Abstract
Objective
Human estrogen receptor α (ESR1), a member of the nuclear receptor superfamily of ligand-activated transcription factors, is one of the key mediators of hormonal response in estrogen-sensitive tissues. Accumulating evidence has demonstrated that two of the most widely studied single-nucleotide polymorphisms in ESR1 – PvuII (T/C, rs223493) and Xbal (A/G, rs9340799) – are possibly associated with Alzheimer’s disease (AD). However, individual study results are still controversial.
Materials and methods
We searched PubMed, Embase, Web of Science, Science Direct, SpringerLink, and the Chinese National Knowledge Infrastructure databases for eligible studies assessing the association of ESR1 polymorphisms and AD risk (last search performed in November 2013). Thereafter, a meta-analysis of 13,192 subjects from 18 individual studies was conducted to evaluate the association between ESR1 polymorphisms and susceptibility to AD.
Results
The results indicated that a significant association was found between the ESR1 PvuII polymorphism and AD risk in Caucasian populations (CC + CT versus TT, odds ratio [OR] 1.14, 95% confidence interval [CI] 1.02–1.28, P=0.03; CT versus TT, OR 1.16, 95% CI 1.02–1.31, P=0.02), whereas no evidence of association was found in Asian populations. Nevertheless, we did not find any significant association between the ESR1 XbaI polymorphism and AD risk for any model in Caucasian and Asian populations (all P>0.05).
Conclusion
Based on this meta-analysis, we conclude that the ESR1 PvuII polymorphism might be a risk factor in AD development in Caucasian populations, not in Asian populations. Further confirmation is needed from better-designed and larger studies.
Keywords: Alzheimer’s disease, estrogen receptor, polymorphism, meta-analysis
Introduction
Alzheimer’s disease (AD) is one of the main causes of dementia among elderly individuals, and is characterized by memory impairments and loss of cognitive functions, which eventually leads to complete incapacity and death of patients within 3–9 years after diagnosis.1 As the elderly population continues to grow, the prevalence of AD has increased remarkably worldwide. At present, AD is one of the leading causes of disability and death among the elderly.2–4 AD has emerged as a serious public health concern, affecting patients’ quality of life and placing an immense burden on the individual, family, and community. Therefore, elucidating the pathogenesis and risk factors of AD is of great significance for early detection, prevention, and control of the susceptible population.
Genetic, metabolic, and environmental factors play a role in the development and progression of AD.5 Recent genome-wide association studies have identified many genetic variances and single-nucleotide polymorphisms (SNPs) that are associated with AD risk.6 Estrogen-receptor gene polymorphisms are possible candidates for AD susceptibility. In women, estrogen loss associated with menopause status has been suggested to contribute to the development of AD.7,8 Estrogen has been shown to act as a neuroprotectant and a neurotrophic agent.9 Estrogen promotes neuronal cell survival, reduces neuronal injury, protects against neurotoxins, facilitates axonal sprouting and neuronal repair, and enhances synaptic transmission and neurogenesis.10 Recently, estrogen-replacement therapy has been proposed as a therapeutic approach to reduce the risk of developing AD and help patients with AD maintain their cognitive function.11 Estrogen exerts most of its effects through at least two major classes of receptors – estrogen receptor a (ESR1) and estrogen receptor β (ESR2).12 Human ESR1, a member of the nuclear receptor superfamily of ligand-activated transcription factors, is located on human chromosome 6q25, and is one of the key mediators of hormonal response in estrogen-sensitive tissues. After binding to estrogen, ESR1 acts as a transcriptional factor that regulates gene expression and function by interacting with regulatory regions of target genes.13 Many studies have demonstrated that ESR1 polymorphisms might influence ESR1 expression and affect estrogen function. To date, associations between ESR1 polymorphisms and cancer,14,15 coronary artery disease,16 hip fracture,17 and bone mineral density18 have been identified.
Accumulating evidence has demonstrated that two of the most widely studied SNPs in ESR1 – PvuII (T/C, rs223493) and Xbal (A/G, rs9340799) – are possibly associated with AD. However, the results of studies seeking associations of ESR1 with AD risk have not always been consistent in different population analyses.19 Therefore, we performed a meta-analysis of all eligible studies to provide a more comprehensive and reliable conclusion by evaluating the association between ESR1 gene polymorphisms and susceptibility to AD.
Materials and methods
Search strategy
We searched PubMed, Embase, Web of Science, Science Direct, SpringerLink, and the Chinese National Knowledge Infrastructure databases for eligible studies assessing the association of ESR1 polymorphisms and AD risk (last search updated to November, 2013). The search terms were “Alzheimer’s disease (AD) or dementia” in combination with “estrogen receptor or oestrogen receptor or estrogen” in combination with “polymorphism or variant or mutation”. There was no restriction on time period, sample size, population, language, or type of report in order to minimize potential publication bias.
