Abstract
Mannose-binding lectin (MBL) is a key factor in innate immunity, and lung infections are the leading cause of morbidity and mortality in cystic fibrosis (CF). Accordingly, we investigated whether MBL variant alleles, which are associated with recurrent infections, might be risk factors for CF patients. In 149 CF patients, different MBL genotypes were compared with respect to lung function, microbiology, and survival to end-stage CF (death or lung transplantation). The lung function was significantly reduced in carriers of MBL variant alleles when compared with normal homozygotes. The negative impact of variant alleles on lung function was especially confined to patients with chronic Pseudomonas aeruginosa infection. Burkholderia cepacia infection was significantly more frequent in carriers of variant alleles than in homozygotes. The risk of end-stage CF among carriers of variant alleles increased 3-fold, and the survival time decreased over a 10-year follow-up period. Moreover, by using a modified life table analysis, we estimated that the predicted age of survival was reduced by 8 years in variant allele carriers when compared with normal homozygotes. Presence of MBL variant alleles is therefore associated with poor prognosis and early death in patients with CF.
J. Clin. Invest. 104:431-437 (1999).
Introduction
Cystic fibrosis (CF) is a multiorgan disease arising from mutations in the gene coding for the cystic fibrosis transmembrane regulator (CFTR) protein. This protein regulates cAMP-mediated chloride conductance at the apical surface of secretory epithelia (1). A majority of the patients acquire a chronic endobronchial infection with Pseudomonas aeruginosa in late childhood or early adulthood. It is primarily this infection that leads to a sustained immune response causing chronic inflammation and gradual tissue destruction, which eventually results in premature death from respiratory insufficiency in CF patients (2). However, the course of the lung disease often differs markedly between patients — even in those carrying the same CFTR mutation(s), including siblings — and despite identical methods of control and treatment.
Mannose-binding lectin (MBL) is a serum protein participating in innate immune defense. It belongs to a family of proteins that share a similar structure and function (lung surfactant proteins A and D) (3, 4). Human MBL is derived from a single gene on chromosome 10, MBL2 (5, 6). On the same chromosome, there is also an MBL pseudogene (MBL1) (7). The ligands for MBL, high mannose and N-acetylglucosamine oligosaccharides, are present on a variety of microorganisms. MBL may activate the complement system by MBL-associated serine proteases and may interact with novel receptors on phagocytes (8–10). This protein is thought to be of particular importance in protecting against bacterial and viral infections during the vulnerable period of infancy before specific immune protection is established by the adaptive immune system. It has been shown that MBL variant alleles causing low MBL serum levels are associated with an increased risk of different types of infections that occur primarily in children (11–13), but also in adults (14, 15). Moreover, MBL deficiency has also been shown to play a role in autoimmune diseases (16–19).
In exon 1 of the MBL2 gene, 3 single-base substitutions independently cause low serum levels of MBL at codon 54 (glycine with aspartic acid, allele B), at codon 57 (glycine with glutamic acid, allele C), and finally at codon 52 (arginine with cysteine, allele D) (11, 20, 21). The common designation for these variant alleles is 0, whereas the normal allele has been named A. Each of the 3 variants reduces the amount of functional MBL subunits in heterozygous individuals 5- to 10-fold (22). Moreover, several nucleotide substitutions in the promoter region of the MBL2 gene affect the MBL serum level (23, 24). Particularly, a polymorphism in codon –221 (X/Y type) has a significant downregulating effect on the MBL serum concentration (22–24).
We investigated whether the presence of structural MBL variant alleles and promoter variants, which give rise to low MBL levels, influences the course of lung disease and survival in patients with CF.
Methods
Patients.
In 1989, there were 235 patients with CF who were exclusively controlled and treated at the Danish Cystic Fibrosis Center in Copenhagen. After exclusion of those too young to perform a reliable lung function test (usually less than 7 years of age), and those in whom DNA (used for CF mutation analysis) was not available, all of the remaining 149 patients (72 females) were included. All were Caucasian and were unrelated, except for 3 pairs of siblings; the median age was 16.2 years (range: 7.2–40.7 years).
