Abstract
Objectives
Serine protease inhibitor Kazal type-5 (SPINK5) plays a crucial role in deciding the timing of desquamation of the skin. Its gene expression is limited at the very surface of the stratum granulosum (SG), whereas expression of kallikreins (KLKs) encoding proteases is usually found throughout the stratum spinosum and SG.
Methods
To explore the difference in expression regulation of these proteases/inhibitors, the function of SPINK5 promoter was examined using luciferase assay.
Results
Luciferase assay targeting the SPINK5 promoters (nucleotide −676/−532 and −318/−146 from the major transcription start site) showed high intensity in NHEK human keratinocyte. These two sites had neither common cis-elements nor GATA3 element but electrophoretic mobility shift assay showed similar retardation bands. Moreover, DNA footprinting did not display specific protected bands. Thus, we could not identify cis-element(s) that controlled these elements. Differentiation induced by high Ca2+ medium failed to alter their luciferase activities. Transfection of GATA3 expressing vector significantly but slightly increased them and that of vector expressing its dominant negative form decreased.
Conclusions
Although GATA3 is reportedly important for inhibition of proliferation and induction of differentiation of keratinocytes, its effect on SPINK5 expression was indirect and GATA3 alone was insufficient for final differentiation of keratinocytes where full SPINK5 expression was observed.
Keywords: Serine protease inhibitor Kazal type-5 (SPINK5), Human keratinocyte, GATA3, Transcription regulation
Introduction
To maintain a steady number of the stratum corneum (SC) layers is an important factor for the solid skin barrier function. In the SC adjacent to the stratum granulosum (SG), corneocytes are tightly bound to each other through the corneodesmosomes consisted of extracellular corneodesmosin, transmembrane desmoglein-1 and desmocollin-1, and cytoplasmic desmosomal plaque proteins (plakoglobin) [1, 2]. These firm contacts reduce toward the surface and finally disappear on the surface where desquamation of the corneocytes occurs [3]. This reduction is due to corneodesmosome degradation caused by serine protease activities supplied by the tissue kallikrein gene (KLK) family proteins consisting of fifteen members known as KLK1 to KLK15 [4]. Their activities are regulated by serine protease inhibitor Kazal type-5 (SPINK5) [5, 6] to match the shedding schedule to the proliferation rate of keratinocytes [7, 8]. This turnover is very steady and maintained regardless of age [9, 10] on behalf of serine protease-SPINK5 balance. In fact, the loss of function mutation of SPINK5 gene causes hyperdesquamation and loss of the SC known as Netherton syndrome [11–13].
Thus, KLK and SPINK5 proteins co-localize and interact with each other at the SC [14]. Nonetheless, expression of KLKs is usually found throughout the stratum spinosum (SS) and SG [5] and, on the other hand, expression of SPINK5 is limited at the very surface of SG [12]. GATA3 plays a very important role for the ubiquitous expression of KLKs [15], whereas the major regulators of local expression of SPINK5 are yet known. Limited expression of SPINK5 plays a crucial role on avoidance of premature- and/or hyperdesquamation. To clarify the basis of such limited expression of SPINK5, the function of its promoter was explored in the present study.
Materials and methods
Keratinocyte culture
Normal human keratinocyte (NHEK; Kurabo, Osaka, Japan, several different single donors) was inoculated at a concentration of 2,500 cells/cm2 in culture media, HuMedia-KG2 (Kurabo) supplemented with insulin 10 µg/ml, human epidermal growth factor 0.1 ng/ml, hydrocortisone 0.5 µg/ml, bovine pituitary extract (BPE) 0.4 % v/v, gentamycin 50 µg/ml and amphotericin B 50 ng/ml. The culture was maintained at 37 °C in a 95 % O2/5 % CO2 humidified chamber. The cells were subcultured twice in the media of which calcium concentration was as low as 0.15 mM to avoid differentiation. In vivo differentiation was induced as described elsewhere [16]. In brief, at the second passage when it became 40–60 % confluent, the media was changed to normal Ca2+ medium (0.15 mM Ca2+; low Ca2+) or high Ca2+ medium (1.5 mM Ca2+) in the absence of BPE and cultured for 2 days.