Inclusion and exclusion criteria
Studies included in this meta-analysis had to meet the following criteria: 1) case-control studies or cohort studies, 2) studies investigating the association between ESR1 gene polymorphisms and AD risk, 3) sufficient data available to calculate an odds ratio (OR) with 95% confidence interval (CI), and 4) clinical diagnosis of AD based on standards of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association. The exclusion criteria of the meta-analysis were: 1) case-control studies not focusing on the correlation between ESR1 polymorphisms and AD risk; 2) insufficient original data was available for data extraction; and 3) meta-analyses, letters, reviews, and editorial articles. If more than one study was published by the same author using the same patient population, the study with the largest size of samples was included.
Data extraction
Two authors (DC and BL) independently extracted the data from all eligible publications based on the inclusion criteria. The following information was recorded: name of first author, year of publication, country, ethnicity, number of cases and controls, the source of control, genotype method, distribution of genotypes, and Hardy–Weinberg equilibrium in controls. According to the source of control, eligible studies were defined as hospital-based and population-based. Ethnicity was simply categorized as Asian or Caucasian. Discrepancies were resolved by consensus and by consulting a third author.
Statistical analysis
Crude ORs with their corresponding 95% CIs were used to assess the strength of association between ESR1 polymorphisms and AD risk. The statistical significance of pooled ORs was assessed by the Z-test. Statistical heterogeneity across studies included in the meta-analysis was assessed by Cochran’s Q statistic and the I2 test.20 P<0.10 and I2>50% were considered to be statistically significant heterogeneity, and the random-effects model or the fixed-effects model were used. Sensitivity analysis was performed using the leave-one-out method to test the reliability of the overall pooled results.21 Publication bias was evaluated by funnel plot22 and further assessed by Egger’s linear regression test,23 and P<0.05 was considered representative of statistically significant publication bias. Data analyses were performed using Stata 11.0 (StataCorp, College Station, TX, USA) and RevMan 5.0 (Cochrane, Oxford, UK).
Results
Eligible studies
A flowchart of the process of study selection is shown in Figure 1. Based on the inclusion and exclusion criteria, a total of 17 articles were included in the meta-analysis after full-text review.9,24–39 The main characteristics of included studies are presented in Table 1. Of the 18 case-control studies included in the 17 articles, 18 studies investigated the ESR1 PvuII polymorphism (3,902 cases and 9,290 controls) and 17 studies investigated the ESR1 XbaI polymorphism (2,841 cases and 8,094 controls). Eight studies were performed in Caucasian populations,9,24,25,29–31,36,37 and ten studies were performed in Asian populations.26–28,32–35,37–39 The genotype distributions among the controls of all studies were in agreement with the Hardy–Weinberg equilibrium, except for one study for the ESR1 PvuII polymorphism25 and one study for the ESR1 XbaI polymorphism.34
Figure 1.
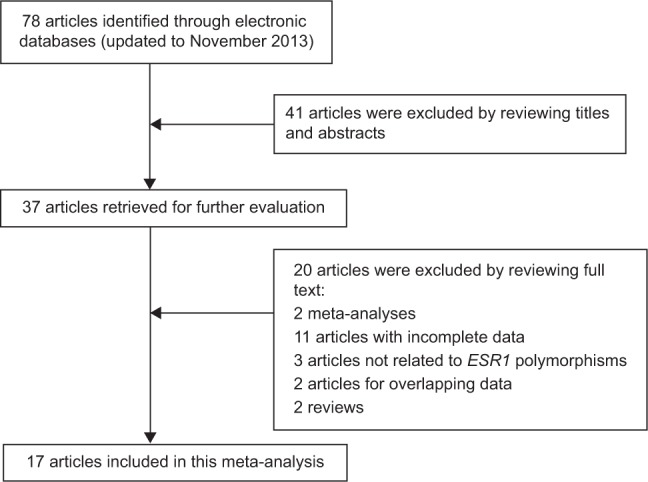
Flowchart of study selection.
Abbreviations: ESR1, estrogen receptor α.
Table 1.
