Summary
Adult hematopoietic stem cells (HSCs) are maintained in specialized niches within the bone marrow under steady-state conditions and mobilized for extramedullary hematopoiesis during periods of stress such as bacterial infections. However, the underlying mechanisms are unclear. We show that systemic infection of mice with Escherichia coli, commonly associated with bacteremia in humans, mobilizes functional HSCs to the spleen. Accumulation of splenic HSCs (CD150+CD48-Lin−/lowScal1+cKit+) was diminished in TLR4-deficient and RIPK2-deficient mice, implicating TLRs and cytosolic NOD1/NOD2 signaling in the process. Accordingly, dual stimulation of NOD1 and TLR4 in radio-resistant cells alone was sufficient to mobilize HSCs, while TLR4 expression on HSCs was dispensable. Mechanistically, TLR4 and NOD1 synergistically induced granulocyte-colony stimulating factor (G-CSF), which was required for extramedullary HSC accumulation. Mobilized HSCs and progenitor cells gave rise to neutrophils and monocytes and contributed to limiting secondary infection.
Introduction
Hematopoietic stem cells are defined by their ability to give rise to all cells of the blood system and to self-renew, where cell division results in at least one daughter that retains the full developmental potential of its parent. The majority of HSCs reside in the bone marrow where they are surrounded by a network of supporting cells, collectively termed the stem cell niche, while a smaller subset of HSCs reside in the spleen, which serves as a site for hematopoiesis during embryogenesis and periods of duress (Morrison and Spradling, 2008). While residency in a niche is essential for HSC maintenance, HSCs regularly traffic through the blood stream in a process that may facilitate competition for niches to ensure a robust pool of stem cells (Wright et al., 2001). Moreover, HSCs have been isolated from lymphatic ducts, indicating that HSCs travel through peripheral tissues and have the potential to provide a local source of cell production (Massberg et al., 2007). While much is known about HSC activity under homeostatic conditions, how HSCs function during periods of stress is less clear.
Bacterial infection is a common form of stress that can induce profound effects on the fate of hematopoietic stem and progenitor cells (HSPCs). Host-derived pattern recognition receptors (PRRs) sense components of bacteria and respond by activating pro-inflammatory signaling pathways that aid in the defense against infection. Escherichia coli is a gram negative bacterium that normally resides in the intestine, but is also a major cause of sepsis in hospitalized patients (Laupland, 2013). The cell wall of E. coli contains lipopolysaccharide (LPS), which is sensed by Toll-like receptor 4 (TLR4), and peptidoglycan, whose cleavage products are sensed by the nucleotide-binding oligomerization domain containing (NOD)-like-receptors (NLRs) NOD1 and NOD2. TLR4 signals via the adaptor proteins myeloid differentiation primary response 88 (Myd88) and TIR-domain-containing adapter inducing interferons (TRIF), while NOD1 and NOD2 signaling requires the adaptor protein receptor-interacting serine-threonine kinase 2 (RIPK2), which leads to the activation of NF-κB and MAPK pathways (Franchi et al., 2009; Sartor, 2008).
TLR4 and TLR2 are expressed on the surface of Lineage−/low Sca1+ cKit+ (LSK) cells, which mark both HSCs and non-self-renewing progenitors, suggesting that HSPCs may actively participate in innate immune responses (Nagai et al., 2006). Activation of TLRs has been proposed to alter the fate and function of HSCs either by direct intracellular signaling, or indirectly via production of inflammatory cytokines or alterations in the bone marrow niche (Baldridge et al., 2010; Chen et al., 2010; Esplin et al., 2011; Essers et al., 2009; Johns et al., 2009; Rodriguez et al., 2009; Scumpia et al., 2010; Takizawa et al., 2011). To date, only one study has tested the function of HSCs following live bacterial infection (Baldridge et al., 2010). However, HSC activity was not assessed in unfractionated bone marrow or in sites of extramedullary hematopoiesis such as the spleen and the surface marker profile of functional HSCs during infection still remains largely uncharacterized. Thus, it is unclear whether bacterial infection alters the phenotype and function of HSCs in the bone marrow and spleen. Furthermore, it remains to be determined whether HSCs directly sense and respond to components of bacteria or whether infection merely alters the bone marrow microenvironment and modulates their fate indirectly.
Activation of TLRs can also affect the localization of HSPCs. Systemic administration of LPS results in the accumulation of HSPCs in the spleen (Esplin et al., 2011; Vos et al., 1972), but the signals and cell types responsible for this phenomenon are unknown. Repeated administration of granulocyte-colony stimulating factor (G-CSF), which can be produced in response to infection or LPS (Hareng and Hartung, 2002), induces the mobilization of HSPCs from bone marrow to peripheral blood and spleen and is the preferred mobilizing agent used in the clinic (Duhrsen et al., 1988; Molineux et al., 1990; Morrison et al., 1997; To et al., 2011). While the mechanisms of G-CSF induced mobilization are well characterized (Levesque and Winkler, 2008; Mazo et al., 2011), it is currently unclear whether the production of endogenous G-CSF mobilizes HSCs during infection. In the current study, we investigated the mechanisms by which bacterial infection influences the localization and function of HSCs.
Results
Systemic E. coli infection reduces functional HSCs in bone marrow
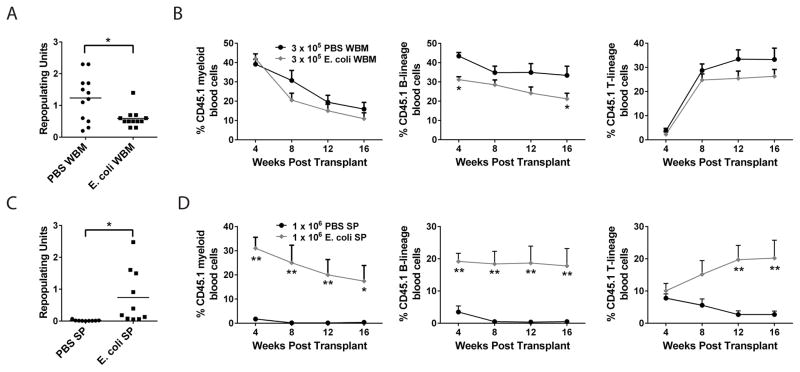
To understand the mechanisms underlying the regulation of HSCs during infection, we developed a model of systemic infection using the gram negative bacterium Escherichia coli K12. This bacterium was chosen because E. coli is commonly isolated from the blood of humans suffering from bacteremia (Laupland, 2013) and because systemic administration was sub-lethal in wild type (WT) mice for all conditions tested, thus allowing an extended window of time to assess HSC activity. To determine whether HSC activity is altered in the bone marrow after E. coli infection, we performed competitive transplantation experiments in which WT (CD45.1) mice were treated with PBS or E. coli and after six days, 3x105 whole bone marrow (WBM) were mixed with 3x105 untreated (CD45.2) WBM and transplanted into lethally irradiated (CD45.2) recipients and peripheral blood reconstitution was followed for 16 weeks (Figure 1A and B). To quantitatively compare levels of reconstitution, we converted percent chimerism after 16 weeks into repopulating units (RUs) (Harrison et al., 1993). In two independent experiments, WBM from E. coli infected mice had on average 2.1-fold fewer RUs compared with PBS treated WBM (Figure 1A). The contribution of E. coli infected WBM to the B cell-lineage, but not the myeloid or T-cell lineage, was significantly reduced after 16 weeks (Figure 1B). These results indicate that total HSC activity in the bone marrow of mice infected with E. coli for six days was moderately diminished compared to that of uninfected mice.