Reporter vectors
The proximal promoter of the human SPINK5 gene [nucleotide −1 to −1141 from the major transcriptional start site (Accession No; Chromosome 5—NC_000005.9)] was PCR amplified using Sac I- and Nhe I-tailed primers, respectively, −1141 and −1 (Table 1), from the genome of NHEK as a template. Evolutional conservation search between human and Rhesus macaque (rheMac2) using ECR browser (http://ecrbrowser.dcode.org) displayed that −1 to −1214 was a putative promoter enhancer. Unfortunately, the primer search program (DNasis-Mac v3.6) selected a primer start with −1141 instead of −1214 was suitable for PCR amplification. After digestion with Sac I and Nhe I, the amplified products were initially cloned into pGL4.10[luc2] (Promega, Madison, WI, USA), designated as −1141/−1. To generate clones with different 5′ terminal length, PCR was performed using different 5′ primers for deletion and the 3′ primer to conserve pGL4 sequence (Table 1). After digestion with Sac I, the products were re-ligated to obtain deletion clones. All cloned promoters in the reporter construct were sequenced to confirm that their sequence was conserved after cloning.
Table 1.
Primers to obtain SPINK5 promoter
| For initial clone | Downstream primer | Promoter included | |
|---|---|---|---|
| −1141 | SacI-CTGGAATGAAACAATTTGGCTCTGG | −1 | −1141/−1 |
| −1 | NheI-TGCTCAGCTGGTGCAGTATGACT | ||
| For deletion | |||
| −1076 | SacI-GCTATTAGGGCAAAATAAGCTGGTA | pGL4 | −1076/−1 |
| −932 | SacI-GAGACTAAATAATGGGATGATACAGG | pGL4 | −932/−1 |
| −861 | SacI-CGGTAATATGGGTAATCATAGGCTA | pGL4 | −861/−1 |
| −676 | SacI- GGATGAAGCAGTGTACAGAGAGG | pGL4 | −676/−1 |
| −555 | SacI-AGTTCCTGCCTCCCTATATATTCA | pGL4 | −555/−1 |
| −437 | SacI-CCCTGGATTTCTCTGGATGTGGA | pGL4 | −437/−1 |
| −318 | SacI-GTCTACGAGGTAGTATATCTGGTAT | pGL4 | −318/−1 |
| −169 | SacI-ATTCTCTGTTCAAACCTGACACCC | pGL4 | −169/−1 |
| pGL4 | Sac I-AGGCCAGAGAAATGTTCTGGCACC | ||
| For fragmentation | |||
| −555c | TGAATATATAGGGAGGCAGGAACT | −677 | −676/−532 |
| −169c | GGGTGTCAGGTTTGAACAGAGAAT | −318 | −318/−146 |
Numbers indicate the location of 5′ terminal base counted from the transcription initiation site. Primers −555c and −169c were complementary to −555, and −169, respectively, and hence the numbers were the locations of 3′ terminal base
Other vectors used
pGL−954/+40; the vector possessed the highest luciferase activity among reporter vectors containing KLK1 promoter [15], pcDNA3.1 (Invitrogen, Carlsbad, CA, USA), pcGATA3; the coding region of GATA3 (the open reading frame with 119 bp upstream to the start codon and 33 bp downstream from the stop codon) was cloned into pcDNA3.1, and pcGATA3mut; to obtain dominant negative mutant of pcGATA3, its sequence corresponding to 305KRR was changed to AAA [15].
SPINK5 promoter activity in NHEK cells
NHEK cells cultured on 24-well plates were transfected with 0.75 μg of reporter vectors pGL4.10[luc2] cloned with different fragments from the proximal promoter of the human SPINK5 using Tfx™-20 (Promega, Madison, WI, USA). After 48 h, the cells were lysed using a luciferase reporter assay system (Promega, Madison, WI, USA) and the firefly luciferase activities were measured with a Lumat LB9507 (Berthold Technologies, Tokyo, Japan). All the experiments were performed at least three times for each reporter plasmid and the relative luciferase activity was calculated. Results were presented as the mean ± SD. To confirm that the putative sequence was really active, −676/−532 or −318/−146 was PCR amplified using a set of primers (Table 1), used for electrophoretic mobility shift assay (EMSA) and cloned into pGL4.10[luc2] for the luciferase assay.
Search for putative cis-elements
To find putative cis-elements, sequence of −1141/−1 was subjected to TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html), ALGGEN PROMO [17, 18] and PhysBinder (http://bioit.dmbr.ugent.be/physbinder/ [19]).