Characteristics of the 18 eligible studies included in the meta-analysis
| Study | Year | Country | Ethnicity | Source of controls | Sample size, (cases/controls) | SNP studied | Genotyping method | HWE |
|---|---|---|---|---|---|---|---|---|
| Brandi et al24 | 1999 | Italy | Caucasian | PB | 193/202 | PvuII, XbaI | PCR-RFLP | 0.827, 0.364 |
| Boada et al25 | 2012 | Spain | Caucasian | PB | 1,069/1,215 | PvuII | Real-time PCR | 0.024 |
| Lambert et al9 | 2001 | UK | Caucasian | PB | 186/405 | PvuII, XbaI | PCR-RFLP | 0.943, 0.159 |
| Lin et al26 | 2003 | People’s Republic of China | Asian | PB | 30/125 | PvuII, XbaI | PCR-RFLP | 0.841, 0.051 |
| Ji et al27 | 2000 | Japan | Asian | PB | 234/134 | PvuII, XbaI | PCR-RFLP | 0.659, 0.679 |
| Usui et al28 | 2006 | Japan | Asian | PB | 205/92 | PvuII, XbaI | PCR-RFLP | 0.385, 0.150 |
| Corbo et al29 | 2006 | Italy | Caucasian | PB | 277/212 | PvuII, XbaI | PCR-RFLP | 0.193, 0.512 |
| Monastero et al30 | 2006 | Italy | Caucasian | PB | 172/172 | PvuII, XbaI | PCR-RFLP | 0.445, 0.062 |
| Porrello et al31 | 2006 | Italy | Caucasian | PB | 131/109 | PvuII, XbaI | PCR-RFLP | 0.103, 0.751 |
| Li et al32 | 2004 | People’s Republic of China | Asian | PB | 66/143 | PvuII, XbaI | PCR-RFLP | 0.288, 0.867 |
| Xu and Jia33 | 2002 | People’s Republic of China | Asian | PB | 49/55 | PvuII, XbaI | PCR-RFLP | 0.736, 0.869 |
| Hou34 | 2009 | People’s Republic of China | Asian | PB | 203/138 | PvuII, XbaI | PCR-RFLP | 0.798, 0.036 |
| Deng et al35 | 2013 | People’s Republic of China | Asian | HB | 236/236 | PvuII, XbaI | PCR-RFLP | 0.240, 0.475 |
| den Heijer et al36 | 2004 | Netherlands | Caucasian | PB | 230/5,514 | PvuII, XbaI | Taqman | 0.802, 0.627 |
| Maruyama et al37 | 2000 | Japan | Asian | PB | 183/133 | PvuII, XbaI | PCR-RFLP | 0.068, 0.061 |
| Maruyama et al37 | 2000 | UK | Caucasian | PB | 156/120 | PvuII, XbaI | PCR-RFLP | 0.903, 0.661 |
| Ma et al38 | 2009 | People’s Republic of China | Asian | PB | 219/215 | PvuII, XbaI | PCR-RFLP | 0.424, 0.962 |
| Zhou et al39 | 2008 | People’s Republic of China | Asian | PB | 63/70 | PvuII, XbaI | PCR-RFLP | 0.858, 0.142 |
Abbreviations: PB, population-based; HB, hospital-based; HWE, Hardy–Weinberg equilibrium; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; SNP, single-nucleotide polymorphism.
Quantitative data synthesis
The results of this meta-analysis are presented in Table 2. The heterogeneity was significantly observed under all models (P<0.05) for the ESR1 PvuII and XbaI polymorphisms, which might have resulted from differences in ethnicity, country, source of controls, and genotype methods, so the random-effects model was used.
Table 2.
Association between ESR1 polymorphisms and Alzheimer’s disease risk
| Allele model
|
Dominant model
|
Recessive model
|
Homozygous comparison
|
Heterozygous comparison
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| PvuII (T/C) | C allele versus T allele | CC + CT versus TT | CC versus CT + TT | CC versus TT | CT versus TT | |||||
| Overall | 1.05 (0.95–1.17) | 0.35 | 1.13 (0.98–1.32) | 0.10 | 0.95 (0.80–1.13) | 0.60 | 1.03 (0.84–1.28) | 0.75 | 1.15 (1.00–1.33) | 0.05 |
| Caucasian | 1.06 (0.98–1.15) | 0.12 | 1.14 (1.02–1.28) | 0.03 | 1.01 (0.88–1.15) | 0.89 | 1.10 (0.94–1.28) | 0.23 | 1.16 (1.02–1.31) | 0.02 |
| Asian | 1.06 (0.87–1.30) | 0.55 | 1.23 (0.91–1.67) | 0.18 | 0.84 (0.61–1.17) | 0.31 | 0.98 (0.65–1.47) | 0.91 | 1.28 (0.95–1.72) | 0.10 |
| XbaI (A/G) | G allele versus A allele | GG + GA versus AA | GG versus GA + AA | GG versus AA | GA versus AA | |||||
| Overall | 1.05 (0.90–1.23) | 0.53 | 1.07 (0.89–1.30) | 0.47 | 1.00 (0.75–1.34) | 0.99 | 1.04 (0.74–1.45) | 0.84 | 1.04 (0.93–1.16) | 0.50 |
| Caucasian | 1.02 (0.85–1.22) | 0.84 | 1.03 (0.83–1.28) | 0.78 | 1.00 (0.70–1.42) | 0.99 | 1.01 (0.67–1.53) | 0.97 | 1.03 (0.88–1.20) | 0.71 |
| Asian | 1.10 (0.84–1.43) | 0.50 | 1.14 (0.82–1.57) | 0.44 | 1.03 (0.62–1.70) | 0.92 | 1.09 (0.62–1.91) | 0.78 | 1.13 (0.83–1.55) | 0.44 |
Abbreviations: ESR1, estrogen receptor α; OR, odds ratio; CI, confidence interval.