The patients were seen on a regular monthly basis, and control included microscopy and culture of lower respiratory tract secretions obtained by expectoration or nasolaryngeal suction, as well as spirometry (Pneumotach, Dräger, Germany) to record forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). Both were expressed as percent of expected values corrected for sex and height (25). The individual patient values are the mean of all recorded values during each calendar year (minimum: 1 value each month). The patients’ lung functions were followed until the end of 1997. Two patients emigrated, and 5 patients were transferred to another regional Danish Cystic Fibrosis Center after the first year of follow-up. Thus, for these patients, only the lung function data from 1989 were available.
The patients’ survival was recorded from inclusion at January 1, 1989 and followed until March 1, 1999 using either time of death or time of lung transplantation as the end point. Information about time of death and lung transplantation were available for all patients, except for one who emigrated at the end of 1989.
Chronic P. aeruginosa infection is defined as growth of P. aeruginosa in 6 consecutive monthly cultures, or for a shorter period, when accompanied by an increase in specific serum anti–P. aeruginosa antibodies detected by crossed immunoelectrophoresis (26, 27).
The CF mutations were determined according to established molecular-based techniques that are regularly updated (28, 29). Of the 149 patients, 126 were homozygous for the ΔF508 mutation, 22 were heterozygous for the ΔF508 mutation, with the other CF mutation being identified in 19. One patient carried 2 non-ΔF508 mutations. None of the patients carried CF mutations known to be associated with mild disease.
The controls were 250 unrelated, healthy Danes. They consisted of 190 blood donors and 60 members of the laboratory staff. All patients or parents received verbal and written information and gave signed consent. The study was approved by the local ethical committee.
Detection of MBL protein concentrations and genotypes.
MBL concentrations in serum and bronchial expectorations were measured in a double-enzyme immunoassay as described previously (30). Genomic DNA was isolated from EDTA blood cells and stored at –20°C. DNA was amplified by general PCR and site-directed mutagenesis PCR, and the MBL alleles were detected as described (22, 23). All 3 structural variant alleles (B, C, and D) have a considerable effect on MBL concentrations, and to avoid small groups, the 3 alleles were grouped in 1 category (allele 0). The normal allele is designated A. The 6 MBL genotypes were defined as follows. The A/A group: 2 normal structural alleles with high-expression promoter activity in position –221 (YA/YA), or 1 high-expression promoter and 1 low-expression promoter (YA/XA), or 2 low-expression promoters (XA/XA). The A/0 group: 1 variant structural allele (i.e., defective allele) and 1 normal structural allele combined with a high-expression promoter (YA/0) or a low-expression promoter (XA/0). The 0/0 group: 2 defective structural alleles.
Because A/0 individuals carrying the low-expressing X promoter allele on the functional A chromosome have very low serum MBL levels, we pooled these patients with those homozygous for 2 defective structural alleles (0/0) into an MBL-insufficient group (n = 19) and compared it with the rest of the patients (MBL-sufficient group; n = 130) in some of the analyses.
Statistical analyses.
Contingency table analyses and Fisher’s exact test were used to compare frequencies. Both the Mann-Whitney U test and the Kruskal-Wallis test were used to compare continuous data between carriers of the different MBL genotypes. The Spearman rank correlation test was used for correlation analyses. Survival analyses were performed with a Kaplan-Meier plot and were assessed by the log rank test. Additionally, because the patients’ ages varied at the time of their inclusion in this study, a modified life table analysis was performed (annual age-specific mortality rate), which adjusts for age differences (31, 32). This is a cross-sectional method, where each patient contributes 1 year of exposure during each year of their life in which she or he is followed in the observational period. The resultant cumulative value for survival probability represents the mortality in the population for that period. P values for this test are calculated by Poisson regression analysis. Two-tailed tests were used throughout. Only the eldest siblings were included when comparing gene frequencies with controls. All patients were included in the other analyses.