Electrophoretic mobility shift assay (EMSA)
According to the results of luciferase assay, −676/−532, and −318/−146 supposed to contain activating element, were PCR amplified using primers listed in Table 1. Nuclear protein was obtained from 1 × 106 NHEK cells cultured with KG2, or 0.15 and 1.5 mM Ca2+ media without BPE, based on the protocol [20]. In brief, after washing with ice-cold PBS (Ca2+ and Mg2+ free), cells were resuspended with 5 volumes of ice-cold cell homogenization buffer (10 mM HEPES–KOH, pH7.8, 1.5 mM MgCl2, 10 mMKCl, 0.5 mM DTT, 0.5 mM PMSF, and 1 × proteinase inhibitor cocktail (Nakalai tesque, Kyoto, Japan) to stand on ice for 10 min, and collected by centrifuging at 250g for 10 min. The cell pellet was resuspended in 3 volumes of ice-cold cell homogenization buffer containing 0.05 % (v/v) Nonidet P-40, and homogenized on ice with 20 strokes of a tight-fitting Dounce homogenizer. The nuclei were collected by centrifugation at 250g for 10 min at 4 °C. After removing the supernatant, the nuclei pellet was resuspended with 20 ml of cell suspension buffer (40 mM HEPES–KOH, pH 7.8, 0.4 M KCl, 10 % glycerol, 1 mM DTT, 0.1 mM PMSF, and 1 × proteinase inhibitor cocktail). NaCl was added to a final concentration of 300 mM, and the resuspended nuclei were incubated on ice for 30 min then centrifuged at 15,093g at 4 °C for 15 min. The supernatant containing nuclear protein was divided into aliquots and stored at −80 °C until use. Protein concentration was determined by the Bradford method on Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA). After 100 ng of each DNA was mixed with 6 μg of nuclear protein in a final volume of 20 μl of binding buffer containing 10 mM Tris/HCl (pH 8.0), 50 mM KCl, 3.5 mM DTT, 0.25 % Tween 20, and 0.025 μg salmon sperm DNA and allowed to stand for 25 min at room temperature, electrophoretic mobility shift assay (EMSA) was performed on 1 % agarose/ethidium bromide gel. Poly(dI-dC) as DNA and BSA as protein were served as negative control.
DNA footprinting
To clarify the location that nuclear protein was attached, DNA footprinting using DNase I protection was performed on pGLSP−676/−532 and pGLSP−318/−146 that showed high luciferase activities. pGL−954/+40 was served as positive control. These DNAs were bound with nuclear protein as described above. To this mixture, 1 U RQ1 RNase-free DNase I (Promega) and supplied DNase I buffer were added and allowed to stand at room temperature for 1 min. The digested DNAs were phenol/chloroform extracted and ethanol precipitated. Footprinting ladder was PCR synthesized using PCR primer, RVprimer3 by BigDye (Applied Biosystems, CA). The sequence was analyzed by ABI Prism 3100 (Applied Biosystems).
Statistical analysis
To clarify statistical significance among luciferase activities, one-way ANOVA with Tukey’s HSD test as post hoc test was used.
