Abstract
Background
Antibiotic de-escalation is a potential strategy advocated to conserve the effectiveness of broad-spectrum antibiotics. The aim of this study was to examine the safety and feasibility of antibiotic de-escalation in patients admitted with bacteremic pneumonia.
Methods
A retrospective chart review was done for patients with bacteremic pneumonia admitted to Northwest Texas Hospital in Amarillo, TX, USA, during 2008. Antibiotic de-escalation was defined as changing the empiric antibiotic regimen to a culture-directed single agent with a narrower spectrum than the original regimen.
Results
Sixty-eight patients were admitted with bacteremic pneumonia. Eight patients were not eligible for de-escalation. Among the 60 patients who were eligible for de-escalation, the treating physicians failed to de-escalate antibiotics in 27 cases (45.0%). Discharge to a long-term care facility predicted failure to de-escalate antibiotics, while an infectious diseases consultation was significantly associated with antibiotic de-escalation. The average daily cost of antibacterial therapy in the de-escalation group was $25.7 compared with $61.6 in the group where de-escalation was not implemented. The difference in mean length of hospital stay and mortality between the two groups was not statistically significant.
Conclusion
Antibiotic de-escalation is a safe management strategy but unfortunately is not widely adopted. Although bacterial resistance poses a significant threat and is rising, antimicrobial de-escalation has emerged as a potential intervention that can conserve the effectiveness of broad-spectrum antibiotics without compromising the patient’s outcome. This practice is becoming important in the face of slow development of new anti-infective agents.
Keywords: bacteremia, antibiotic de-escalation strategy, lung infection
Introduction
Pneumonia is a common cause of death worldwide and is a frequent reason for hospital admission.1 Bacteremia complicates about 20% of cases of pneumonia.2–4 Isolating the pathogen in blood confirms the identity of the microbe causing this infection, but does not seem to predict mortality.5 However, bacteremia tends to be associated with other high-risk features known to herald a poor outcome, eg, hypotension, acute kidney injury, and hypothermia.2–4
Giving appropriate and adequate antibiotics in a timely fashion has been shown to decrease mortality.6 This holds true whether we are dealing with pneumonia or any other serious infection.6 Multidrug-resistant bacteria are becoming more prevalent, and in combination with clinicians’ awareness of the need for early appropriate therapy, use of broad-spectrum antibiotics is increasing. The downside to the above approach is that unjustified lengthy use of this finite resource could precipitate further bacterial resistance.7
Antibiotic de-escalation refers to the practice of starting with a broad-spectrum empiric antibiotic regimen, designed to avoid inadequate therapy, combined with a commitment to change from broad-spectrum to narrow-spectrum therapy and from multiple agents to fewer medications and possible even a single agent.8 It also entails reducing the duration of therapy and stopping it in selected patients, as indicated by clinical response, microbiological data, and novel inflammatory markers.
The aim of this retrospective study was to highlight potential opportunities for antibiotic de-escalation in patients admitted to a community hospital with bacteremic pneumonia. Factors associated with lack of antibiotic de-escalation, when it could have been possible, are identified. Further, several important outcomes are compared between bacteremic patients in whom empiric antibiotic coverage was de-escalated based on culture and susceptibility data and patients in whom antibiotics were not de-escalated.
Materials and methods
In this retrospective cohort study, medical records were reviewed for all patients older than 18 years who had bacteremic pneumonia during hospitalization at Northwest Texas Hospital from January 1, 2008, to December 31, 2008. Northwest Texas Hospital is a 400-bed teaching community hospital for Texas Tech University Health Science Center and serves a large catchment area in West Texas. Searching the computerized microbiology laboratory records for patients with positive blood cultures and subsequently reviewing their records for evidence of pneumonia with an identical bacterium in sputum culture identified the above cohort. The investigational review boards at Northwest Texas Hospital and Texas Tech University Health Science Center approved the study protocol.
The following information regarding the aforementioned group of patients was collected: demographic data, Acute Physiologic and Chronic Health Evaluation (APACHE) II score, Charlson comorbidity index, implicated bacteria, antibiotic susceptibility, and empiric choice of antibiotic therapy. The patient’s need for admission to intensive care, vasopressor support, or mechanical ventilation was also noted. Information about antibiotic de-escalation or the lack of it, the daily cost of antibiotic therapy, request for an infectious diseases consultation, length of stay in intensive care and in hospital, and mortality was collected.