Figure 1. Infection reduces HSC activity in bone marrow and increases HSC activity in spleen.
(A–D) WT mice were injected with ~1x108 E. coli K12 i.p. and after six days (A, B) 3x105 whole bone marrow (WBM) or (C, D) 1x106 splenocytes were mixed with 3x105 WBM and transplanted into lethally irradiated recipients. Peripheral blood chimerism of myeloid (Mac1+), B-lineage (B220+) and T-lineage (CD3+) cells was analyzed by flow cytometry every 4 weeks. (A, C) n = 12 mice per condition from 2 experiments. *p<0.05 by t-test. (B, D) n= 9–10 mice per condition from two experiments. *p<0.05 **p<0.05 by Two way ANOVA versus recipients of PBS cells at each time point. Error bars represent SEM.
Systemic E. coli infection promotes the accumulation of functional HSCs in spleen
The spleen can serve as a site for extramedullary hematopoiesis during periods of stress and previous studies have found that treatment of mice with LPS, which mimics some aspects of infection, induces the accumulation of HSPCs in the spleen (Esplin et al., 2011; Vos et al., 1972). To determine whether HSC activity is altered in the spleen after live bacterial infection, we treated WT (CD45.1) mice with PBS or E. coli and after six days mixed 1x106 splenocytes with 3x105 untreated (CD45.2) WBM and transplanted these cells into lethally irradiated (CD45.2) recipients and followed peripheral blood reconstitution for 16 weeks (Figure 1C and D). In two independent experiments, splenocytes from E. coli infected mice had on average 38.9-fold more RUs than splenocytes from PBS treated mice (Figure 1C). We defined long term multilineage reconstitution (LTMR) activity, characteristic of functional HSCs, as the presence of greater than 0.3% of donor-derived myeloid, T-lineage and B-lineage cells at 16 weeks post transplant. Whereas splenocytes from PBS treated donors contributed minimally to myeloid and lymphoid lineages over 16 weeks (2/9 LTMR), consistent with a very small resident population of splenic HSCs under steady-state conditions, splenocytes from E. coli infected donors contributed robustly to myeloid and lymphoid lineages over 16 weeks (9/9 LTMR) (Figure 1D). These results indicate that functional HSC activity is enhanced in the spleens of mice infected with E. coli for six days compared with spleens of uninfected mice.
The majority of HSC activity is within the CD150+, CD48−, Lineage−/low, cKit+ and Sca1+ fractions of bone marrow and spleen during infection
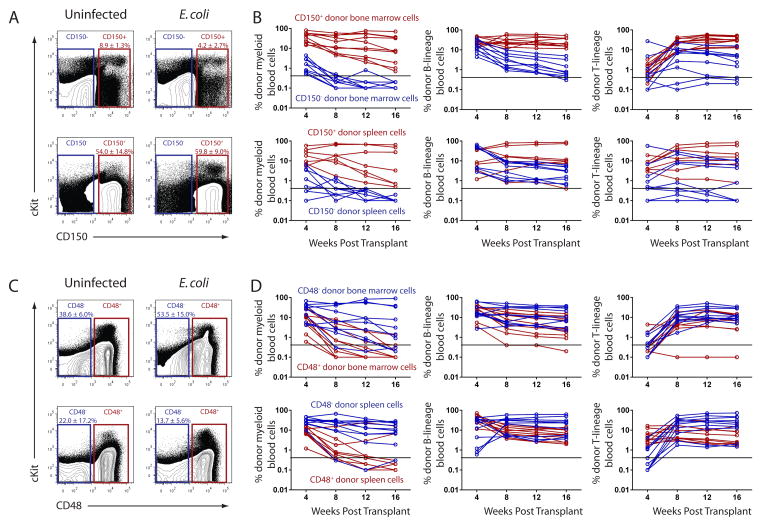
All functional HSC activity is present within the CD150+ CD48− Lineage−/low Sca1+ cKit+ fraction of bone marrow under steady-state conditions (Kiel et al., 2005), yet administration of live bacteria, LPS or inflammatory cytokines can alter the expression of these surface markers on various cell types (Howie et al., 2002; Munitz et al., 2006; Zhang et al., 2008). Therefore we set out to determine whether functional HSCs retain their surface marker profile during infection. We performed competitive reconstitution experiments where bone marrow cells or splenocytes from E. coli infected mice were fractionated based on positive or negative expression of CD150 or CD48 (Figure 2; Figure S1). Donor cells were transplanted based on the percentage of each population within infected bone marrow or spleen (Table S1). In two independent experiments, all mice that received CD150+ bone marrow cells (10/10 LTMR) or splenocytes (7/7 LTMR) were long term multilineage reconstituted whereas no mice that received CD150− bone marrow cells (0/9 LTMR) or splenocytes (0/9 LTMR) were long term multilineage reconstituted (Figure 2A and B; Table S2). Transplanted CD150− bone marrow cells and splenocytes gave transient myeloid reconstitution with sustained yet limited lymphoid reconstitution, consistent with previously published data indicating that CD150− bone marrow cells contain transiently reconstituting multipotent progenitors (Kiel et al., 2008).
Figure 2. The majority of HSC activity exists in the CD150+ CD48− fraction of bone marrow and spleen during infection.
(A–D) WT mice were injected with ~1x108 E. coli K12 i.p. and after six days, (top) bone marrow or (bottom) splenocytes were sorted and resorted based on positive or negative expression of (A, B) CD150 or (C, D) CD48. (B, D) Positive or negative fractions from (top) bone marrow or (bottom) spleen were mixed with 3x105 WBM and transplanted into lethally irradiated recipients. Peripheral blood chimerism of myeloid (Mac1+), B-lineage (B220+) and T-lineage (CD3+) cells was analyzed by flow cytometry. Connected data points represent individual mice bled every 4 weeks. Data are from two experiments for each condition. The black line represents the background threshold of 0.3% below which we could not detect chimerism. See also Figure S1, Figure S2, Table S1 and Table S2.
Under steady-state conditions, all LTMR activity in adult bone marrow is contained within the CD48 negative fraction of cells (Kiel et al., 2005). In E. coli infected mice, while 7/10 and 9/10 recipients of CD48− bone marrow and splenocytes, respectively, were LTMR, we found that 2/10 and 2/10 recipients of CD48+ bone marrow and splenocytes, respectively, were LTMR (Figure 2C and D; Table S2). These results indicate that all HSC activity is present in the CD150+ fraction and a majority is present in the CD48− fraction of bone marrow and spleen, while a minority of HSC activity is present in the CD48+ fraction of bone marrow and spleen six days after E. coli infection.
We performed similar experiments by sorting bone marrow cells and splenocytes from infected mice based on positive or negative expression of Sca1, cKit or Lineage markers (Figure S1; Figure S2). We found that all HSC activity was present in the Sca1+ and cKit+ fractions and a majority of HSC activity was present in the Lineage−/low fraction of bone marrow and spleen during infection. A minority of HSC activity was present in the Lineage+ fraction of the bone marrow but not the spleen (Figure S2; Table S2). Altogether, we find that the majority of functional HSCs are contained within the CD150+ CD48− LSK fraction of bone marrow and spleen six days after infection with E. coli.
