP, Silver PA. A subset of membrane-associated proteins is ubiquitinated in response to mutations in the endoplasmic reticulum degradation machinery. Proc. Natl. Acad. Sci. USA. 2003;100:12735–12740. doi: 10.1073/pnas.2135500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleghpour K, Pyronnet S, Gingras AC, Sonenberg N. Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol. Cell. Biol. 1999;19:4302–4310. doi: 10.1128/mcb.19.6.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–1212. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- Lavoie CA, Lachance PE, Sonenberg N, Lasko P. Alternatively spliced transcripts from the Drosophila eIF4E gene produce two different Cap-binding proteins. J. Biol. Chem. 1996;271:16393–16398. doi: 10.1074/jbc.271.27.16393. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lin TA, Kong X, Saltiel AR, Blackshear PJ, Lawrence JC. Jr. Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J. Biol. Chem. 1995;270:18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín ME, Perez MI, Redondo C, Alvarez MI, Salinas M, Fando JL. 4E binding protein 1 expression is inversely correlated to the progression of gastrointestinal cancers. Int. J. Biochem. Cell Biol. 2000;32:633–642. doi: 10.1016/s1357-2725(00)00007-8. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimotohno K. Ubiquitination and proteasome-dependent degradation of human eukaryotic translation initiation factor 4E. J. Biol. Chem. 2006;281:20788–20800. doi: 10.1074/jbc.M600563200. [DOI] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Tuschl T. On the art of identifying effective and specific siRNAs. Nat. Methods. 2006;3:670–676. doi: 10.1038/nmeth911. [DOI] [PubMed] [Google Scholar]
- Pintard L, Willis JH, Willems A, Johnson JL, Srayko M, Kurz T, Glaser S, Mains PE, Tyers M, Bowerman B, Peter M. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature. 2003;425:311–316. doi: 10.1038/nature01959. [DOI] [PubMed] [Google Scholar]
- Poulin F, Gingras AC, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 1998;273:14002–14007. doi: 10.1074/jbc.273.22.14002. [DOI] [PubMed] [Google Scholar]
- Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, Sonenberg N. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA. 2008;14:1318–1327. doi: 10.1261/rna.950608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. doi: 10.1016/s0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat. Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M, Elledge SJ, Harper JW. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425:316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yoshida K, Kozlov G, Lim NS, De Crescenzo G, Pang Z, Berlanga JJ, Kahvejian A, Gehring K, Wing SS, Sonenberg N. Poly(A) binding protein (PABP) homeostasis is mediated by the stability of its inhibitor, Paip2. EMBO J. 2006;25:1934–1944. doi: 10.1038/sj.emboj.7601079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.