Results
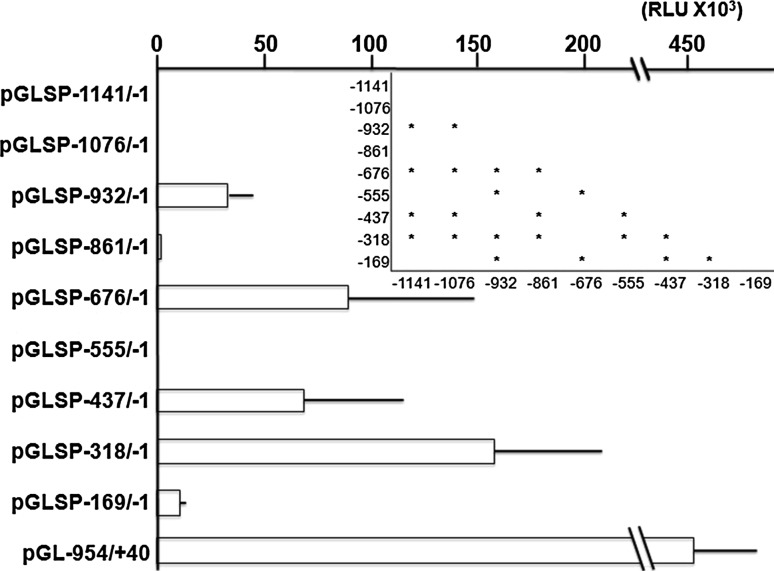
To examine the location of putative activating and/or inhibitory cis-elements, reporter vectors were transfected into NHEK cells and luciferase assay was performed. Transcriptional activity was almost null when pGLSP−1141/−1 and pGLSP−1076/−1 were transfected to NHEK (Fig. 1). When pGLSP−932/−1, a clone deleted −1141 to −932, was transfected, a significantly higher intensity than pGLSP−1141/−1 and pGLSP−1076/−1 was observed. Moreover, transfection of pGLSP−676/−1, a clone deleted −1141 to −676, and pGLSP−318/−1, a clone deleted −1141 to −318, gave significantly higher intensity than other clones. The intensities of these two clones did not show significant difference. The intensity of pGL−954/+40 containing KLK1 promoter was more than three times higher than that of pGLSP−318/−1.
Fig. 1.
Relative luciferase activities of reporter plasmids containing different lengths of putative SPINK5 promoter in NHEK. Each column represents the mean of three independent experiments, each done in triplicate; bars, ± SD. Inset; Significant differences in the intensity between the groups (P < 0.05 One-way ANOVA and post hoc test using Tukey’s HSD test). When pGL−954/+40 was transfected, the intensity was higher than 450 × 103 RLU
Effect of high Ca2+ medium on transcriptional activity of SPINK5 putative promoter
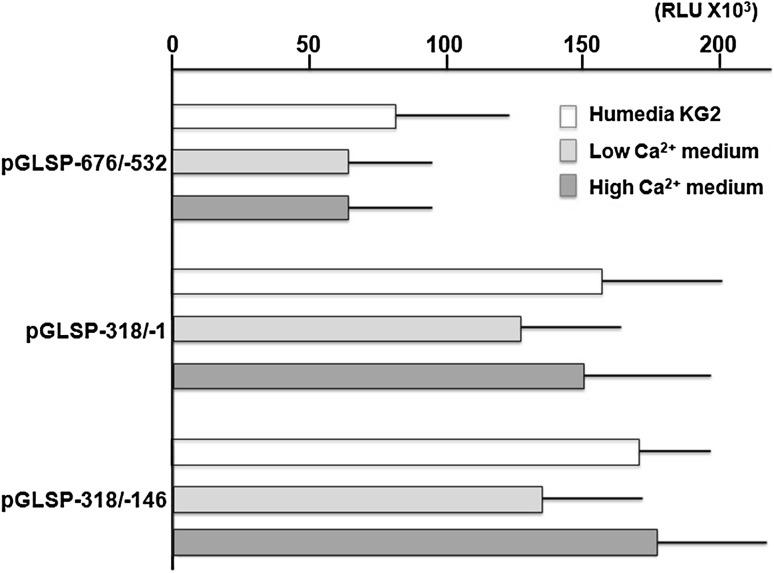
Intensities of pGLSP−676/−1 and pGLSP−318/−1 were significantly higher than that of pGLSP−932/−1, and those of pGLSP−318/−1 and pGLSP−318/−146 were almost the same (Fig. 2). Thus, pGLSP−676/−1, pGLSP−318/−1 and pGLSP−318/−146 were subjected to examine the effects of high Ca2+ medium. Removal of BPE from Humedia-KG showed no significant differences in the intensities of pGLSP−676/−532, pGLSP−318/−1 and pGLSP−318/−146 (Fig. 2). High Ca2+ media-cultured NHEK also did not show any differences in their intensities.
Fig. 2.