Antibiotic de-escalation was defined as changing the empiric antibiotic regimen to a culture-directed single agent with a narrower spectrum than the original empiric regimen. Patients who were treated empirically with broad-spectrum antibiotics or an antibiotic regimen consisting of multiple agents were considered candidates for de-escalation. On the other hand, patients prescribed narrow-spectrum antibiotics on admission and those who grew resistant bacteria prohibiting use of narrow-spectrum antibiotics were not considered candidates for de-escalation. Antibiotics considered acceptable to de-escalate the empirical regimen to, included fluoroquinolones, first-generation and second-generation cephalosporins, broad-spectrum penicillins, and macrolides. De-escalation was evaluated on the fifth day following diagnosis of bacteremia. A broad-spectrum antibiotic was defined as a third-generation or fourth-generation cephalosporin, extended-spectrum penicillin, or a carbapenem. At the time of this study, our hospital did not have an active antibiotic stewardship team.
Statistical analysis
Continuous data are expressed as a mean with standard deviation. Categorical data were presented as the number of variables with the corresponding percentage. Comparison of continuous variables was carried out using the independent t-test. A box plot was used to visualize the distribution of these variables. The association of categorical variables with different end points of interest was assessed by the χ2 test with Yate’s continuity correction or Fisher’s exact test. The statistical analysis was done using Stata 12 (StataCorp LP, College Station, TX, USA). A P-value <0.05 was considered to be statistically significant.
Results
During the study period, 814 patients had a positive blood culture, of whom 68 (36 males and 32 females) had bacteremic pneumonia. All of these infections were monomicrobial and there were no other associated infections. Eight of the 68 patients received only levofloxacin, a fluoroquinolone, hence were not eligible for de-escalation and excluded from further analysis. Pathogens isolated from concomitant blood and sputum cultures in the remaining 60 patients are shown in Table 1. Among Streptococcus pneumoniae isolates, all were fluoroquinolone-susceptible and all but one was penicillin-susceptible. There were four methicillin-resistant Staphylococcus aureus isolates. Among the isolated Enterobacteriaceae, all but one case of Enterobacter cloacae were resistant to ampicillin, all were susceptible to fluoroquinolones except for one case each of Escherichia coli and one Klebsiella pneumoniae, and all were susceptible to cefazolin except for two cases involving Klebsiella spp. and one case of E. cloacae. There were no extended-spectrum beta-lactamase-producing or carbapenemase-producing bacteria. An isolated case of Pseudomonas aeruginosa was fluoroquinolone-susceptible. In all of the above cases, the empiric antibiotic regimen prescribed by the treating physician was active against the isolated bacteria.
Table 1.
Pathogens isolated from blood and sputum samples of the study cohort
| Pathogen | n (%) |
|---|---|
| Gram-positive bacteria | |
| Streptococcus pneumoniae | 37 (61.6) |
| Staphylococcus aureus | 9 (15.0) |
| Streptococcus agalactiae | 1 (1.7) |
| Gram-negative bacteria | |
| Klebsiella spp. | 7 (11.7) |
| Escherichia coli | 3 (5.0) |
| Enterobacter cloacae | 2 (3.3) |
| Pseudomonas aeruginosa | 1 (1.7) |
The mean age of this cohort was 56.0±17.1 years. Twenty-seven patients (45%) required intensive care unit admission, 15 and 17 of whom, respectively, required vasopressor support and mechanical ventilation. There were four immunocompromised patients in the cohort, one with human immunodeficiency virus infection and the others with multiple myeloma. The mean length of stay in the intensive care unit was 11.3±14.8 days. Fourteen patients were discharged to a long-term care facility (nine went to long-term acute care hospitals and five were discharged to a nursing home). The cohort’s length of hospital stay was 10.5±9.2 days and the overall mortality rate was 16.7%.
Antibiotics were de-escalated in 33 (55.5%) of 60 eligible patients. This group is referred to as the de-escalation group, whereas the group in which initial antibiotics were not de-escalated is referred to as the broad-spectrum group. Among the 60 patients who were candidates for de-escalation, a single broad-spectrum antibiotic (carbapenem or an extended-spectrum penicillin) was initiated empirically in four cases (6.7%), while two or more antibiotics, including broad-spectrum agents, were used in the other 56 patients (93.3%).
The mean length of hospital stay in the de-escalation group was 10.1±8.4 days versus 10.9±10.2 days in the broad-spectrum group (P=0.73). Two patients (6.1%) in the de-escalation group died versus five patients (18.5%) in the other group (P=0.23). The average daily cost of antibiotic therapy in the de-escalation group was 26.0±12.0 dollars versus 61.8±89.7 dollars in the other group (P=0.03). Among patients whose antibiotic therapy was de-escalated, an infectious diseases specialist was consulted 15 times compared with only twice in patients without de-escalation (P<0.01). The distribution of age, APACHE II score, and Charlson comorbidity index in the two groups is shown using a box plot in Figure 1. A comparison of the clinical and demographic characteristics of the de-escalation group and the broad-spectrum group is presented in Table 2.
Figure 1.