Results
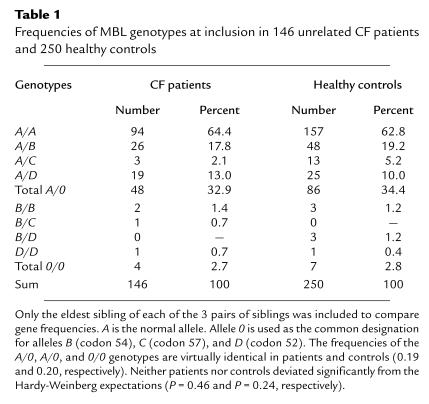
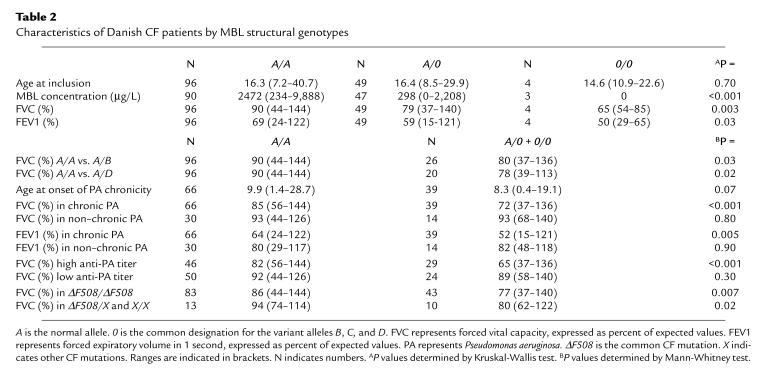
The frequency of MBL variant alleles at inclusion was virtually identical in CF patients and controls (Table 1). No significant difference in age was observed between A/A patients and carriers of variant alleles (Table 2). The median MBL serum concentration was 10-fold lower in variant allele carriers than in A/A individuals (Table 2).
Table 1.
Frequencies of MBL genotypes at inclusion in 146 unrelated CF patients and 250 healthy controls
Table 2.
Characteristics of Danish CF patients by MBL structural genotypes
Both FVC and FEV1 percentages were higher in A/A patients than in carriers of the variant alleles (Table 2), and this pattern was found each year in the follow-up period (data not shown). When patients heterozygous for B (n = 26) and D (n = 20) alleles were tested separately against A/A individuals, it was revealed that both alleles contributed independently to the decrease in lung function (Table 2).
Differences in MBL genotypes (A/A vs. variant alleles) did not affect whether the patients had chronic P. aeruginosa infection or not (Fisher’s exact test, P = 0.60), but there was a trend in which the variant allele carriers were younger at onset of chronic P. aeruginosa infection than their A/A counterparts (Table 2).
Significantly higher FVC and FEV1 percentages were observed in A/A patients with chronic P. aeruginosa infection compared with those carrying the variant alleles (Table 2). In contrast, no significant difference was observed between the 2 genotypes in patients without chronic P. aeruginosa infection. Again, the same pattern was found each year in the follow-up period (data not shown).
No significant difference was observed in FVC values between those with and without chronic P. aeruginosa infection when A/A patients were investigated separately (Mann-Whitney U test, P = 0.20). However, a significant difference was observed when the same analysis was performed on variant allele carriers (Mann-Whitney U test, P = 0.002) (Table 2).
In CF patients with complicating chronic P. aeruginosa infection, high levels of specific antibodies are closely associated with progressive decrease in lung function (2, 26). Including all patients with detectable (>0) anti–P. aeruginosa precipitins (n = 115), we found a significant inverse correlation between the number of precipitins and the FVC values in patients carrying variant alleles (r = –0.32, P = 0.04, n = 40), but no correlation in A/A patients (r = –0.18, P = 0.10, n = 75). The MBL genotype did not influence the level of precipitins, because A/A patients and carriers of variant alleles were equally distributed between those with precipitins above (n = 46 and n = 29, respectively) and below (n = 50 and n = 24, respectively) the median (Fisher’s exact test, P = 1.00).
The difference in lung function between A/A patients and variant allele carriers was observed both in ΔF508 homozygous and heterozygous patients (Table 2). This was expected, because none of the patients carried mutations associated with mild disease.
Chronic Burkholderia cepacia infection was present in 2 patients in 1989; during the 9-year follow-up period, 8 more contracted this infection. Among these 10 patients, 4 were heterozygous and 3 homozygous for variant alleles (χ2 = 31.9, 2 degrees of freedom, P < 0.001). Excluding the patients with B. cepacia infection did not change the overall conclusion regarding the influence of the MBL genotype on lung function (data not shown).