Effect of high Ca2+ medium on relative luciferase activities of SPINK5 putative promoters. pGLSP−676/−532, pGLSP−318/−1 and pGLSP−318/−146 were transfected to normal HuMedia-KG2-, low Ca2+ medium-, and high Ca2+ medium-cultured NHEK. Each column represents the mean of three independent experiments, each done in triplicate; bars, ± SD. No significant difference was observed in any of pGLSP−932/−1, pGLSP−676/−1 and pGLSP−318/−1 among different culture media (One-way ANOVA and post hoc test using Tukey’s HSD test)
TFSEARCH, ALGGEN PROMO and PhysBinder to search putative cis-elements
Sequence of −1141/−1 was subjected to TFSEARCH, ALGGEN PROMO and PhysBinder for searching putative cis-elements. Among elements hit by these programs, those that did not exist in −1141/−933, −860/−677, −554/−319, −169/−1 were listed (Table 2). Excluding SRY, no common cis-elements existed in the activating regions −932/−837, −676/−532 and −318/−146. A point mutation on −206 G to A reportedly produces GATA3-binding site [21], but our −206 was G. Thus, no GATA3-binding site was extracted in any of the activating regions.
Table 2.
Putative cis-elements located at −932/−837, −676/−532, and −318/−146
| −932/−862 | −676/−532 | −318/−146 | |
|---|---|---|---|
| POU2F2 | STAT5A | HNF-4a | HSF1 |
| SRY | AML-1a | LEF-1 | HSF2 |
| E2F | Nkx-2 | TCF-4 | Oct-1 |
| YY-1 | SRY | CDP-CR | |
| TCF-4E | MZF-1 | ||
| STAT1 | |||
Putative cis-elements were searched using TFSEARCH (www.cbrc.jp/research/db/TFSEARCH.html) and ALGGEN PROMO [17, 18] and PhysBinder [19]. Those did not exist in −1141/−933, −860/−677, −554/−319, −169/−1 were listed
Electrophoretic mobility shift assay (EMSA)
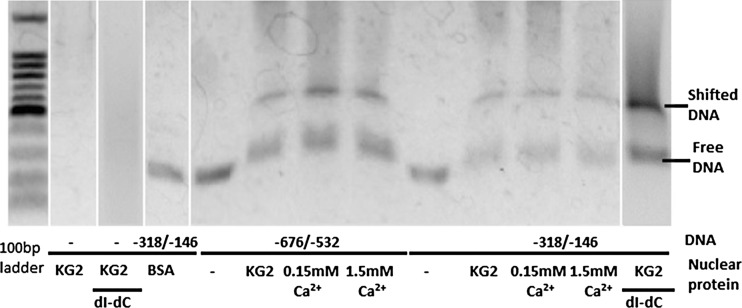
−676/−532 and −318/−146 supposed to contain activating element were PCR amplified using primers listed in Table 1 and subjected to EMSA (Fig. 3). Mobility of both fragments was delayed by all of nuclear extracts from normal HuMedia-KG-, low Ca2+ medium-, and high Ca2+ medium-cultured NHEK. Location of retarded bands was almost identical regardless of −676/−532 or −318/−146 and regardless of culture media.
Fig. 3.
EMSA for fragments −676/−532 and −318/−146. To avoid non-specific binding, poly(dI-dC) was utilized. Aside from nuclear proteins, bovine serum albumin (BSA) was served as negative control
Effect of pcGATA3 and pcGATA3mut on SPINK5 promoter
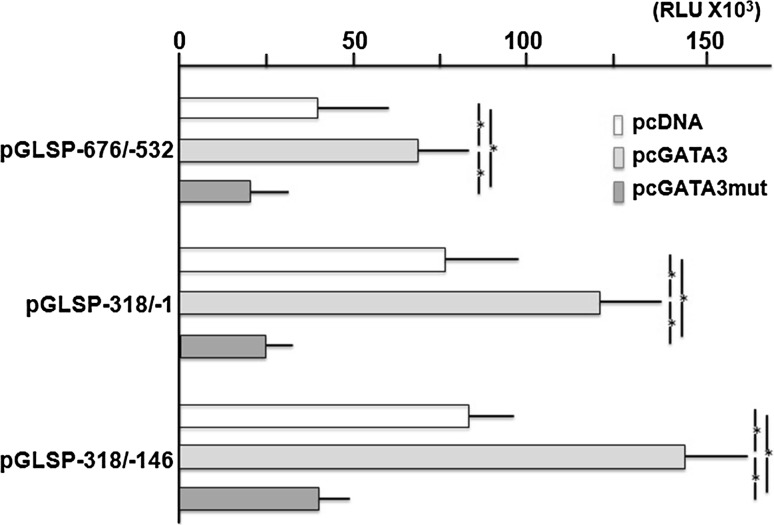
Co-transfection of pcGATA3 with pGLSP−676/−532, −318/−146 and −318/−1 increased the intensities significantly (Fig. 4). However, their intensities were still <1/3 of that of pGL−954/+40. On the other hand, pcGATA3mut co-transfection decreased the activities significantly in any of the plasmids.