For the ESR1 PvuII polymorphism, a total of 18 studies including 3,902 cases and 9,290 controls were included in the meta-analysis. In the overall analysis, we did not find any significant association between the ESR1 PvuII polymorphism and AD risk in any comparison model (C allele versus T allele, OR 1.05, 95% CI 0.95–1.17, P=0.35; CC + CT versus TT, OR 1.13, 95% CI 0.98–1.32, P=0.10; CC versus CT + TT, OR 0.95, 95% CI 0.80–1.13, P=0.60; CC versus TT, OR 1.03, 95% CI 0.84–1.28, P=0.75; CT versus TT, OR 1.15, 95% CI 1.00–1.33, P=0.05). Subgroup analysis stratified by ethnicity showed a significant association between the ESR1 PvuII polymorphism and AD risk under the dominant model (CC + CT versus TT, OR 1.14, 95% CI 1.02–1.28, P=0.03; Figure 2) and heterozygous comparison (CT versus TT, OR 1.16, 95% CI 1.02–1.31, P=0.02), whereas no evidence of association was found under the allele model (C allele versus T allele, OR 1.06, 95% 0.98–1.15, P=0.12), recessive model (CC versus CT + TT, OR 1.01, 95% CI 0.88–1.15, P=0.89), or homozygous comparison (CC versus TT, OR 1.10, 95% CI 0.94–1.28, P=0.23) in Caucasian populations. Nevertheless, we did not find any significant association between the ESR1 PvuII polymorphism and AD risk under any model in the Asian population (all P>0.05).
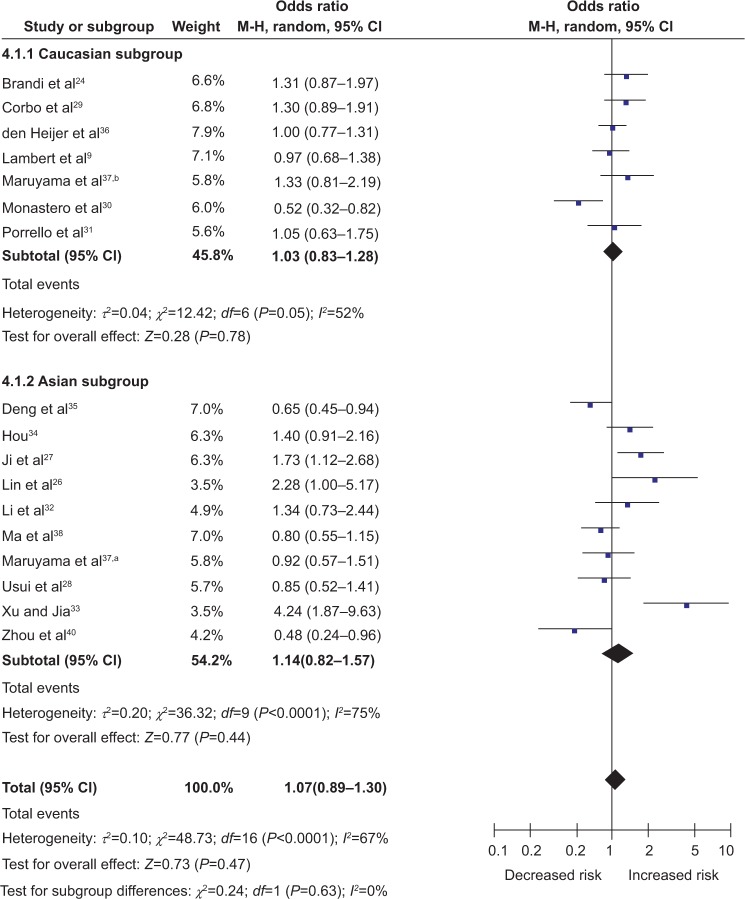
Figure 2.