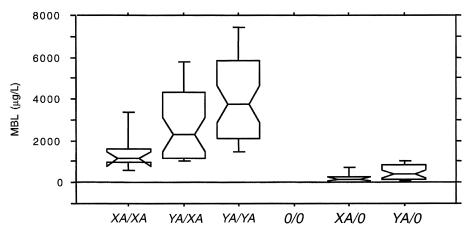
Figure 1 shows the MBL serum concentrations in the 6 genotypes according to the combination of both the structural and the X/Y promoter alleles. This shows that the A/0 patients carrying the X promoter on the functional A chromosome resembles the 0/0 homozygous individuals with regard to MBL serum concentration.
Figure 1.
MBL serum concentration in CF patients in relation to MBL structural alleles as well as the MBL promoter alleles in position –221 (X/Y). Boxes are interquartile ranges. Bars show range from 10th to 90th percentiles. The detection limit in the assay is 20 μg/L.
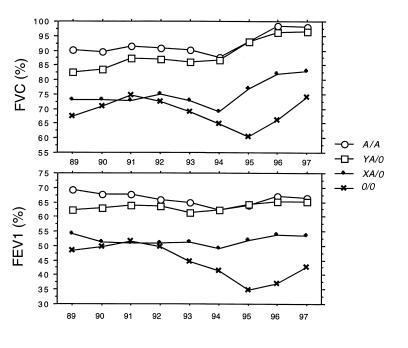
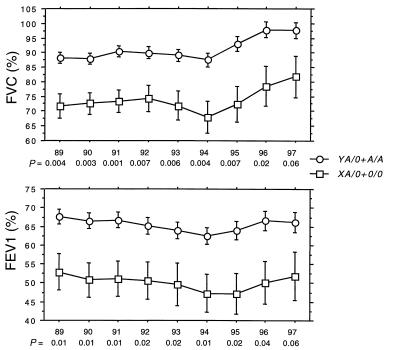
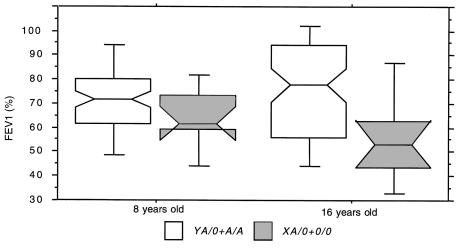
Figure 2 shows that the mean lung function measured from 1989 to 1997 is virtually identical in A/A individuals and in heterozygotes carrying the high MBL–expressing Y promoter on the functional A chromosome (YA/0), and that those carrying X promoter on the functional A chromosome (XA/0) resemble those with the homozygous defect (0/0). The XA/0 and 0/0 genotypes were pooled into an MBL-insufficient group (n = 19) and compared with the rest (A/A and YA/0 in the MBL-sufficient group; n = 130) to evaluate statistically the effect of the low-expressing MBL genotypes on lung function. Figure 3 shows that from 1989 to 1997, the lung function in MBL-insufficient patients is reduced significantly compared with MBL-sufficient patients. In 85 patients, lung function data (FEV1 values) were available at 8 and 16 years of age (75 MBL sufficient and 10 MBL insufficient). Figure 4 shows that a trend toward the relative reduction in lung function in MBL-insufficient patients could already be seen at the age of 8 years (Mann-Whitney U test, P = 0.12). The difference in lung function between the MBL-sufficient and MBL-insufficient patients became significant at 16 years of age (Mann-Whitney U test, P = 0.017).
Figure 2.
The lung function (FVC and FEV1, expressed as percentages) measured from 1989 to 1997 was stratified according to A/A (n = 96) and the X/Y promoter (base substitution in position –221) on the functional A chromosome in structural MBL allele heterozygotes (YA/0, n = 34; and XA/0, n = 15) and those with homozygous defect (0/0, n = 4). The numbers refer to those included in the study in 1989. Mean is indicated. The general improvement in lung function data from 1995 to 1997 probably is due to a combination of the change in analyzing equipment, the number of dropouts, and improved treatment.
Figure 3.
The lung function (FVC and FEV1, expressed as percentages) measured from 1989 to 1997 was stratified according to whether it belonged to the MBL-sufficient group (A/A and YA/0; n = 130) or the MBL-insufficient group (XA/0 and 0/0; n = 19). The numbers refer to those included in the study in 1989. Mean and SE are indicated. The individual P values (Mann-Whitney U test) each year are given.