Systemic E. coli infection dynamically affects HSPCs in bone marrow and spleen
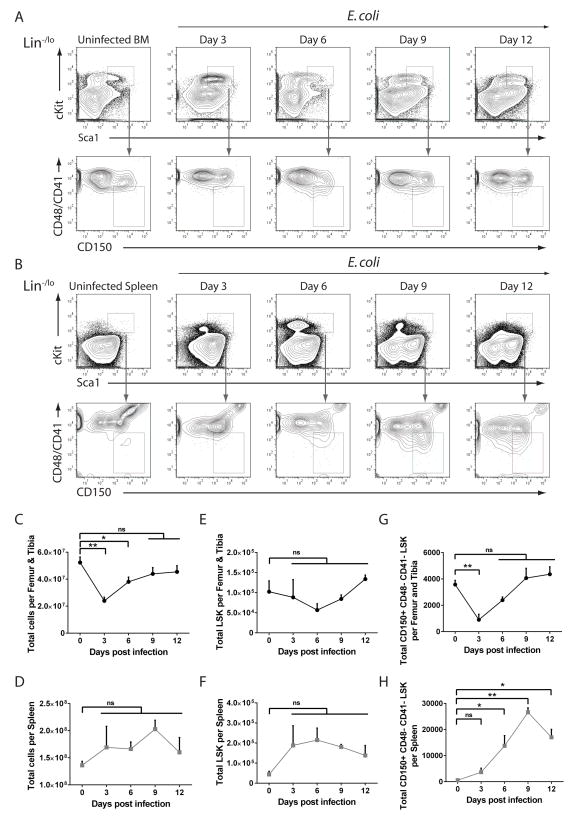
Having confirmed the surface marker profile of HSCs following infection, we measured the frequency of HSPCs (LSK) and HSCs (CD150+ CD48− CD41− LSK) in bone marrow and spleen over twelve days of infection (Figure 3A and B). Although CD41 expression on HSCs increases with age, it is very low on HSCs from young adult mice and its inclusion helps to exclude contaminating megakaryocytes (Kiel et al., 2005). E. coli infection resulted in transient bone marrow hypocellularity at days 3 and 6 that normalized by day 9 along with mild splenomegaly that did not reach significance (Figure 3C and D). Total LSK cells non-significantly reduced in the bone marrow by day 6 and recovered thereafter, while LSK cells in the spleen were non-significantly increased by days 3 and 6 and diminished by day 12 (Figure 3E and F). Total CD150+ CD48− CD41− LSK cells were significantly reduced in the bone marrow by day 3 and recovered to near normal levels by day 9, while CD150+ CD48− CD41− LSK cells in the spleen were significantly increased by day 6, peaked by day 9 and began to diminish by day 12 (Figure 3G and H). These results suggest that systemic infection induces a reduction and subsequent recovery of HSCs in bone marrow along with a delayed accumulation of HSCs in the spleen.
Figure 3. Kinetics of hematopoietic stem and progenitor cells during systemic E. coli infection.
(A–H) WT mice were injected with ~1x108 E. coli K12 i.p. and femur and tibia or spleens were isolated at indicated time points. Shown are representative flow plots from (A) bone marrow or (B) spleen and quantification of (C) total BM cells, (D) total splenocytes, (E) total BM Lineage−/low Sca1+ cKit+ (LSK), (F) total splenic LSK, (G) total BM HSCs (CD150+ CD48− CD41− LSK) and (H) total splenic HSCs. n = 3–8 mice per time point. Error bars indicate SEM. *p<0.05 **p<0.01 by One way ANOVA. (ns) not significant. See also Figure S3 and Figure S4.
To determine whether the accumulation of extramedullary HSCs during infection was specific to the spleen or also occurred in other tissues, we injected WT mice with either PBS or E. coli and after nine days harvested bone marrow, spleen, liver and inguinal lymph nodes and stained these tissues for markers of HSCs (Figure S3). In contrast to the spleen, we did not observe any CD150+ CD48− CD41− LSK cells in the liver or inguinal lymph nodes of infected mice (Figure S3). These data suggest that the spleen may serve as a preferential site for HSC accumulation during E. coli infection.
Mobilization is the primary mechanism for HSC accumulation in spleen
Given that a small subset of functional HSCs exists in the spleen under steady-state conditions (Morrison and Spradling, 2008), it was initially unclear whether the accumulation of splenic HSCs during infection was a result of local cell division, mobilization of HSCs from the bone marrow to the spleen, or both. To address this issue, we infected mice with E. coli and after six days sorted HSCs from the bone marrow or spleen and stained them for Ki67, a marker of cycling cells (Figure S4). We found no significant difference in the percentage of Ki67+ HSCs from uninfected bone marrow (2.7% ± 2.0), infected bone marrow (2.4% ± 1.4) or infected spleen (4.0% ± 2.5) (Figure S4). Since this level of proliferation cannot account for the greater than 30-fold expansion of HSCs in spleen by six days, the studies suggest that mobilization is the primary mechanism underlying the accumulation of splenic HSCs during E. coli infection.
Accumulation of splenic HSCs during infection is dependent on PRRs
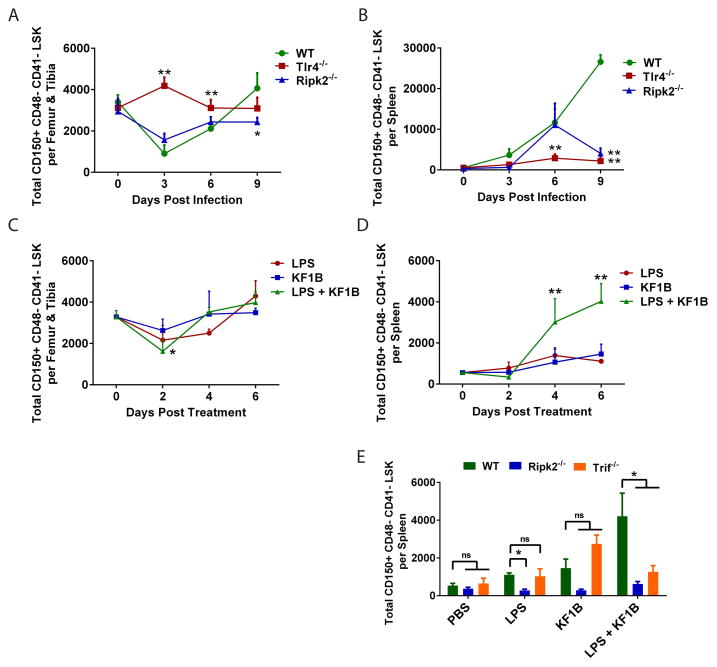
Systemic administration of LPS, which is sensed by TLR4, can promote extramedullary hematopoiesis in the spleen (Esplin et al., 2011; Vos et al., 1972). However, most preparations of LPS are contaminated with peptidoglycan that activates NLRs (Inohara et al., 2001). To determine whether TLRs and NLRs play a role in driving the changes observed in HSC populations during infection, we quantified LSK and CD150+ CD48− CD41− LSK cells in bone marrow and spleen of WT, Tlr4−/− and Ripk2−/− mice before and after infection with E. coli (Figure 4A and B; Figure S5). We did not observe any differences in the total number of CD150+ CD48− CD41− LSK cells in the bone marrow or spleens of uninfected Tlr4−/− or Ripk2−/− mice compared with uninfected WT mice (Figure 4A and B). Similar to WT mice, we observed a reduction of total CD150+ CD48− CD41− LSK cells in bone marrow of Ripk2−/− mice three days after infection, but this was not observed in Tlr4−/− mice (Figure 4A). CD150+ CD48− CD41− LSK cells were increased in the spleen of Ripk2−/− mice by day 6 after infection, yet by day 9 after infection there were significantly fewer CD150+ CD48− CD41− LSK cells in spleens of Ripk2−/− mice compared with WT mice (Figure 4B). Strikingly, we observed almost no accumulation of CD150+ CD48− CD41− LSK cells in spleens of Tlr4−/− mice at any point during infection (Figure 4B). These results implicate NLR and TLR signaling in the accumulation of extramedullary HSCs during infection.