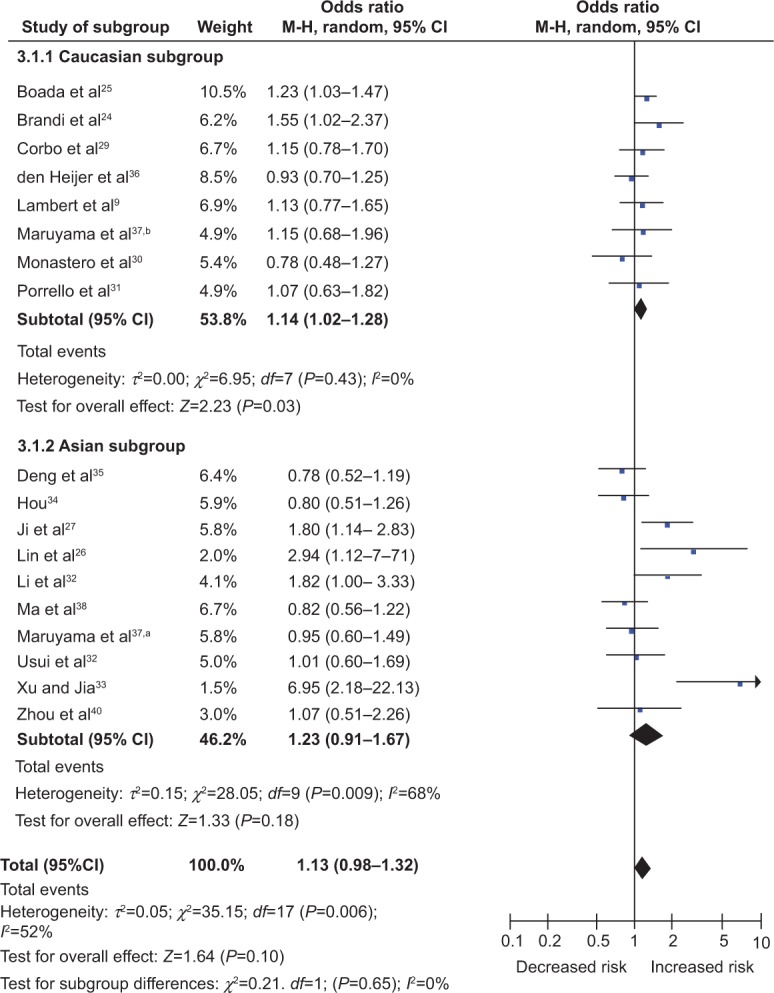
Forest plots of ESR1 PvuII polymorphism and Alzheimer’s disease risk in Caucasian and Asian populations (dominant model, CC + CT versus TT).
Notes: A random model of meta-analysis was employed for calculation of the combined ORs and P-values. Caucasian population, OR 1.14, CI 1.02–1.28, P=0.03; Asian population, OR 1.23, CI 0.91–1.67, P=0.18. The study of Maruyama et al37 was performed in Asian populations (a), and Caucasian populations (b).
Abbreviations: M-H, Mantel-Haenszel; OR, odds ratio; CI, confidence interval; ESR1, estrogen receptor α.
For the ESR1 XbaI polymorphism, a total of 17 studies including 2,841 cases and 8,094 controls were included in the meta-analysis. Overall, there was no evidence of an association between the ESR1 XbaI polymorphism and AD risk in different genetic models when all the eligible studies were pooled into the meta-analysis (G allele versus A allele, OR 1.05, 95% CI 0.90–1.23, P=0.53; GG + GA versus AA, OR 1.07, 95% CI 0.89–1.30, P=0.47 [Figure 3]; GG versus GA + AA, OR 1.00, 95% CI 0.75–1.34, P=0.99; GG versus AA, OR 1.04, 95% CI 0.74–1.45, P=0.84; GA versus AA, OR 1.04, 95% CI 0.93–1.16, P=0.50). The association of the ESR1 XbaI polymorphism with AD was further stratified by ethnicity. Neither Caucasian populations nor Asian populations showed a significant association between the ESR1 XbaI polymorphism and AD risk in any model (all P>0.05) (Figure 3).
Figure 3.
Forest plots of ESR1 XbaI polymorphism and Alzheimer’s disease risk in Caucasian and Asian populations (dominant model, GG + GA versus AA).
Notes: Random model of meta-analysis was employed for calculation of the combined ORs and P-values. Caucasian population, OR 1.03, CI 0.83–1.28, P=0.78; Asian population, OR 1.14, CI 0.82–1.57, P=0.44. The study of Maruyama et al37 was performed in Asian populations (a), and Caucasian populations (b).