Fig. 4.
Effects of co-transfection of pcGATA3 and pcGATA3mut with pGLSP−676/−532, pGLSP−318/−1 and pGLSP−318/−146. Each column represents the mean of three independent experiments, each done in triplicate; bars, ± SD. *Significant difference (One-way ANOVA and post hoc test using Tukey’s HSD test)
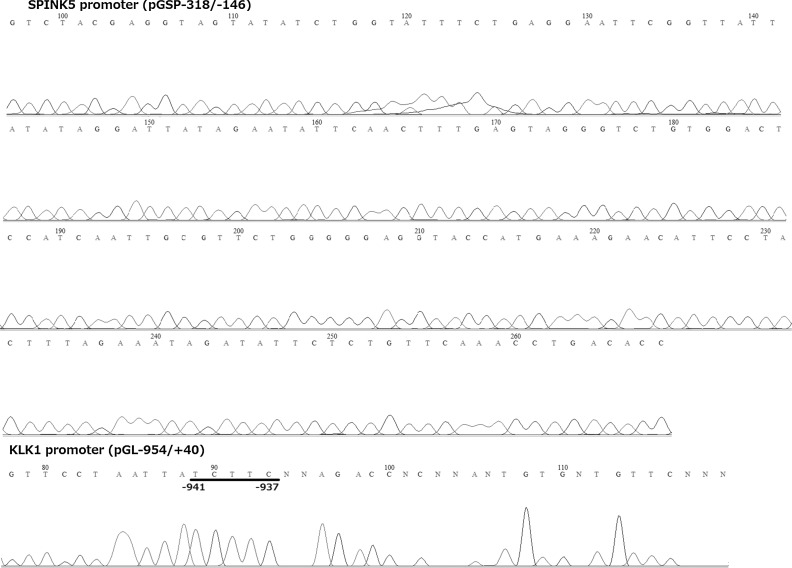
DNA foot printing
When pGLSP−676/−532 and pGLSP−318/−146 were subjected to DNA footprinting, no specific sequence was protected (pGLSP−676/−532; data not shown and pGLSP−318/−146, Fig. 5). However, in case of pGL−954/+40, protected band was found at −941/−937 where STAT4, c-Ets-1, c-Ets-2, Elk-1, Pax-5 and p53 could be putative transcription factors. Thus, we could not specify cis-elements for pGLSP−676/−532 and pGLSP−318/−146 and EMSA using oligo-DNA was currently unavailable.
Fig. 5.
Sequence ladder of pGSP−318/−146 (Upper) and pGL−954/+40 (Lower; positive control) after digestion by DNaseI along with nuclear protein. Sequence ladder was made by the primer, RV3 (supplied for pGL4.10[luc2]). Underline; protected sequence where TFSEARCH, ALGGEN PROMO, and PhysBinder selected STAT4, c-Ets-1, c-Ets-2, Elk-1, Pax-5 and p53 as putative cis-elements
Discussion
It was surprising that pGLSP−676/−1 and pGLSP−318/−1 displayed high luciferase activities in NHEK cells. NHEK cells were cultured in culture media containing 0.15 mM Ca2+ to avoid differentiation. Expression of SPINK5 is limited at the very surface of SG, namely SPINK5 expression is limited at the very last step of differentiation just before denucleation [5]. Thus, in NHEK cells without induction of differentiation, no luciferase activity was expected in any of reporter constructs. However, their intensities were only <1/3 of that of pGL−954/+40 containing KLK1 promoter. Expression of KLK1 is observed throughout the SS and SG [5]. It is not surprising that, independent of 0.15 mM Ca2+, KLK1 promoter appeared to have an activity in NHEK cells with very high intensity. Therefore, some NHEK seemed to differentiate to the final step in this medium even when Ca2+ concentration was maintained at 0.15 mM and growth factors like BPE were included. Growth factors are considered to suppress keratinocyte differentiation [22, 23]. However, neither removal of growth factors alone (low Ca2+ medium) nor removal of growth factors and an increase in Ca2+ concentration (high Ca2+ medium) altered the intensity of luciferase and the movability of EMSA bands, indicating that such stimulation is not suitable for the final differentiation. On the other hand, high cell density reportedly promotes keratinocyte differentiation [23]. It is conceivable that several colonial clones may differentiate further.