Figure 4. Combined signaling of NOD-like Receptors and Toll-like Receptors is important for HSC accumulation in spleen during infection.
(A, B) WT, Tlr4−/− or Ripk2−/− mice were infected with ~1x108 E. coli K12 i.p. and WBM or splenocytes were isolated. Total HSCs (CD150+ CD48− CD41− LSK) were enumerated in (A) bone marrow and (B) spleen. n = 3–8 mice per time point. *p<0.05 **p<0.01 by Two way ANOVA versus WT mice at same time point. (C, D) WT mice were injected with PBS, the TLR4 agonist LPS (100 ug), the NOD1 agonist KF1B (50 ug) or both i.p. and HSCs were quantified in (C) bone marrow and (D) spleen at indicated time points. n = 3–6 mice per time point. *p<0.05 **p<0.01 by Two way ANOVA versus PBS treated mice. (E) WT, Ripk2−/− or Trif−/− mice were injected as in C–D and HSCs were quantified in spleen after six days. n = 3–6 mice per condition. **p<0.01 by One way ANOVA. See also Figure S5.
NOD1 and TLR4 signaling act synergistically to mobilize HSCs
To investigate whether activation of TLR and NLR signaling pathways are sufficient to mobilize HSCs, we treated WT mice with either ultrapure LPS, which is devoid of contaminating peptidoglycan, or KF1B, a bacterial dipeptide that specifically activates NOD1 (Masumoto et al., 2006), or both (Figure 4C and D). A single i.p. injection of ultrapure LPS or KF1B alone induced minimal alterations in the number of CD150+ CD48− CD41− LSK cells in bone marrow or spleen over six days, whereas dual administration of LPS and KF1B resulted in a reduction of CD150+ CD48− CD41− LSK cells in bone marrow after 2 days and an accumulation of CD150+ CD48− CD41− LSK cells in spleen after six days (Figure 4C and D), reminiscent of changes observed after E. coli infection. These results indicate that dual activation of TLR4 and NOD1 cooperate to mobilize HSCs.
We challenged Ripk2−/−, Trif−/− and Myd88−/− mice with PBS, LPS, KF1B or both and quantified CD150+ CD48− CD41− LSK cells after six days (Figure 4E). Accumulation of CD150+ CD48− CD41− LSK cells in the spleen after LPS and KF1B administration was dependent on RIPK2 and TRIF (Figure 4E). Myd88−/− mice had increased numbers of splenic CD150+ CD48− CD41− LSK cells under steady-state conditions and no significant differences were observed after LPS and KF1B administration (data not shown). These results confirm that KF1B induces the activation of NOD1, which signals through the adaptor RIPK2 in this setting, and suggests that TLR4 signaling through TRIF is important for inducing the mobilization of HSCs.
TLR4 expression on HSCs is not required for their accumulation in spleen
To test whether expression of TLR4 on HSCs influences their reconstitution ability, we transplanted lethally irradiated WT (CD45.1) mice with a mixture of 2.5x106 WT (CD45.1) WBM and 2.5x106 Tlr4−/− (CD45.2) WBM and measured peripheral blood chimerism (Figure 5A and B). After 16 weeks, myeloid reconstitution was equivalent between WT and Tlr4−/− cells, while B-lineage reconstitution was unbalanced in favor of Tlr4−/− cells and T-lineage reconstitution was unbalanced in favor of WT cells (Figure 5B). These results indicate that Tlr4−/− HSCs are functionally comparable to WT HSCs, but may harbor bias with respect to lymphoid reconstitution.
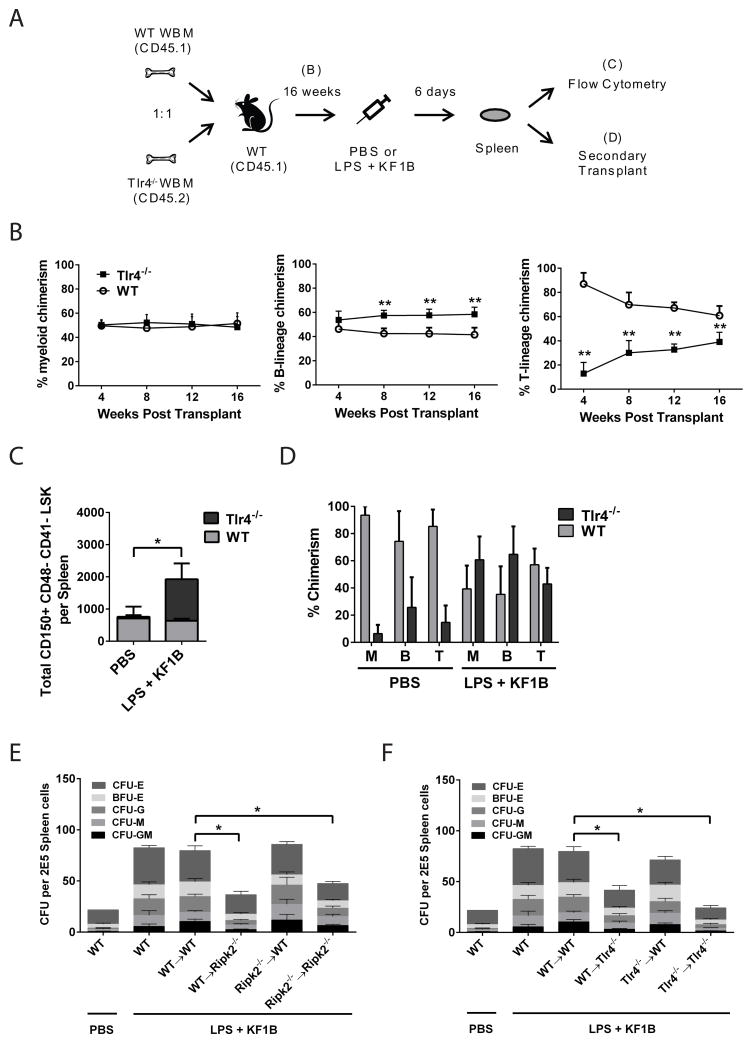
Figure 5. Radio-resistant cells are important for NOD1 and TLR4 induced HSC accumulation in spleen.