Figure 4.
Lung function at 8 and 16 years of age. Parallel lung function (FEV1, expressed as a percentage) in CF patients at 8 and 16 years of age was stratified according to whether they belonged to the MBL-sufficient group (A/A and YA/0; n = 75) (open boxes) or the MBL-insufficient group (XA/0 and 0/0; n = 10) (gray boxes). Boxes are interquartile ranges. Bars show range from 10th to 90th percentiles.
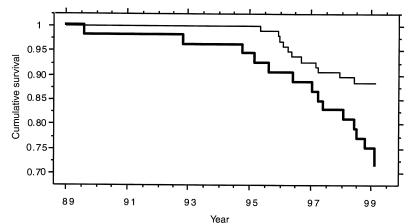
By the end of February 1999, 14 patients had died (8 of whom carried variant alleles [5 heterozygous and 3 homozygous]) and 12 patients had undergone lung transplantation (7 of whom were heterozygous for variant alleles). Thus, an increased frequency of the variant allele carriers reached end-stage CF lung disease during the observation period compared with the A/A patients (15 of 53 [28%] vs. 11 of 96 [11%]) (Fisher’s exact test, P = 0.013; odds ratio = 3.0; 95% confidence interval (CI) = 1.3–7.0). Of the 26 patients with lung disease, 24 had chronic P. aeruginosa infection, and 2 had chronic B. cepacia infection at time of death or transplantation. Estimating the time from inclusion in the study on January 1, 1989 to the date of death or lung transplantation, it was calculated that survival was significantly reduced for carriers of variant alleles when compared with A/A patients (log rank, P = 0.009; proportional hazard ratio = 2.7; 95% CI = 1.2–5.9) (Figure 5). The reduced survival was more pronounced when the MBL-sufficient and MBL-insufficient groups were compared (log rank, P = 0.007; proportional hazard ratio = 3.0; 95% CI = 1.3–7.2) (data not shown).
Figure 5.
Kaplan-Meier plot for 10 years, ranging from date of inclusion in 1989 to 1999, of survival rate of CF patients. Events are defined as date of death (n = 14) and lung transplantation (n = 12) (end-stage CF). Thin line indicates A/A patients, and thick line indicates variant allele carriers (A/0 and 0/0).
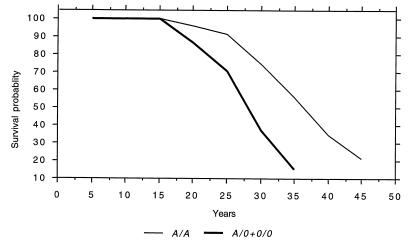
Because the age of the patients varied considerably at inclusion in 1989, we performed a modified life table analysis that takes age differences into account (Figure 6). This showed that the median expected survival age in carriers of MBL variant alleles was 28 years, compared with 36 years in A/A individuals (Poisson regression analysis, P = 0.01). This disparity was even more pronounced when the patients were divided into the MBL-insufficient and MBL-sufficient groups, in whom the median expected survival age was 25 and 34 years, respectively (Poisson regression analysis, P = 0.007).
Figure 6.
A modified life table analysis (annual age-specific mortality rate) that adjusts for age differences at inclusion. The figure shows that the expected median survival age in A/A patients was 36 years, compared with 28 years in carriers of variant alleles (A/0 and 0/0).
Discussion
The course of lung disease in patients with CF varies considerably, even among patients with the same CFTR genotype who are receiving standardized care. This variation is largely unexplained. We found that patients carrying 1 defective copy of the MBL gene may have an overall 11% decrease in lung function, and those carrying 2 copies have an overall 25% decrease in lung function, compared with patients homozygous for the A/A genotype. As shown in Figure 1, the MBL serum levels can, in addition to the structural alleles, be further subdivided because of the effect of a base substitution in codon –221 (X/Y variant) (22–24).
In agreement with the MBL serum level findings, the lung function in the low-expressing heterozygotes is virtually identical to those with the homozygous defect. During the 9-year observation period, the lung function was more or less unchanged in the whole group of patients. However, when paired data were analyzed from data collected at 8 and 16 years of age, it became evident that patients with genotypes encoding low MBL serum levels (the MBL-insufficient group) had a slightly lower lung function at age 8, but then experienced a marked drop in lung function over the ensuing 8-year period, whereas it remained more or less constant in the MBL-sufficient group. Thus, the effect of MBL on lung function is likely initiated quite early in life and becomes progressively more prominent with increasing age.