Abbreviations: M-H, Mantel-Haenszel; CI, confidence interval; OR, odds ratio; ESR1, estrogen receptor α.
Sensitivity analysis
A sensitivity analysis was performed to assess the influence of each individual study on the pooled ORs through omitting individual studies. Statistically similar results were obtained after sequentially excluding each study, suggesting the stability of this meta-analysis.
Publication bias
The shapes of the funnel plots did not reveal any evidence of obvious asymmetry visually under the dominant model of PvuII and XbaI polymorphisms (Figure 4). Also, there was no statistical evidence of publication bias among studies using Egger’s regression test for the PvuII (P=0.296) and XbaI (P=0.206) polymorphisms.
Figure 4.
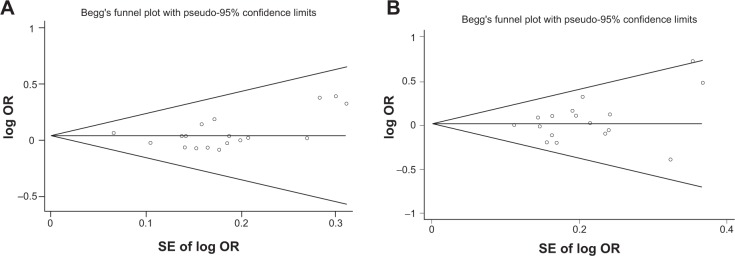
Egger’s funnel plots of publication-bias analysis for the polymorphisms.
Notes: (A) ESR1 PvuII polymorphism; (B) ESR1 XbaI polymorphism.
Abbreviations: SE, standard error; OR, odds ratio; ESR1, estrogen receptor α.
Discussion
The ESR1 gene is critical for hormone binding, deoxyribonucleic acid binding, and activation of transcription, because it encodes an estrogen receptor that is key in the mediation of hormonal response in estrogen-sensitive tissues.40 Given that the effect of a hormone is given by the interaction with its receptor, genetic changes in the ESR1 gene may lead to differences in ESR1 expression and estrogen metabolism, and thereby possibly explain interindividual differences in cognitive impairment or AD risk. Several SNPs have been identified in ESR1, and among these identified SNPs, PvuII (T/C, rs2234693) and XbaI (A/G, rs9340799), which are located in intron 1 of the ESR1 gene, 397 and 351 base pairs upstream of exon 2, respectively, are the most studied. Many previous genetic studies have investigated the function of ESR1 PvuII and XbaI polymorphisms in the etiology of AD, but the results remain inconclusive. Bertram et al reported that ESR1 PvuII and XbaI polymorphisms were associated with AD risk, and the ESR1 gene could be a candidate gene in the development of AD using a systematic meta-analysis.41 Another meta-analysis by Luckhaus and Sand indicated that ESR1 PvuII and XbaI polymorphisms were confirmed to modulate susceptibility to AD in Asian individuals, but not in Europeans.42 However, only eleven studies and eight studies were included in Bertram et al’s and Luckhaus and Sand’s studies, respectively. Therefore, we performed a meta-analysis to update previous meta-analyses, and provide a more comprehensive and reliable analysis of the association between ESR1 PvuII and XbaI polymorphisms and AD risk.
This meta-analysis included 18 case-control studies with 3,902 cases and 9,290 controls for the PvuII polymorphism, and 17 case-control studies with 2,841 cases and 8,094 controls for the XbaI polymorphism. In the overall analysis, the ESR1 PvuII polymorphism was not associated with AD risk. In the analysis stratified by ethnicity, the ESR1 PvuII polymorphism was associated with AD susceptibility in Caucasian populations, while there was no evidence of an association between the ESR1 PvuII polymorphism and AD risk in Asian populations, which suggested that the differences in genetic background may affect AD susceptibility due to different ethnicities with different allele frequencies. Although the exact mechanism of the PvuII polymorphism in the development of AD is not yet clear, a possible reason could be that the PvuII polymorphism produces a binding site for a specific transcription factor that may affect gene expression,43 while the presence of the C allele in the PvuII site was associated with decreased ESR1 transcription and, consequently, a low number of receptors.44 However, neither Caucasian populations nor Asian populations showed statistically significant associations between the ESR1 XbaI polymorphism and AD risk. Given that the PvuII and XbaI polymorphisms are in strong linkage disequilibrium,45 it is difficult to determine which of the two polymorphic sites is driving the association. In our meta-analysis, the results suggested that the ESR1 PvuII polymorphism may be of importance in AD risk in Caucasian populations. These findings are not consistent with previous meta-analyses;41,42 our results indicated that only the ESR1 PvuII polymorphism may alter the risk of AD in Caucasian populations, not in Asian populations, suggesting multiple and different genes are involved in the pathophysiological process of AD in different ethnicities.