(A) Schematic of experiments in (B–D). (B) Peripheral blood chimerism mice transplanted with a 1:1 mixture of WT and Tlr4−/− WBM and bled every 4 weeks. n = 7 mice **p<0.01 by Two way ANOVA. (C) After 16 weeks, chimeric mice were injected with PBS or LPS + KF1B and 6 days later, the absolute number of HSCs (CD150+ CD48− CD41− LSK) in spleen was enumerated. n = 3–4 mice per condition. *p<0.05 by One way ANOVA. (D) 3x107 splenocytes from mice in B–C were transplanted non-competitively into lethally irradiated recipients and peripheral blood chimerism measured 16 weeks after transplant. (E, F) Chimeras were generated as described in text. Mice were injected with PBS or LPS + KF1B and after 6 days, splenocytes were plated in Methocult 3434. n = 3–7 mice per condition. *p<0.05 by One way ANOVA. Error bars represent SEM. Burst Forming Unit (BFU) –E (Erythroid); Colony Forming Unit (CFU) –E (Erythroid); –G (Granulocyte); –M (Macrophage); –GM (Granulocyte-Macrophage).
To determine whether TLR4 expression on HSCs themselves was required for their accumulation in spleen, we challenged the reconstituted mice with PBS or LPS and KF1B and enumerated CD150+ CD48− CD41− LSK cells in bone marrow and spleen after six days (Figure 5C). We detected only minimal Tlr4−/− CD150+ CD48− CD41− LSK cells in the spleens of PBS treated mice, whereas Tlr4−/− CD150+ CD48− CD41− LSK cells were increased in the spleens of LPS and KF1B treated mice (Figure 5C). To confirm these data functionally, we non-competitively transplanted 3x107 splenocytes from PBS or LPS and KF1B treated mice into lethally irradiated CD45.1 recipients and measured peripheral blood chimerism at 16 weeks (Figure 5D). As expected, in recipients of PBS treated splenocytes, the majority of peripheral blood cells were WT derived, whereas in recipients of LPS and KF1B treated splenocytes, Tlr4−/− and WT peripheral blood cells were roughly equivalent (Figure 5D). These data indicate that expression of TLR4 on HSCs is not required for their accumulation in spleen after TLR4 and NOD1 stimulation.
NLR and TLR signaling in the radio-resistant compartment is important for accumulation of splenic HSPCs
To determine which cellular compartment is required for mediating TLR and NLR induced extramedullary hematopoiesis, we generated reverse chimeras in which WBM from WT, Ripk2−/− or Tlr4−/− mice was transplanted into lethally irradiated WT, Ripk2−/− or Tlr4−/− recipients. Chimeric mice were administered LPS and KF1B and after six days splenocytes were plated in Methocult 3434 medium to quantify myeloid and erythroid progenitors. Ripk2−/− recipients of Ripk2−/− WBM and Tlr4−/− recipients of Tlr4−/− WBM exhibited blunted accumulation of myeloid and erythroid progenitors in spleen, consistent with a requirement for both TLR and NLR signaling in promoting HSPC mobilization (Figure 5E and F). Accumulation of myeloid and erythroid progenitors in the spleens of WT mice that received either Ripk2−/− or Tlr4−/− WBM was comparable to WT controls, whereas Ripk2−/− or Tlr4−/− mice that received WT WBM exhibited reduced accumulation of myeloid and erythroid progenitors compared to WT controls (Figure 5E and F). These results indicate that NLR and TLR signaling in radio-resistant cells are required for mobilizing HSPCs and suggest that indirect factors are involved.
G-CSF signaling is required for NLR and TLR induced mobilization of HSCs
Repeated administration of G-CSF induces the mobilization of HSCs from bone marrow to peripheral blood and spleen in both mouse and man (Duhrsen et al., 1988; Molineux et al., 1990; Morrison et al., 1997) and systemic administration of LPS can increase G-CSF in the serum (Hareng and Hartung, 2002), but a role for endogenous G-CSF in bacteria- or LPS-induced mobilization has not been addressed. Compared with naive mice, infection of WT mice with E. coli resulted in a 744-fold increase of G-CSF in sera after two days (Figure 6A). Injection of WT mice with ultrapure LPS resulted in 621-fold increase of G-CSF in sera by four hours compared with 4-fold increase by KF1B (Figure 6B). Moreover, the level of G-CSF in sera was synergistically enhanced to 1627-fold by four hours with dual administration of LPS and KF1B (Figure 6B). Treatment of Nod1−/− mice with LPS and KF1B resulted in G-CSF levels similar to WT mice treated with LPS alone, confirming that KF1B acts exclusively through NOD1 (Figure 6B).
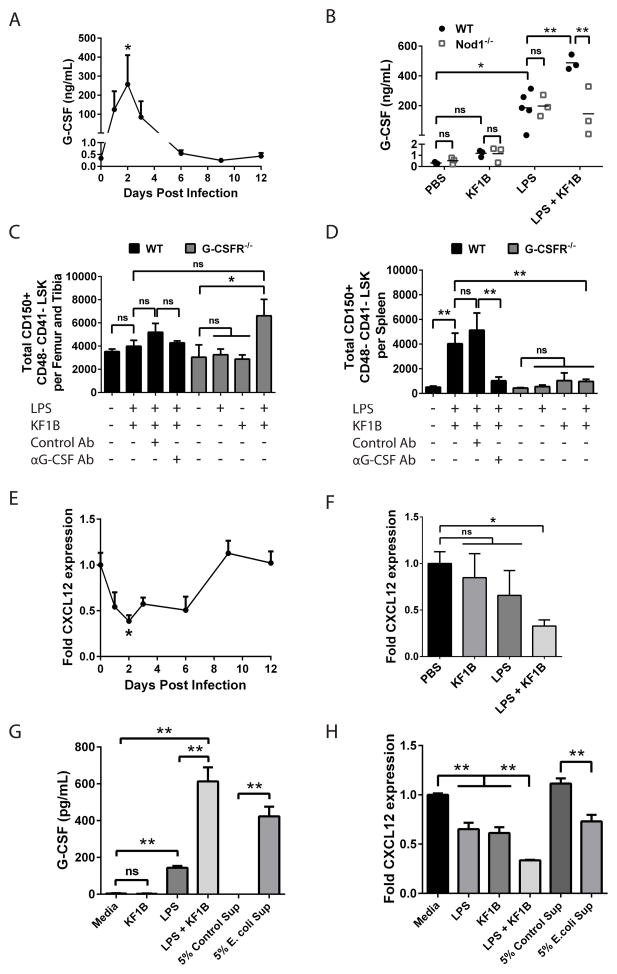
Figure 6. NOD1 and TLR4 induced HSC accumulation in spleen is dependent on G-CSF.
(A) G-CSF in sera after i.p. injection of WT mice with ~1 x 108 E. coli. n = 3–7 mice per time point. (B) G-CSF in sera 4 hours after i.p. injection of WT or Nod1−/− mice with KF1B (50 ug), LPS (100 ug) or both. Each data point represents one mouse. (C, D) Quantification of HSCs (CD150+ CD48− CD41− LSK) in (C) bone marrow or (D) spleen 6 days after challenge. Where indicated, WT mice received 250 ug of isotype control or G-CSF-blocking antibody (Ab) i.p. 1 hour before challenge. n = 3–5 mice per condition. (E) CXCL12 expression in WBM following infection of WT mice as in A. (F) CXCL12 expression in WBM 2 days after injection of WT mice as in B. n = 3–5 mice per condition. (G) G-CSF in supernatants from cultured C166 endothelial cells 24 hours after stimulation with KF1B (1 ug/mL), LPS (10 ng/mL), both or E. coli supernatant (Sup). (H) CXCL12 expression in cultured C166 endothelial cells 4 hours after stimulation as in G. Data are representative of at least 3 independent experiments. * p < 0.05 ** p < 0.01 by One-way ANOVA for all graphs. (ns) not significant. Error bars represent SEM.