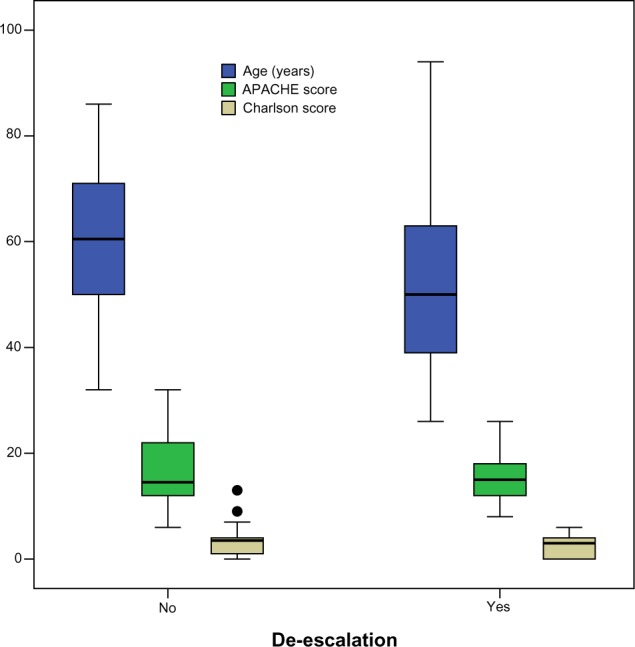
Distribution of age, APACHE score, and Charlson comorbidity index among patients with and without antibiotic de-escalation.
Abbreviation: APACHE, Acute Physiologic and Chronic Health Evaluation.
Table 2.
Comparison of patients with and without antibiotic de-escalation
| Factors | De-escalation
|
P-value | ||
|---|---|---|---|---|
| No (n=27) | Yes (n=33) | Total (n=60) | ||
| Age, years | ||||
| Mean | 59.1 | 53.5 | 56.0 | 0.212 |
| Sex | ||||
| Female | 10 | 18 | 28 (46.7%) | 0.275 |
| Male | 17 | 15 | 32 (53.3%) | |
| Charlson comorbidity index score | ||||
| Mean | 3.3 | 2.6 | 2.9 | 0.269 |
| APACHE score | ||||
| Mean | 16.6 | 15.4 | 15.9 | 0.494 |
| Isolated bacterium | ||||
| Streptococcus pneumoniae | 20 | 21 | 41 (68.3%) | 0.679 |
| MSSA/MRSA | 3 | 6 | 9 (15%) | |
| Others | 4 | 6 | 10 (16.7%) | |
| Need for ICU admission | ||||
| No | 13 | 20 | 33 (55%) | 0.481 |
| Yes | 14 | 13 | 27 (45%) | |
| Mechanical ventilation use | ||||
| No | 19 | 24 | 43 (71.7%) | 1.0 |
| Yes | 8 | 9 | 17 (28.3%) | |
| Vasopressor use | ||||
| No | 22 | 23 | 45 (75%) | 0.454 |
| Yes | 5 | 10 | 15 (25%) | |
| Disposition | ||||
| Expire | 7 | 3 | 10 (16.7%) | 0.040 |
| Home | 12 | 24 | 36 (60%) | |
| Long-term care facility | 8 | 6 | 14 (23.3%) | |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; APACHE, Acute Physiologic and Chronic Health Evaluation; ICU, intensive care unit.
Fourteen patients were sent to a long-term care facility. Their mean age was 66.7±14.9 years, their mean APACHE II score was 18.6±6.8, and their mean Charlson comorbidity index was 3.9±2.6, with all values being higher than for the rest of the cohort (P≤0.01). The mean length of hospital stay in this group was 15.1±10.9 days, which was significantly different from the rest of the cohort (P≤0.01). These patients grew seven S. pneumoniae (all were penicillin-susceptible and fluoroquinolone-susceptible), four Enterobacteriaceae (all fluoroquinolone-susceptible and cefazolin-susceptible), and three S. aureus, two of which were methicillin-resistant. Empiric antibiotic therapy consisted of two or more antibiotics (at least a fluoroquinolone and a broad-spectrum antibiotic or vancomycin and a broad-spectrum antibiotic) in all cases. Among the 14 patients who were candidates for de-escalation, empiric antibiotic therapy was de-escalated in only six patients (42.9%).
Discussion
In this retrospective study, the practice of antibiotic de-escalation was examined in 60 eligible patients with bacteremic pneumonia in a community hospital. The initial antibiotic regimen was de-escalated in about 55% of patients without increasing the length of hospital stay or inhospital mortality. The above data attest to the safety of this strategy. Discharge to a long-term care facility predicted failure to de-escalate empiric antibiotic therapy whereas an infectious diseases consultation was associated with antimicrobial de-escalation. De-escalation was cost-effective, saving approximately 35 dollars per patient per day. This finding should encourage hospitals and physicians alike to embrace this safe and effective strategy.
