Abstract
The neural and genetic factors underlying chronic tolerance to alcohol are currently unclear. The GluN2A NMDAR subunit and the NMDAR-anchoring protein PSD-95 mediate acute alcohol intoxication and represent putative mechanisms mediating tolerance. We found chronic intermittent ethanol exposure (CIE) did not produce tolerance (loss of righting reflex/LORR) or withdrawal-anxiety in C57BL/6J, GluN2A or PSD-95 knockout mice assayed 2–3 days later. However, significant tolerance to LORR was evident 1 day after CIE in C57BL/6J and PSD-95 knockouts, but absent in GluN2A knockouts. These data suggest a role for GluN2A in tolerance, extending evidence that human GluN2A gene variation is involved in alcohol dependence.
Alcoholism is associated with the development of tolerance (DSM-5, 2013) to the effects of alcohol, but the neural and genetic mechanisms underlying chronic tolerance are still not well understood. Glutamate signaling via N-methyl-D-aspartate receptors (NMDAR) is a putative mechanism mediating tolerance and withdrawal (Krystal et al., 2003). Two glutamate signaling molecules, the GluN2A NMDAR subunit and the NMDAR-anchoring protein PSD-95, have previously been shown to have a role in regulating acute alcohol intoxication (Boyce-Rustay and Holmes, 2005; Boyce-Rustay and Holmes, 2006; Camp et al., 2011). Variation of the GluN2A gene is associated with alcohol dependence in humans (Domart et al., 2012; Schumann et al., 2008). Here, we investigated the development of tolerance and withdrawal-associated anxiety-like behavior following chronic intermittent EtOH exposure (CIE) via vapor inhalation as to determine the contribution of GluN2A and PSD-95 to these effects.
C57BL/6J-background knockout (KO) mice lacking either GluN2A (Boyce-Rustay and Holmes, 2006) or PSD-95 (Camp et al., 2011), together with their respective wild-type (WT) littermate controls, were tested for evidence of withdrawal-anxiety and tolerance after CIE for a total of 16 × 16-hour exposures over a 4 week period (for full methods, see Supplementary Information). This CIE procedure has previously been shown to cause tolerance to EtOH conditioned taste aversion in C57BL/6J mice (Lopez et al., 2012). Each cohort of mice received only one regimen of CIE.
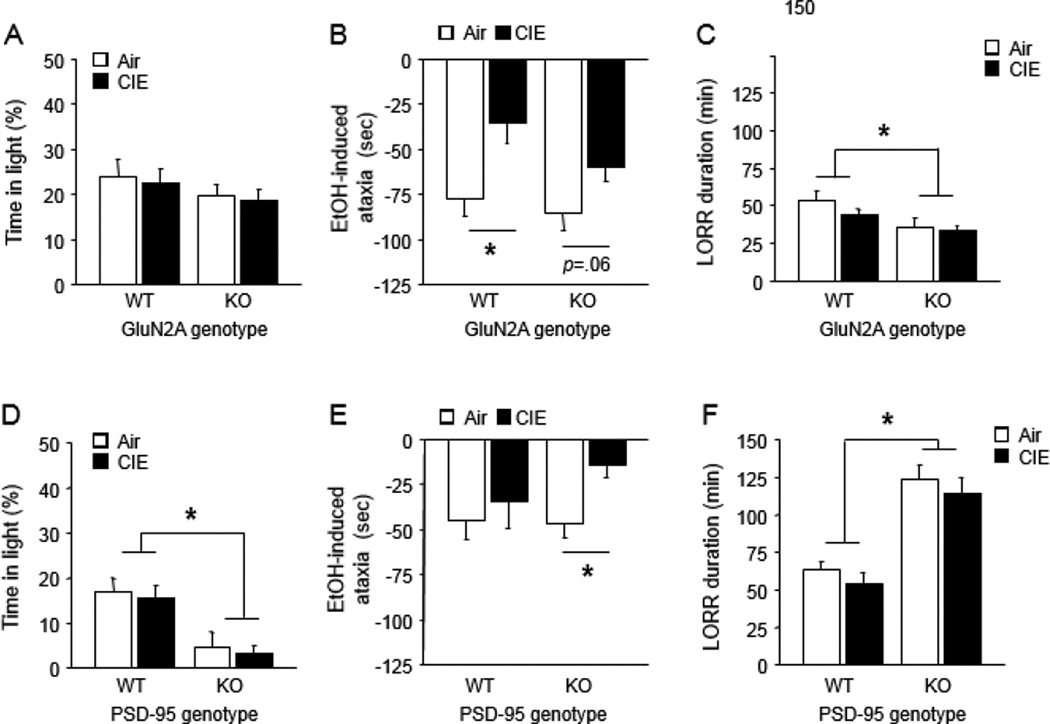