To test whether G-CSF was required for HSC accumulation in spleen, we injected WT mice with G-CSF blocking antibody or isotype control one hour prior to injection with LPS and KF1B and quantified CD150+ CD48− CD41− LSK cells in bone marrow and spleen after six days (Figure 6C and D). Antibody pre-treatment did not influence the levels of CD150+ CD48− CD41− LSK cells in the bone marrow, yet accumulation of CD150+ CD48− CD41− LSK cells in spleen was reduced in mice pre-treated with G-CSF blocking antibody but not isotype control antibody (Figure 6C and D). We treated Csf3r−/− (G-CSFR−/−) mice with PBS or LPS and KF1B and found that the accumulation of CD150+ CD48− CD41− LSK cells in spleens of these mice was almost completely blocked (Figure 6D). These results indicate that G-CSF signaling acts downstream of TLR4 and NOD1 to promote the mobilization of HSCs.
CXCL12 is a chemokine critical for retention of HSCs in the bone marrow niche and downregulation of CXCL12 in bone marrow is a common feature of G-CSF-mediated mobilization (Levesque and Winkler, 2008; Petit et al., 2002). CXCL12 expression was reduced 2-fold in WBM two days after E. coli infection (Figure 6E). Single administration of LPS or KF1B did not significantly alter CXCL12 expression in WBM after two days, whereas dual administration resulted in a 3-fold reduction of CXCL12 expression (Figure 6F). Thus, E. coli infection results in downregulation of CXCL12 in bone marrow and NOD1 and TLR4 can act synergistically to mediate this phenomenon.
Endothelial cells are important producers of CXCL12 in the bone marrow and contribute to the maintenance of HSCs under steady-state conditions (Ding and Morrison, 2013; Ding et al., 2012; Greenbaum et al., 2013). To test whether endothelial cells could respond to bacterial components, we cultured the mouse endothelial cell line C166 in the presence of LPS, KF1B, or supernatants from E. coli grown in vitro (Figure 6G and H). KF1B alone did not induce G-CSF production in C166 cells, while LPS alone increased G-CSF production by 39-fold and co-administration synergistically enhanced G-CSF production by 167-fold (Figure 6G). E. coli supernatants were also sufficient to increase G-CSF production by 115-fold (Figure 6G). LPS or KF1B treatment alone reduced CXCL12 expression by 1.6 and 1.5 fold, respectively, whereas co-administration reduced CXCL12 expression by 3-fold (Figure 6H). Culture with E. coli supernatants resulted in 1.5-fold reduction of CXCL12 expression (Figure 6H). These results suggest that endothelial cells may be important for sensing bacterial components through NOD1 and TLR4 and promoting mobilization during infection.
NOD1 and TLR4 mobilized HSPCs give rise to neutrophils and monocytes and contribute to limiting infection
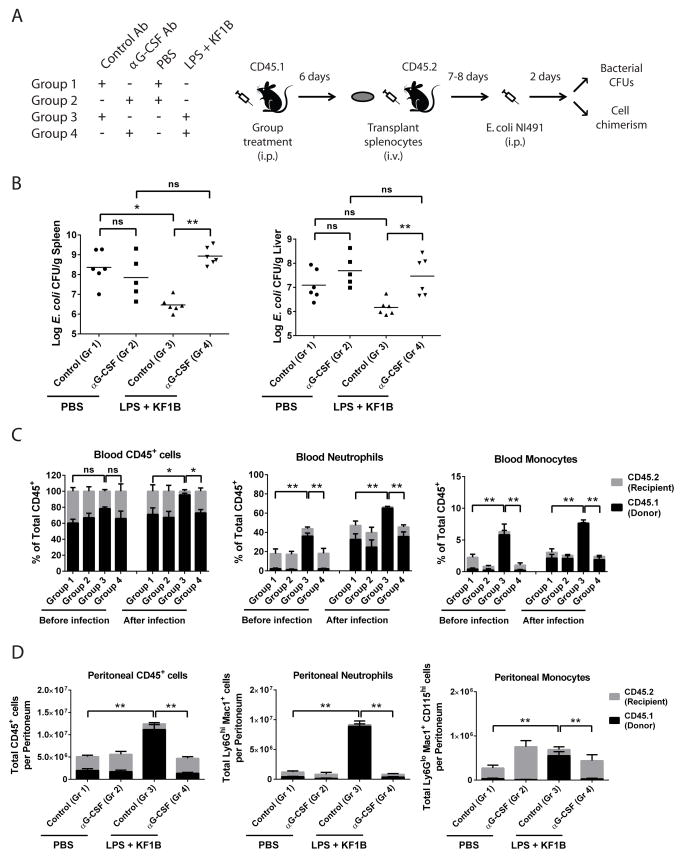
Finally, we wished to determine whether PRR-mediated mobilization of HSPCs influenced subsequent bacterial infection. To address the contribution of mobilized splenic HSPCs and their progeny to infection, we devised the experiment described in Figure 7A. Wild type (CD45.1) mice were pre-treated with isotype control or αG-CSF antibody to permit or block mobilization, respectively, followed by injection of PBS or LPS and KF1B. After six days, 4x107 unfractionated splenocytes were transplanted into sub-lethally irradiated WT (CD45.2) mice. Seven or eight days later, recipient mice were infected i.p. with ~1x107 CFU of E. coli and sacrificed after two days to measure bacterial CFUs and cell chimerism. Mice that received splenocytes from mobilized mice (Group 3) were more efficient at reducing the systemic load of E. coli compared with mice receiving un-mobilized splenocytes (Group 1) and mice receiving splenocytes where mobilization was blocked (Group 4) (Figure 7B). Donor splenocytes containing mobilized HSPCs contributed robustly to Ly6Ghi Mac1+ neutrophils and CD115+ Mac1+ monocytes in the blood and peritoneum compared with recipients of other groups (Figure 7C and D), which correlated with reduced systemic bacterial load in these mice. Together, these data indicate that splenic HSPCs, mobilized by dual NLR and TLR signaling, give rise to enhanced numbers myeloid cells and contribute to the fight against infection.
Figure 7. NOD1 and TLR4 mobilized HSPCs contribute to limiting infection.
(A) Schematic of experiments in (B–D). (B) Colony forming units (CFUs) of E. coli NI491 per gram of spleen or liver two days after infection. Each data point represents CFUs from one mouse. (C) Peripheral blood chimerism one day before and two days after infection. Neutrophils were defined as Ly6Ghi Mac1+ and monocytes were defined as CD115+ Mac1+. (D) Total cell numbers and chimerism from peritoneal cavity two days after infection. Black bars represent donor splenocyte derived cells, while grey bars represent residual recipient derived cells. n = 5–6 mice per group combined from two independent experiments. * p < 0.05 ** p < 0.01 by One-way ANOVA for all graphs. Error bars represent SEM.