Several limitations should not be ignored when interpreting the results. First, all eligible studies were from Caucasian and Asian populations; therefore, our results were limited to these two populations. More studies containing the full range of possible ethnic differences are needed to avoid selection bias. Second, AD is a multifactorial disease involving complex gene–gene or gene–environment interactions. In this study, we had insufficient data to evaluate such interactions for the independent role of ESR1 polymorphisms in AD risk. Third, we did not perform subgroup analysis by sex, age, or different stage of AD, due to limited data in primary studies. Fourth, because only published studies were included in this study, publication bias may have occurred, even though no statistical test bias was found.
Conclusion
In summary, this meta-analysis indicates that the ESR1 PvuII polymorphism is associated with increased AD risk in Caucasian populations, but not in Asian populations. However, no significant association was observed for the ESR1 XbaI polymorphism and AD risk. This result should be interpreted cautiously. To confirm or refute this result, well-designed studies with larger sample sizes and more ethnic groups are required to elucidate this association further.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (C120108).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Shi Z, Liu M, Liu S, Wang J. Association between the COMTVal158Met genotype and Alzheimer’s disease in the Han Chinese population. Dement Geriatr Cogn Dis Extra. 2014;4(1):14–21. doi: 10.1159/000357161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 8.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32(4):604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert JC, Harris JM, Mann D, et al. Are the estrogen receptors involved in Alzheimer’s disease? Neurosci Lett. 2001;306(3):193–197. doi: 10.1016/s0304-3940(01)01806-7. [DOI] [PubMed] [Google Scholar]
- 10.Pirskanen M, Hiltunen M, Mannermaa A, et al. Estrogen receptor beta gene variants are associated with increased risk of Alzheimer’s disease in women. Eur J Hum Genet. 2005;13(9):1000–1006. doi: 10.1038/sj.ejhg.5201447. [DOI] [PubMed] [Google Scholar]
- 11.Birge SJ, Mortel KF. Estrogen and the treatment of Alzheimer’s disease. Am J Med. 1997;103(3A):36S–45S. doi: 10.1016/s0002-9343(97)00258-1. [DOI] [PubMed] [Google Scholar]
- 12.Enmark E, Gustafsson JA. Oestrogen receptors – an overview. J Intern Med. 1999;246(2):133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu CY, Jiang ZN, Zhou Y, Li JJ, Huang LM. Estrogen receptor alpha roles in breast cancer chemoresistance. Asian Pac J Cancer Prev. 2013;14(7):4049–4052. doi: 10.7314/apjcp.2013.14.7.4049. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Chen D, Hu LP, et al. Estrogen receptor alpha gene polymorphisms and breast cancer risk: a case-control study with meta-analysis combined. Asian Pac J Cancer Prev. 2013;14(11):6743–6749. doi: 10.7314/apjcp.2013.14.11.6743. [DOI] [PubMed] [Google Scholar]
- 15.Gu Z, Wang G, Chen W. Estrogen receptor alpha gene polymorphisms and risk of prostate cancer: a meta-analysis involving 18 studies. Tumour Biol. 2014 Mar 1; doi: 10.1007/s13277-014-1785-4. Epub. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Xu H, Yin X, et al. Estrogen receptor alpha gene PvuII polymorphism and coronary artery disease: a meta-analysis of 21 studies. J Zhejiang Univ Sci B. 2014;15(3):243–255. doi: 10.1631/jzus.B1300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang L, Cheng GL, Xu ZH. Association between estrogen receptor alpha gene (ESR1) PvuII (C/T) and XbaI (A/G) polymorphisms and hip fracture risk: evidence from a meta-analysis. PloS One. 2013;8(12):e82806. doi: 10.1371/journal.pone.0082806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang KJ, Shi DQ, Sun LS, et al. Association of estrogen receptor alpha gene polymorphisms with bone mineral density: a meta-analysis. Chin Med J. 2012;125(14):2589–2597. [PubMed] [Google Scholar]