As compared to yoked controls exposed to vaporized air, CIE-exposed GluN2A KO and WT showed no increase in anxiety-related behavior (light/dark exploration) 2 days after CIE (Figure 1A, S1A). CIE mice did, however, exhibit lesser ataxia responses to 2 g/kg EtOH challenge in the rotarod test 3 days post-CIE, relative to air controls, indicating tolerance to this effect. This was statistically significant in WT and trended in the same direction in KO (CIE effect: F1,61=11.10, P<.01, followed by planned post hoc comparisons by genotype, n=16–17) (Figure 1B, S2A). However, there was no indication of lesser LORR duration in either genotype after challenge with 3.5 g/kg EtOH 4 days post-CIE. GluN2A KO generally had lower scores than WT (genotype effect: F1,53=10.17, P=.05, n=12–16) (Figure 1C).
Figure 1. Effects of CIE on withdrawal-anxiety and tolerance in GluN2A KO and PSD-95 KO.
(A) Effect of CIE and GluN2A genotype on light/dark exploration time in light. (B) Effect of CIE and GluN2A genotype on EtOH-induced rotarod ataxia. (C) Effect of CIE and GluN2A genotype on EtOH-induced LORR. (D) Effect of CIE and PSD-95 genotype on light/dark exploration time in light. (E) Effect of CIE and PSD-95 genotype on EtOH-induced rotarod ataxia. (F) Effect of CIE and PSD-95 genotype on EtOH-induced LORR. Data are Means ±SEM. *P<.05
PSD-95 KO showed increased baseline anxiety-like behavior (as seen previously (Feyder et al., 2010) but, as in the GluN2A KO experiment, CIE did not alter this behavior (genotype effect: %light time: F1,35=18.58, P<.01, light entries: F1,35=21.71, P<.01, n=8–11) (Figure 1D, S1B). EtOH-induced ataxia scores were diminished after CIE due to a tolerance effect in PSD-95 KO only (CIE effect: F1,36=4.00, P=.05, n=8–11) (Figure 1E, S2B). CIE had no effect on LORR duration and, as previously reported (Camp et al., 2011), PSD-95 KO had higher LORR scores than WT, irrespective of CIE (F1,36=48.55, P<.01, n=8–11) (Figure 1F).
These results indicate that 16 × 16-hour CIE exposure did not produce anxiety-like withdrawal or LORR tolerance and did not produce significant tolerance to ataxia tolerance across experiments. These negative data could reflect the relative resistance of the C57BL/6 background strain to these specific assays for tolerance and withdrawal (Metten et al., 2010). Indeed, in commercially obtained C57BL/6Tac mice exposed to the same CIE procedure, there was also no evidence of CIE-induced tolerance or withdrawal-anxiety (all P<.05, n=8–10) (Figure S3A-E).