Discussion
A growing body of evidence has emerged supporting a role for hematopoietic progenitors, including long term multilineage repopulating HSCs, in the fight against infection, yet mechanisms governing the response of HSCs to infection are poorly understood. Here we have demonstrated that systemic infection with the Gram negative bacterium Escherichia coli drives the mobilization and accumulation of long term multilineage repopulating HSCs in the spleen along with a moderate reduction of HSCs in the bone marrow. Moreover, we find that activation of both Toll-like receptors and NOD-like receptors are important for the accumulation of splenic HSCs after E. coli infection. Together, our results illustrate that detection of bacterial components via pattern recognition receptors is a crucial step in the initiation of extramedullary hematopoiesis following infection.
Systemic administration of LPS results in the mobilization of HSPCs (Vos et al., 1972), but the cell types and signaling pathways responsible for this phenomenon are unclear. We found that the accumulation of splenic HSCs following infection was largely dependent on TLR4 signaling and partially dependent on NLR signaling via the adaptor protein RIPK2. Importantly, administration of ultrapure LPS alone was not sufficient to induce the accumulation of HSCs in the spleen, whereas dual administration of LPS and the synthetic NOD1 agonist KF1B was sufficient. Thus, our studies highlight a previously unappreciated role for NLR signaling in promoting extramedullary hematopoiesis in the context of infection and suggest that contamination of LPS preparations with NLR-activating peptidoglycan moieties likely contributed to the mobilization of HSPCs observed previously. Interestingly, whereas a recent report found that multipotent progenitors are capable of directly responding to TLR ligands and producing cytokines to coordinate inflammatory responses (Zhao et al., 2014), our data point towards radio-resistant cells such as endothelial cells, as initiators of the mobilization response following infection. In support of this concept, HSCs did not require the expression of TLR4 on their surface to accumulate in spleen following PRR activation. In fact, more Tlr4−/− HSCs accumulated in spleen compared with WT HSCs. The reason for this is unclear, but one possibility is that Tlr4−/− HSCs may have weaker associations with bone marrow niches and might be preferentially mobilized when directly competing with WT HSCs.
It was originally unclear whether the accumulation of splenic HSCs that occurs following infection was due to expansion of resident HSCs or mobilization of HSCs from the bone marrow. In support of mobilization, we found that less than 5% of splenic HSCs were actively cycling at a time when their numbers were rapidly increasing. Moreover, we found that dual activation of TLR4 and NOD1 resulted in the synergistic production of G-CSF in the serum, which correlated with accumulation of HSCs in the spleen. Inhibition of G-CSF signaling either by a G-CSF blocking antibody or lack of the G-CSF receptor was sufficient to limit HSC accumulation in the spleen, indicating that G-CSF plays a crucial role downstream of PRR activation to promote HSC mobilization during infection.
The spleen serves as a site for extramedullary hematopoiesis during periods of stress, yet the spleen is not essential for life. The biological significance for an expanded pool of splenic HSCs during infection is not clear. We found that HSPCs which had been mobilized to the spleen by PRR stimulation preferentially gave rise to neutrophils and monocytes and reduced the systemic bacterial burden following transplantation and infection with E. coli. Given the immense need for cell replenishment during systemic infection, it is likely that mobilization of HSPCs to the spleen provides an additional site for generation of effector cells during infection. This is supported by evidence that the spleen serves as a reservoir for monocytes that contribute to the resolution of sterile inflammation (Swirski et al., 2009).
Another non-exclusive possibility is that excessive inflammatory signaling within the bone marrow microenvironment might transiently compromise the stem cell niche. We and others have found that CXCL12 is downregulated in bone marrow after exposure to bacteria or their components and this was associated with bone marrow hypocellularity (Delano et al., 2011; Johns and Borjesson, 2012; Ueda et al., 2005). Interestingly, CXCL12 also contributes to the retention of neutrophils in bone marrow and mobilization of neutrophils during polymicrobial sepsis is required for bacterial clearance and host survival (Delano et al., 2011). Moreover, physiologic trafficking of HSPCs between the bone marrow and blood stream is influenced by the level of circulating neutrophils and removal of aged neutrophils by bone marrow macrophages promotes HSPC mobilization (Casanova-Acebes et al., 2013). Egress of HSCs could merely represent a bystander effect secondary to an immediate requirement for peripheral granulocytes, or it could serve as a protective mechanism to maintain these cells until the infection is cleared and the bone marrow niche is repaired. Surprisingly, we did not observe any accumulation of CD150+ CD48− LSK cells in the liver or inguinal lymph nodes of E. coli infected mice, suggesting that the spleen may serve as a specialized niche to support HSC function during systemic infection.
The mobilization of HSCs during bacterial infection highlights the dynamic nature of stem cells and their niche(s) in the setting of stress. Our results support the hypothesis that the initiation of extramedullary hematopoiesis during infection is a consequence of alterations in the HSC microenvironment initiated by recognition of bacterial components via host pattern recognition receptors. A better understanding of the signals that influence HSCs and their niche(s) during infection may lead to improved methodologies for isolation and expansion of HSCs in the clinical setting.
Experimental Procedures
Mice
CD45.2 (C57BL/6) and CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice were purchased from Jackson Laboratories. Nod1−/− mice were kindly provided by Grace Chen (University of Michigan, USA). Csf3r−/− (G-CSFR−/−) mice were kindly provided by Daniel Link (Washington University, USA). Myd88−/−, Trif−/− and Tlr4−/− were kindly provided by Shizuo Akira (Osaka University, Japan). All mice were maintained under SPF conditions at the University of Michigan. All experiments were approved by the University of Michigan Committee on the Care and Use of Animals.
Reagents
KF1B has been reported (Masumoto et al., 2006). E. coli K12 was obtained from ATCC (ATCC 19798). E. coli NI491 was isolated from the intestine of a C57BL/6 mouse and the bacterial species verified by 16S rRNA sequencing. Ultrapure LPS (E. coli K12) was purchased from Invivogen. Antibodies targeting G-CSF (clone 67604) and rat IgG1 isotype control were a gift from CSL Limited, Australia, and used as described previously (Lawlor et al., 2004).
Bacterial culture and infection
E. coli K12 or NI491 was picked from frozen glycerol stock into LB broth, shaken at 37° C overnight, and sub-cultured in LB for another 2–3 hours. Bacteria were washed multiple times in PBS and numbers were estimated by optical density at 600 nm. Mice were injected i.p. with bacteria diluted in PBS and residual bacteria was serially diluted in PBS and plated on LB agar to quantitate CFU.
Cell isolation and flow cytometry
Bone marrow cells were isolated by flushing one femur and tibia with 3 mL Ca2+ and Mg2+ free HBSS supplemented with 2% HI FBS, filtered through a 50 μm mesh and counted using a hemocytometer. Splenocytes were obtained by mashing between two glass slides and filtered through a 100 μm mesh. Lineage cocktail was comprised of biotin labeled lineage panel (CD3e 145-2C11, B220 RA3-6B2, TER119, Gr-1 RB6-8C5, Mac1 M1/70 eBioscience) along with biotin CD4 (GK1.5 BioLegend) and CD8a (53-6.7 BioLegend) followed by staining with Streptavidin Pacific Orange (Invitrogen). Antibodies used to stain HSPCs included PE CD150 (TC15-12F123.2 BioLegend), PE Cy7 CD48 (HM48-1 BioLegend), PE Cy7 CD41 (MWReg eBiosciences), APC Sca1 (E13-161.7 BioLegend), APC Cy7 cKit (2B8 BioLegend). Antibodies used to stain peripheral blood included: FITC CD45.2 (104 BioLegend), PE CD3e (145-2C11 BioLegend), PE CD115 (AFS98 BioLegend), PE Cy5 B220 (RA3-6B2 eBioscience), PE Cy7 Gr-1 (RB6-8C5 BioLegend), PE Cy7 Ly6G (1A8 BD Pharmingen), APC Mac1 (M1/70 eBioscience), APC Cy7 CD45.1 (A20 eBioscience), biotin CD3e (145-2C11, eBioscience), biotin CD4 (GK1.5 BioLegend) and biotin CD8a (53-6.7 BioLegend).