TFSEARCH, ALGGEN PROMO and PhysBinder did not find common transcription factors in the activating regions −932/−837, −676/−532 a3d −318/−146. Only SRY (Sex-determining region Y) was common to −932/−837 and −318/−146. However, SRY was encoded on Y chromosome and regulated sex differentiation [24], indicating that this transcription factor was not responsible for skin function. Judging from the results of EMSA, the size of transcription factor binding to −676/−532 and −318/−146 was almost the same. It is possible that the same transcription factor which could not be detected by these programs occupied −932/−837 and −318/−146. Otherwise, the results of DNA footprinting might indicate that more than one protein occupied these sequences.
An epidemiological research with case–control design in Chinese Han population indicated that −206 G > A mutation produced GATA3 binding site in SPINK5 promoter and that the G allele was significantly more common among patients with asthma than among the controls [21]. Existence of GATA3 site might directly increase SPINK5 expression. However, our −206 was G, hence no GATA3 binding site was extracted in neither −676/−532 nor −318/−146. Therefore, GATA3 unlikely affected directly on these sites. Although intensity was still <1/3 of pGL−954/+40, induction of GATA3 in NHEK increased their luciferase activity. On the other hand, transfection of dominant negative GATA3mut suppressed it. The effect of GATA3 was limited and indirect. This may be via transcription factor(s) downstream to GATA3. For example, GATA3 reportedly react with Smads [25] but none of TFSEARCH, ALGGEN PROMO, and PhysBinder found any Smads site in −676/−532 and −318/−146. It seems necessary to explore transcription factors downstream to GATA3 that could regulate these sites. However, unfortunately, we could not yet identify the transcription factor(s) that regulated SPINK5 expression in keratinocytes. Otherwise, inhibition of proliferation and induction of differentiation of keratinocytes by GATA3 [26] might trigger the next step of differentiation of keratinocytes and SPINK5 expression that was regulated by transcription factor(s)/cis-element(s) different from GATA3 system. That is, GATA3 is a very important factor for the differentiation of keratinocytes, but GATA3 itself seems to be insufficient for the final differentiation and/or SPINK5 expression.
It is very difficult to obtain sufficient amount of nuclear protein from human skin where SPINK5 expresses. It seems also very difficult to induce final differentiation to keratinocyte culture. High Ca2+ stimulation in the present study was insufficient for the final differentiation. Further examination on expression regulation mechanism of −676/−532 and −318/−146 will supply important information related to a key regulator of SPINK5 expression and final differentiation of keratinocytes.
Conflict of interest
We acknowledge that we have no conflict of interest.
References
- 1.North AJ, Bardsley WG, Hyam J, Bornslaeger EA, Cordingley HC, Trinnaman B, et al. Molecular map of the desmosomal plaque. J Cell Sci. 1999;112:4325–4336. doi: 10.1242/jcs.112.23.4325. [DOI] [PubMed] [Google Scholar]
- 2.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 3.Murphy G, et al. Istology of the skin. In: Edler D, et al., editors. Lever’s istopathology of the skin. 8. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 5–29. [Google Scholar]
- 4.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu N, Takata M, Otsuki N, Toyama T, Ohka R, Takehara K, et al. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J Invest Dermatol. 2003;121:542–549. doi: 10.1046/j.1523-1747.2003.12363.x. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu N, Saijoh K, Sidiropoulos M, Tsai B, Levesque MA, Elliott MB, et al. Quantification of human tissue kallikreins in the stratum corneum: dependence on age and gender. J Invest Dermatol. 2005;125:1182–1189. doi: 10.1111/j.0022-202X.2005.23933.x. [DOI] [PubMed] [Google Scholar]
- 7.Halprin KM. Epidermal “turnover time” a re-examination. Br J Dermatol. 1972;86:14–19. doi: 10.1111/j.1365-2133.1972.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 8.Pierard GE, Goffin V, Hermanns-Le T, Pierard-Franchimont C. Corneocyte desquamation. Int J Mol Med. 2000;6:217–221. doi: 10.1007/PL00010728. [DOI] [PubMed] [Google Scholar]