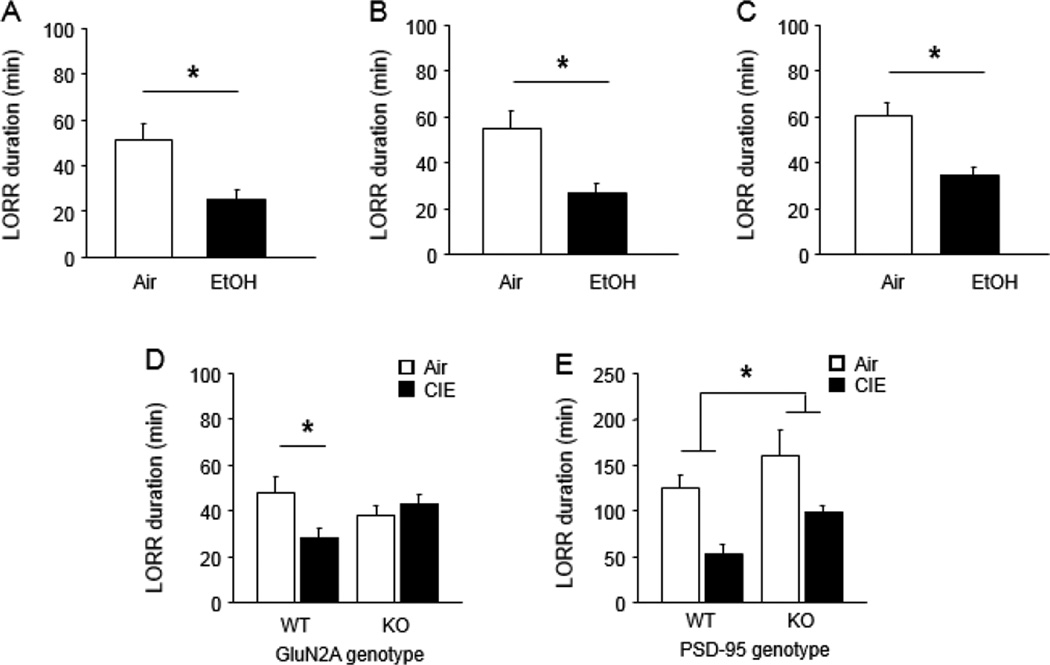
Next, we tested for parametric variables that could potentially reveal CIE-induced tolerance in C57BL/6J mice. Based on the observation that rats exposed to prolonged CIE (7 weeks) can show tolerance to EtOH-induced LORR (Rimondini et al., 2008), C57BL/6J mice were given 32 × 16-hour CIE exposures over 8 weeks and tested for tolerance. Although EtOH-induced ataxia scores (assayed 3 days post-CIE) only tended to be lower in CIE mice than air controls (Air=71.4 ±22.3, CIE=52.2 ±19.2, P>.05, n=9–10), LORR duration (assayed 4 days post-CIE) was significantly reduced after this prolonged CIE regimen (t(14)=3.01, P<.01, n=7–9) (Figure 2A).
Figure 2. Effects of CIE on tolerance in C57BL/6J mice, GluN2A KO and PSD-95 KO.
(A) Effect of prolonged CIE on EtOH-induced LORR in C57BL/6J. (B) Effect of CIE on EtOH-induced LORR 1 day post-CIE in C57BL/6J. (C) Effect of CIE on EtOH-induced LORR 1 day post-CIE in a replicate C57BL/6J sample. (D) Effect of CIE and GluN2A genotype on EtOH-induced LORR 1 day post-CIE. (E) Effect of CIE and PSD-95 genotype on EtOH-induced LORR 1 day post-CIE. Data are Means ±SEM. *P<.05
We also determined whether LORR tolerance could be revealed if CIE was less extensive (i.e., standard 16 × 16-hour exposures) but there was a lesser interval between CIE and tolerance testing. CIE mice tested at an interval of 1 day post-CIE had significantly lesser LORR duration than air controls (t(15)=3.70, P<.01, n=8–9) (Figure 2B). The reliability of this effect was confirmed in a separate cohort of mice (t(8)=3.44, P<.01, n=4–6) (Figure 2C). In fact, in a third cohort, LORR tolerance was still evident at a 3-day post-CIE interval (CIE effect: F1,39=14.72, P<.01, n=7–8) and was reversible by pharmacological blockade of the NMDAR with MK-801 (drug effect: F2,39=14.33, P<.01) (Figure S4) (Debrouse et al., 2013).