- 19.Xing Y, Jia JP, Ji XJ, Tian T. Estrogen associated gene polymorphisms and their interactions in the progress of Alzheimer’s disease. Prog Neurobiol. 2013;111:53–74. doi: 10.1016/j.pneurobio.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3673. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 21.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandi ML, Becherini L, Gennari L, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Bioch Biophys Res Commun. 1999;265(2):335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- 25.Boada M, Antunez C, López-Arrieta J, et al. Estrogen receptor alpha gene variants are associated with Alzheimer’s disease. Neurobiol Aging. 2012;33(1):198. e15–e24. doi: 10.1016/j.neurobiolaging.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Lin GF, Ma QW, Zhang DS, Zha YL, Lou KJ, Shen JH. Polymorphism of alpha-estrogen receptor and aryl hydrocarbon receptor genes in dementia patients in Shanghai suburb. Acta Pharmacol Sin. 2003;24(7):651–656. [PubMed] [Google Scholar]
- 27.Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer’s disease, vascular dementia and alcohol-associated dementia. Dement Geriatr Cogn Disord. 2000;11(3):119–122. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- 28.Usui C, Shibata N, Ohnuma T, et al. No genetic association between the myeloperoxidase gene-463 polymorphism and estrogen receptor-alpha gene polymorphisms and Japanese sporadic Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(5–6):296–299. doi: 10.1159/000091437. [DOI] [PubMed] [Google Scholar]
- 29.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer’s disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 30.Monastero R, Cefalu AB, Camarda C, et al. Association of estrogen receptor alpha gene with Alzheimer’s disease: a case-control study. J Alzheimers Dis. 2006;9(3):273–278. doi: 10.3233/jad-2006-9306. [DOI] [PubMed] [Google Scholar]
- 31.Porrello E, Monti MC, Sinforiani E, et al. Estrogen receptor alpha and APOEε4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur J Neurol. 2006;13(6):639–644. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 32.Li HM, Chen SQ, Liu XH, Wang YP, Huang SK. Relationship between estrogen receptor gene polymorphism and Alzheimer’s disease. Anat Res. 2004;26(2):109–111. Chinese [with English abstract] [Google Scholar]
- 33.Xu M, Jia J. The estrogen receptor alpha gene polymorphism in Chinese sporadic Alzheimer’s disease. Stroke Nerv Dis. 2002;9(5):266–269. [Google Scholar]
- 34.Hou L. Association of Estrogen Receptor Alpha and BDNF Polymorphisms with Late-Onset Alzheimer’s Disease in Han Population in Southern Chinese [master’s thesis] Guangzhou: Guangzhou Medical College; 2009. [Google Scholar]
- 35.Deng J, Shen C, Wang Y, et al. Association between the polymorphism of estrogen receptor alpha and Alzheimer’s disease in Chinese population. Clin Lab. 2013;59(7–8):741–746. doi: 10.7754/clin.lab.2012.120426. [DOI] [PubMed] [Google Scholar]
- 36.den Heijer T, Schuit SC, Pols HA, et al. Variations in estrogen receptor alpha gene and risk of dementia, and brain volumes on MRI. Mol Psychiatry. 2004;9(12):1129–1135. doi: 10.1038/sj.mp.4001553. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57(2):236–240. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 38.Ma SL, Tang NL, Tam CW, et al. Polymorphisms of the estrogen receptor alpha (ESR1) gene and the risk of Alzheimer’s disease in a southern Chinese community. Int Psychogeriatr. 2009;21(5):977–986. doi: 10.1017/S1041610209990068. [DOI] [PubMed] [Google Scholar]
- 39.Zhou BR. Analysis between estrogen receptor alpha gene polymorphisms and cognitive decline. Chin J Gerontol. 2008;28(11):2221–2225. Chinese [with English abstract] [Google Scholar]
- 40.Zhou X, Gu Y, Wang DN, Ni S, Yan J. Eight functional polymorphisms in the estrogen receptor 1 gene and endometrial cancer risk: a meta-analysis. PloS One. 2013;8(4):e60851. doi: 10.1371/journal.pone.0060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 42.Luckhaus C, Sand PG. Estrogen receptor 1 gene (ESR1) variants in Alzheimer’s disease. Results of a meta-analysis. Aging Clin Exp Res. 2007;19(2):165–168. doi: 10.1007/BF03324684. [DOI] [PubMed] [Google Scholar]
- 43.Herrington DM, Howard TD. ER-alpha variants and the cardiovascular effects of hormone replacement therapy. Pharmacogenomics. 2003;4(3):269–277. doi: 10.1517/phgs.4.3.269.22686. [DOI] [PubMed] [Google Scholar]
- 44.Cordts EB, Santos AA, Peluso C, Bianco B, Barbosa CP, Christofolini DM. Risk of premature ovarian failure is associated to the PvuII polymorphism at estrogen receptor gene ESR1. J Assist Reprod Genet. 2012;29(12):1421–1425. doi: 10.1007/s10815-012-9884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Meurs JB, Schuit SC, Weel AE, et al. Association of 5′ estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet. 2003;12(14):1745–1754. doi: 10.1093/hmg/ddg176. [DOI] [PubMed] [Google Scholar]