Long-term reconstitution assays
Ten week or older recipient mice were lethally irradiated using X-ray (Phillips RT250, Kimtron Medical) with two doses of 540 rad (total 1080) delivered between 3 and 24 hours apart. In some experiments, 3x105 WBM or 1x106 splenocytes were mixed with 3x105 competitor WBM and transplanted via tail vein injection. To determine the surface marker profile of HSCs after infection, WBM or spleen was harvested from mice after six days, purity sorted to enrich for the positive or negative fraction, purity sorted again and mixed with 3x105 WBM prior to retro-orbital injection. Recipient mice were maintained on antibiotic water (neomycin sulphate 1.11g/L and polymyxin B sulfate 0.121 g/L, 50 g/L sucrose) for at least 6 weeks. In experiments where live E. coli exposed cells were transplanted, recipient mice received 5 mg/kg enrofloxacin (Bayer) i.v. at day 0 and s.c. at day 5 post transplant. To generate reverse chimeras, 5x106 WBM from WT, Tlr4−/− or Ripk2−/− mice was transplanted via tail vein into lethally irradiated WT, Tlr4−/− or Ripk2−/− mice and recipients were allowed to recover for 16 weeks prior to challenge. For sub-lethal irradiation, recipient mice received a single dose of 800 rad.
C166 endothelial cell culture
C166 yolk sac endothelial cells (ATCC CRL-2581) were cultured in DMEM supplemented with 10% FBS and pen/strep. Ultrapure LPS (Invivogen; E. coli 011:B4) was added to media at final concentration of 10 ng/mL, while KF1B was added at a final concentration of 1 ug/mL. For stimulation with bacterial supernatants, E. coli K12 was shaken overnight at 37° C in LB media, centrifuged and passed through 0.2 um filter.
Quantitative PCR
Cells were homogenized using a column (Omega) and RNA was isolated using a Total RNA kit (Omega). Eluted RNA was reverse transcribed into cDNA using High-Capacity RNA-to-cDNA kit (Applied Biosystems). Quantitative real-time PCR was performed using SYBR Green (Applied Biosystems). Gene expression was normalized to the house keeping gene GAPDH. Primers for CXCL12 were F- TGCATCAGTGACGGTAAACCA and R- GTTGTTCTTCAGCCGTGCAA and for GAPDH were F- TGCGACTTCAACAGCAACTC and R- GCCTCTCTTGCTCAGTGTCC.
Methylcellulose culture
Splenocytes were counted using a hemocytometer, diluted in Methocult M3434 medium (Stem Cell Technologies) and incubated at 37 C for 8–12 days after which myeloerythroid colonies were quantified.
Statistical Analysis
All statistics were performed using Graphpad Prism software. Statistical tests used are described in Figure Legends.
Supplementary Material
Highlights.
Systemic E. coli infection mobilizes expanded bone marrow HSCs to the spleen
Dual stimulation of NOD1 and TLR4 is required for HSC expansion
TLR4 and NOD1 synergistically induce G-CSF, which is required for HSC mobilization
Mobilized HSCs give rise to myeloid cells to limit infection
Acknowledgments
We would like to thank L. Franchi and B. Moore for helpful discussions. We would like to thank J. Whitfield of the University of Michigan Immunology Core for preforming ELISAs and Mizuho Hasegawa for providing E. coli strain NI491. Flow cytometry costs were partially defrayed by the U of M Cancer Center. A. Burberry was partially supported by the Training Program in Organogenesis grant T32HD007505. M. Zeng was supported by Training Grant T32DK094775. This work was supported by grant RO1 DK61707 from the National Institutes of Health to G. Nunez.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, NAG, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. The Journal of clinical investigation. 2010;120:4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Kelly-Scumpia KM, Thayer TC, Winfield RD, Scumpia PO, Cuenca AG, Harrington PB, O’Malley KA, Warner E, Gabrilovich S, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol. 2011;187:911–918. doi: 10.4049/jimmunol.1100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhrsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. [PubMed] [Google Scholar]
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic Exposure to a TLR Ligand Injures Hematopoietic Stem Cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biological chemistry. 2002;383:1501–1517. doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Experimental hematology. 1993;21:206–219. [PubMed] [Google Scholar]
- Howie D, Okamoto S, Rietdijk S, Clarke K, Wang N, Gullo C, Bruggeman JP, Manning S, Coyle AJ, Greenfield E, et al. The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon gamma production. Blood. 2002;100:2899–2907. doi: 10.1182/blood-2002-02-0445. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. The Journal of biological chemistry. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- Johns JL, Borjesson DL. Downregulation of CXCL12 signaling and altered hematopoietic stem and progenitor cell trafficking in a murine model of acute Anaplasma phagocytophilum infection. Innate immunity. 2012;18:418–428. doi: 10.1177/1753425911413794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JL, Macnamara KC, Walker NJ, Winslow GM, Borjesson DL. Infection with Anaplasma phagocytophilum induces multilineage alterations in hematopoietic progenitor cells and peripheral blood cells. Infect Immun. 2009;77:4070–4080. doi: 10.1128/IAI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Morrison SJ. CD150− cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111:4413–4414. doi: 10.1182/blood-2007-12-129601. author reply 4414–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- Lawlor KE, Campbell IK, Metcalf D, O’Donnell K, van Nieuwenhuijze A, Roberts AW, Wicks IP. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11398–11403. doi: 10.1073/pnas.0404328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Current opinion in organ transplantation. 2008;13:53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. The Journal of experimental medicine. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends in immunology. 2011;32:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux G, Pojda Z, Hampson IN, Lord BI, Dexter TM. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153–2158. [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz A, Bachelet I, Eliashar R, Khodoun M, Finkelman FD, Rothenberg ME, Levi-Schaffer F. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. Journal of immunology. 2006;177:77–83. doi: 10.4049/jimmunol.177.1.77. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature immunology. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA, et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064–4076. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118:4530–4540. doi: 10.1182/blood-2011-06-318220. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos O, Buurman WA, Ploemacher RE. Mobilization of haemopoietic stem cells (CFU) into the peripheral blood of the mouse; effects of endotoxin and other compounds. Cell Tissue Kinet. 1972;5:467–479. doi: 10.1111/j.1365-2184.1972.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Zhang P, Nelson S, Bagby GJ, Siggins R, 2nd, Shellito JE, Welsh DA. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Ma C, O’Connell RM, Mehta A, Diloreto R, Heath JR, Baltimore D. Conversion of Danger Signals into Cytokine Signals by Hematopoietic Stem and Progenitor Cells for Regulation of Stress-Induced Hematopoiesis. Cell stem cell. 2014 doi: 10.1016/j.stem.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.