On the basis of these results in the C57BL/6J strain, we tested naïve cohorts of GluN2A and PSD-95 KO using the procedure where tolerance was tested 1-day after 16 × 16-hour CIE exposures. LORR duration was significantly lesser after CIE in WT but not GluN2A KO (genotype × CIE interaction: F1,34=5.01, P<.01, n=7–12) (Figure 2D). By contrast, CIE exposure produced LORR tolerance regardless of PSD-95 genotype (CIE effect: F1,28=16.29, P<.01, n=7–9) (Figure 2E). LORR duration was significantly higher in PSD-95 KO than WT, irrespective of CIE (genotype effect: F1,28=6.04, P<.05). This CIE effect difference was evidence despite relatively high LORR values in air controls, which may reflect mixed genetic background of this mutant line (Feyder et al., 2010) and the greater contribution of longer sleeping ‘129’ inbred mouse strain genes (Chen and Holmes, 2009).
Collectively, these data indicate loss of tolerance to the sedative/hypnotic effects of alcohol following constitutive deletion of GluN2A. Tolerance to these effects was unaltered in mice lacking PSD-95, although these mice did show some evidence of greater chronic tolerance to EtOH-induced ataxia. These genetic effects do not appear to be explained by abnormal EtOH pharmacokinetics, given previous evidence that neither GluN2A or PSD-95 deletion (Boyce-Rustay and Holmes, 2006; Camp et al., 2011) nor CIE (Holmes et al., 2012) alters EtOH metabolism. Further studies are needed to determine whether this function of GluN2A extends to other measures of EtOH tolerance, as well as withdrawal anxiety. Notwithstanding, the current study demonstrates a contribution of GluN2A-containing NMDARs to long-term adaptations to chronic EtOH exposure, and extends the recent finding that human GluN2A gene variation is associated with risk for alcohol dependence (Domart et al., 2012; Schumann et al., 2008).
Supplementary Material
Acknowledgements
We are very grateful to Dr. Howard Becker, Dr. Marcelo Lopez, Dr. Nicolas Jury, Miss Benita Hurd, and Miss Eric Sagalyn for valuable assistance with the CIE procedure. Research supported by the NIAAA Intramural Research Program.
References
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC, Coba MP, Grant SG, Holmes A. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol. 2011;16:428–439. doi: 10.1111/j.1369-1600.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34:1454–1466. doi: 10.1038/npp.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrouse L, Hurd B, Kiselycznyk C, Plitt A, Todaro A, Mishina M, Grant SG, Camp M, Gunduz-Cinar O, Holmes A. Probing the Modulation of Acute Ethanol Intoxication by Pharmacological Manipulation of the NMDAR Glycine Co-Agonist Site. Alcohol Clin Exp Res. 2013;37:223–233. doi: 10.1111/j.1530-0277.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domart MC, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, Reynaud M, Saffroy R. Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism. Addict Biol. 2012;17:783–785. doi: 10.1111/j.1369-1600.2011.00321.x. [DOI] [PubMed] [Google Scholar]
- DSM-5. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition ed. Washington, D.C.: APA Press; 2013. [Google Scholar]
- Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momenan R, Munasinghe J, Scattoni ML, Ihne J, Camp M, Graybeal C, Strathdee D, Begg A, Alvarez VA, Kirsch P, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A, Grant SG, Holmes A. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams' syndrome. Am J Psychiatry. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, Macpherson KP, Debrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcohol Clin Exp Res. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, Crabbe JC. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1552–1564. doi: 10.1111/j.1530-0277.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall'Olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.