- 9.Ya-Xian Z, Suetake T, Tagami H. Number of cell layers of the stratum corneum in 304 normal skin—relationship to the anatomical location on the body, age, sex and physical parameters. Arch Dermatol Res. 1999;291:555–559. doi: 10.1007/s004030050453. [DOI] [PubMed] [Google Scholar]
- 10.Harding K. Challenges for skin and wound care in the new century. Adv Skin Wound Care. 2000;13(212):215. [PubMed] [Google Scholar]
- 11.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu N, Takata M, Otsuki N, Ohka R, Amano O, Takehara K, et al. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J Invest Dermatol. 2002;118:436–443. doi: 10.1046/j.0022-202x.2001.01663.x. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu N, Saijoh K, Jayakumar A, Clayman GL, Tohyama M, Suga Y, et al. Correlation between SPINK5 gene mutations and clinical manifestations in Netherton syndrome patients. J Invest Dermatol. 2008;128:1148–1159. doi: 10.1038/sj.jid.5701153. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu N, Saijoh K, Toyama T, Ohka R, Otsuki N, Hussack G, et al. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br J Dermatol. 2005;153:274–281. doi: 10.1111/j.1365-2133.2005.06754.x. [DOI] [PubMed] [Google Scholar]
- 15.Do NS, Li L, Katsuyama H, Komatsu N, Saito M, Tanii H, et al. Abundant expression of Kallikrein 1 gene in human keratinocytes was mediated by GATA3. Gene. 2009;436:121–127. doi: 10.1016/j.gene.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Matsui T, Hayashi-Kisumi F, Kinoshita Y, Katahira S, Morita K, Miyachi Y, et al. Identification of novel keratinocyte-secreted peptides dermokine-alpha/-beta and a new stratified epithelium-secreted protein gene complex on human chromosome 19q13.1. Genomics. 2004;84:384–397. doi: 10.1016/j.ygeno.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 18.Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucl Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broos S, Soete A, Hooghe B, Moran R, van Roy F, De Bleser P. PhysBinder: improving the prediction of transcription factor binding sites by flexible inclusion of biophysical properties. Nucl Acids Res. 2013;41:W531–W534. doi: 10.1093/nar/gkt288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abmayr SM, Yao T, Parmely T, Workman JL. Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr Protoc Mol Biol. 2006. doi:10.1002/0471142727.mb1201s75. [DOI] [PubMed]
- 21.Liu Q, Xia Y, Zhang W, Li J, Wang P, Li H, et al. A functional polymorphism in the SPINK5 gene is associated with asthma in a Chinese Han Population. BMC Med Genet. 2009;10:59. doi: 10.1186/1471-2350-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchese C, Rubin J, Ron D, Faggioni A, Torrisi MR, Messina A, et al. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: differentiation response distinguishes KGF from EGF family. J Cell Physiol. 1990;144:326–332. doi: 10.1002/jcp.1041440219. [DOI] [PubMed] [Google Scholar]
- 23.Poumay Y, Pittelkow MR. Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins. J Invest Dermatol. 1995;104:271–276. doi: 10.1111/1523-1747.ep12612810. [DOI] [PubMed] [Google Scholar]
- 24.Assumpcao JG, Benedetti CE, Maciel-Guerra AT, Guerra G, Jr, Baptista MT, Scolfaro MR, et al. Novel mutations affecting SRY DNA-binding activity: the HMG box N65H associated with 46, XY pure gonadal dysgenesis and the familial non-HMG box R30I associated with variable phenotypes. J Mol Med. 2002;80:782–790. doi: 10.1007/s00109-002-0376-9. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, He H, Pillai S, Xiong Y, Challa S, Xu L, et al. GATA3 transcription factor abrogates Smad4-mediated fascin overexpression, invadopodium formation and breast cancer cell invasion. J Biol Chem. 2013;288:36971–36982. doi: 10.1074/jbc.M113.506535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masse I, Barbollat-Boutrand L, Kharblili ME, Berthier-Vergnes O, Aubert D, Lamantine J. GATA3 inhibits proliferation and induces expression of both early and late differentiation markers in keratinocytes of the human epidermis. Arch Dermatol Res. 2014;306(2):201–8. doi:10.1007/s00403-013-1435-5. [DOI] [PubMed]