When the data were further analyzed, we found that the difference in lung function between patients homozygous for the normal allele (A/A) and patients carrying 1 or 2 copies of defective alleles (A/0 and 0/0) was restricted to patients with chronic P. aeruginosa infection and high titers of anti–P. aeruginosa serum precipitins. MBL seemed to offer no protection against chronic colonization of P. aeruginosa, because there were no significant differences between patients with normal and defective alleles with respect to the prevalence of chronic infection or the age at onset.
The link between the molecular and cellular abnormalities in CF and colonization of respiratory epithelium with P. aeruginosa and other pathogens is still unclear. Recent evidence has suggested that increased concentrations of sodium chloride in the lungs due to CFTR mutations may impede the killing of P. aeruginosa by innate antibacterial defense systems (defensins) (33). There is also evidence suggesting that the CFTR molecule itself is directly involved in clearance of P. aeruginosa (34), but several other mechanisms have also been proposed. In the early phase of intermittent colonization, there is no detectable anti–P. aeruginosa serum precipitins and presumably little local inflammation. Transition to chronic P. aeruginosa infection coincides with an increase in serum precipitins and activation of genes coding for alginate production (2). This results in growth of P. aeruginosa microcolonies embedded in agar, which protects against host defense systems.
That MBL is synthesized exclusively in the liver may explain why it lacks a protective role against P. aeruginosa colonization and subsequent infection (35). It may reach a localized inflammatory focus by exudation relatively late in the pathophysiological process (36). Unlike lung surfactant protein D, MBL is absent from the normal mouse lung, but appears in lung lavage fluid 3 days after experimental infection with influenza virus (37). We found MBL (median levels: 200 μg/L) in lung expectoration from 4 CF patients (all A/A individuals) out of 100 investigated patients (data not shown). However, it is likely that lysosomal enzymes from polymorphonuclear leukocytes degrade MBL in the lungs of CF patients, because the mixing of normal serum with expectoration or sputum was followed by a rapid decrease in detectable MBL antigen (data not shown), as has been shown for several other serum proteins (38).
As there is probably little or no local inflammation in the early phase of colonization with P. aeruginosa, there may very little, if any, MBL leaving systemic circulation. Once the inflammatory process is initiated, as a consequence of the local reaction of circulating and secretory anti–P. aeruginosa antibodies with microbial antigens at foci of P. aeruginosa microcolonies, there may be exudation of MBL. However, the mechanism by which MBL may have a protective role against the inflammatory tissue destruction that is secondary to chronic P. aeruginosa infection remains elusive at this point, because MBL binds only weakly to whole P. aeruginosa bacteria in vitro (P. Garred, unpublished observations). Thus, a direct, MBL-mediated P. aeruginosa killing or opsonization is not likely in the vivo situation, but MBL might play a role in the clearance or neutralization of P. aeruginosa–derived LPS or other toxic substances released from the bacteria.
Alternatively, MBL may have a protective role against the viral infections suggested to precede P. aeruginosa colonization and exacerbation, which in turn may slow the progression of the disease (39). Therefore, MBL deficiency could be associated with common viral respiratory infections that render the lungs more susceptible to the damage caused by chronic P. aeruginosa colonization. There is also the possibility that MBL deficiency may cause intrinsic immune disturbances: it has recently been shown that lack of C1q, a molecule very similar to MBL, in knockout mice is involved in the clearance of apoptotic material (40). Likewise, MBL could be involved in the clearance of immune complexes of pathophysiological importance in CF by its interaction with a galactosylated IgG (41). None of these hypotheses would appear to be mutually exclusive.
It is very interesting that out of 10 patients with B. cepacia infection (2 already infected in 1989 and 8 who contracted this infection during the follow-up period), 4 were heterozygous and 3 were homozygous for MBL variant alleles, giving a highly significant increased risk of acquiring this complication in carriers of variant alleles. This is in contrast to our finding that the acquisition of P. aeruginosa does not seem to be related to the MBL levels. However, B. cepacia is known to lead to a much higher degree of inflammation than P. aeruginosa, as evidenced by the quite frequent septic form of infection that is rarely, if ever, seen with P. aeruginosa (42). Therefore, MBL may appear on the bronchial surface much earlier in the course of B. cepacia infection than in P. aeruginosa infection. MBL’s protective role against the progression of B. cepacia infection may therefore be disclosed in carriers of variant alleles.
It is of interest that the genes for lung surfactant proteins A and D, which are proteins with similar functions as MBL, are located near the MBL2 gene on chromosome 10 (43). Therefore, it could be argued that the effect of the MBL variant alleles is due to linkage disequilibrium with polymorphisms in one of these or in another gene. Indeed, decreased levels of lung surfactant proteins A and D have been detected recently in lung lavage fluid from CF patients (44, 45). Although we cannot discount the possibility that the MBL effect on lung function and survival is due to linkage disequilibrium, the fact that each of the B and D alleles are significantly associated with decreased lung function argues against this notion.
Because MBL variant alleles are so frequent in the healthy population, it is conceivable that multiple genetic factors may influence susceptibilities and outcomes in which MBL deficiency plays a role. It has been shown that concomitant occurrence of MBL variant alleles and IgG subclass deficiency may increase risk of infections (12), and that concomitant occurrence of polymorphisms in the FcγRIIa receptor and MBL deficiency is associated with autoimmunity in chronic granulomatous disease (46). Likewise, the possession of both complement C4-null alleles and MBL variant alleles has been shown to increase susceptibility to systemic lupus erythematosus (18).
The high frequency of MBL variant alleles in different populations indicates that MBL polymorphisms represent a balanced genetic system favoring variant alleles arising from genetic selection (47). In sub-Saharan Africa, more than 50% of the population carry the C allele, and in certain South American Indian tribes, more than 70% of the population carry the B allele (24). Thus, the normal A allele may, under some circumstances, confer disadvantages to the host.
During the 10-year follow-up period, 14 patients died and 12 underwent lung transplantation. Of the 26 patients, 24 had chronic P. aeruginosa infection and 2 had chronic B. cepacia infection. Of these same 26, 12 were heterozygous and 3 were homozygous for MBL variant alleles. When death and lung transplantation are viewed as end-stage disease, this group of patients had a marked increase in risk of end-stage disease and a significantly reduced survival time. Taking their age at inclusion into consideration, and using a modified life table analysis, revealed that the expected median survival was about 8 years shorter in those carrying MBL variant alleles.
Our data indicate that a shortened life-span in carriers of variant alleles results primarily from the more aggressive course of lung disease caused by chronic P. aeruginosa infection, but they also indicate that carriers of variant alleles are at high risk of acquiring B. cepacia infection and this infection is often associated with an even greater mortality than chronic P. aeruginosa colonization. However, our data also raise the possibility that future patients may benefit from substitution therapy with purified or recombinant MBL.
Acknowledgments
The authors thank Bente Frederiksen and Vibeke Weirup for excellent technical assistance. This work was supported by the Novo Nordisk Research Foundation.
References
- 1.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 3.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 4.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz RAB. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 5.Sastry K, et al. The human mannose-binding protein gene. Exon structure reveals its evolutionary relationship to a human pulmonary surfactant gene and localization to chromosome 10. J Exp Med. 1989;170:1175–1189. doi: 10.1084/jem.170.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor ME, Brickell PM, Craig RK, Summerfield JA. Structure and evolutionary origin of the gene encoding a human serum mannose-binding protein. Biochem J. 1989;262:763–771. doi: 10.1042/bj2620763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo N, Mogues T, Weremowicz S, Morton CC, Sastry KN. The human ortholog of rhesus mannose-binding protein-A gene is an expressed pseudogene that localizes to chromosome 10. Mamm Genome. 1998;9:246–249. doi: 10.1007/s003359900735. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1503. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiel S, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 10.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qRp, the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–126. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 11.Sumiya M, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 12.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–943. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 13.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 1997;314:1229–1232. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summerfield JA, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–889. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 15.Garred P, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–240. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 16.Davies EJ, et al. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–114. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan KE, Wooten C, Goldman D, Petri M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:2046–2051. doi: 10.1002/art.1780391214. [DOI] [PubMed] [Google Scholar]
- 18.Davies EJ, et al. A dysfunctional allele of the mannose binding protein gene associates with systemic lupus erythematosus in a Spanish population. J Rheumatol. 1997;24:485–488. [PubMed] [Google Scholar]
- 19.Graudal N, et al. Mannan-binding lectin in rheumatoid arthritis. A longitudinal study. J Rheumatol. 1998;25:629–635. [PubMed] [Google Scholar]
- 20.Lipscombe RJ, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 21.Madsen HO, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 22.Garred, P., Madsen, H.O., and Svejgaard, A. 1996. Genetics of human mannan-binding protein. In Collectins and innate immunity. R.A.B. Ezekowitz, K. Sastry, and K.B.M. Reid, editors. R.G. Landes. Austin, TX. 139–164.
- 23.Madsen HO, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–3020. [PubMed] [Google Scholar]
- 24.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J Immunol. 1998;161:3169–3175. [PubMed] [Google Scholar]
- 25.Polgar, G.E., and Promadhat, V. 1971. Pulmonary function testing in children: techniques and standards. W.B. Saunders. Philadelphia, PA. 88 pp.
- 26.Høiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determination by means of crossed immunoelectrophoresis. A survey. Acta Pathol Microbiol Scand Suppl. 1977;262:1–96. [PubMed] [Google Scholar]
- 27.Høiby, N. 1995. Microbiology of cystic fibrosis. In Cystic fibrosis. M.E. Hodson and D.M. Geddes, editors. Chapman and Hall Medical. London, United Kingdom. 75–98.
- 28.Schwartz M, Johansen HK, Koch C, Brandt NJ. Frequency of the delta F508 mutation on cystic fibrosis chromosomes in Denmark. Hum Genet. 1990;85:427–428. doi: 10.1007/BF02428297. [DOI] [PubMed] [Google Scholar]
- 29.Johansen HK, Nir M, Høiby N, Koch C, Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for ΔF508 mutation. Lancet. 1991;337:631–634. doi: 10.1016/0140-6736(91)92449-c. [DOI] [PubMed] [Google Scholar]
- 30.Garred P, et al. Diallelic polymorphism may explain variations of blood concentration of mannan-binding protein in Eskimos, but not in black Africans. Eur J Immunogenet. 1992;19:403–412. doi: 10.1111/j.1744-313x.1992.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 31.Warwick WJ, Pogue RE, Gerber HU, Nesbitt CJ. Survival patterns in cystic fibrosis. J Chronic Dis. 1975;28:609–622. doi: 10.1016/0021-9681(75)90074-0. [DOI] [PubMed] [Google Scholar]
- 32.Frederiksen B, Lanng S, Koch C, Høiby N. Improved survival in the Danish center-treated cystic fibrosis patients: results of aggressive treatment. Pediatr Pulmonol. 1996;21:153–158. doi: 10.1002/(SICI)1099-0496(199603)21:3<153::AID-PPUL1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 34.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezekowitz RAB, Day LE, Herman GA. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988;167:1034–1046. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garred P, et al. Mannan-binding protein: levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clin Exp Immunol. 1993;94:99–104. doi: 10.1111/j.1365-2249.1993.tb05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection in mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein W, Doring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Johansen HK, Høiby N. Seasonal onset of initial colonization and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax. 1992;47:109–111. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra R, et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via mannose-binding protein. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 42.Govan JR, Hughes JE, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 43.Kölble K, Lu J, Mole SE, Kaluz S, Reid KBM. Assignment of the human pulmonary surfactant protein D gene (SFTP4) to 10q22-23 close to the surfactant protein A gene cluster. Genomics. 1993;17:294–298. doi: 10.1006/geno.1993.1324. [DOI] [PubMed] [Google Scholar]
- 44.Griese M, Birrer P, Demirsoy A. Pulmonary surfactant in cystic fibrosis. Eur Respir J. 1997;10:1983–1988. doi: 10.1183/09031936.97.10091983. [DOI] [PubMed] [Google Scholar]
- 45.Postle AD, et al. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- 46.Foster CB, et al. Host defense molecule polymorphisms influence the risk for immune-mediated complications in chronic granulomatous disease. J Clin Invest. 1998;102:2146–2155. doi: 10.1172/JCI5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–131. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]