Bacteremic pneumonia is known to occur in a small proportion of patients with lower respiratory tract infection.2–4 Further, several studies have demonstrated the safety and feasibility of antibiotic de-escalation in multiple types of pneumonia, including ventilator-associated pneumonia and hospital-acquired pneumonia.9–11 Nevertheless, we felt this was worthwhile studying further because: having concordant blood and sputum cultures confirms the identity of the culprit pathogen; bacteremic patients tend to be more severely ill, leading to use of multiple broad-spectrum antibiotics initially, which should be de-escalated when culture results and susceptibility become available; once clinicians are comfortable with the concept of antibiotic de-escalation in a culprit-confirmed infection, they might gradually expand implementing this strategy to other types of pneumonia, eg, polymicrobial or culture-negative pneumonia.
Multiple reasons could account for the slow adoption of antibiotic de-escalation,8,12 including reluctance to change an antibiotic regimen that has proven to be effective, especially if the patient was critically ill on presentation, mistrust of microbiological data (which is less likely to happen with concordant blood and sputum cultures), under-recognition of potential opportunities, poor understanding of how to do de-escalate, and, above all, a lack of high-quality evidence.13–16 Looking for other factors associated with failure to de-escalate is worthwhile. In our cohort, there was an association between discharge destination and failure to de-escalate empiric antibiotic therapy. This represents a potential opportunity for the antibiotic stewardship team to pursue.
It is possible that discharge destination may be more of a consequence of de-escalation than a factor associated with it. In this study, patients sent to a long-term care facility were older than the rest of the cohort and had a higher APACHE II score and Charlson comorbidity index, but they did not have more multidrug-resistant bacteria. Despite the factors that dictated disposition, the empiric antibiotic therapy started in this group of patients could have been changed to either a fluoroquinolone or a narrow-spectrum penicillin. The above supports the opposite argument, ie, once disposition is secured, physicians are under less pressure to change the antibiotic regimen. The problem with sending patients to long-term care facilities without de-escalation or a clear plan to de-escalate is that the new physician, who is usually not familiar with the case, might be less likely to change the antibiotic regimen. Moreover, patients are likely to stay in those long-term care facilities for extended periods of time, and this, especially if they stay on broad-spectrum antibiotics, will select for more resistant microbes, contributing to the reputation of such facilities for having high rates of multidrug-resistant bacteria.17,18
An infectious diseases consultation has been associated with reduced mortality in bacteremic patients, especially in those who are critically ill or have multiple comorbidities.19,20 In our cohort, an infectious diseases consultation was requested in 28.3% of cases and was associated, as expected, with more judicious use of antibiotics.
Combination antimicrobial therapy aims mainly to provide a regimen likely to cover the suspected pathogen(s).21,22 It might also provide synergy and prevent emergence of resistance. In bacteremic pneumococcal pneumonia, combination therapy (a beta-lactam and macrolide agent, in particular) has been shown to reduce mortality.23,24 The need for prolonged combination therapy, however, has not been demonstrated to be superior to adequate monotherapy, even for pseudomonal pneumonia or bacteremia.25,26 In our study, we looked for de-escalation by day 5 of bacteremia to allow long enough time for the patient to harness all of the above potential benefits. It should be noted that de-escalation could be performed earlier in the course of treatment if microbiological information was available but it is practiced most safely between days 3 and 5.6 This allows the patient’s clinical condition to show clear trend and ensures complete and final reporting of microbiological data.
This study has some limitations. First, it is a retrospective study that evaluated a small number of patients. Second, it included low-risk patients commonly seen in community hospitals, hence it missed other important groups of patients, eg, those who are immunocompromised. Third, it investigated bacteremic pneumonia only, neglecting other types of pneumonia. Fourth, information on total duration of therapy and intravenous to oral antibiotic switch was not collected, neglecting an important dimension of de-escalation, ie, early oral switch, when feasible, and not treating longer than necessary. Further, no data regarding infection relapse rates or long-term mortality were gathered to demonstrate the safety of the de-escalation strategy in the long run.
In conclusion, antibiotic de-escalation is a strategy advocated to prevent overuse of broad-spectrum antibiotics. It is safe to implement in patients with bacteremic pneumonia, and can be guided by an infectious diseases consultation. Further, there is an untapped opportunity to de-escalate antimicrobials in patients discharged to long-term care facilities, with a potentially significant cost savings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wunderink RG, Waterer GW. Clinical practice. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543–551. doi: 10.1056/NEJMcp1214869. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SG, Marrie TJ, Anstey R, Ackroyd-Stolarz S, Dickinson G. Utility of blood cultures in the management of adults with community acquired pneumonia discharged from the emergency department. Emerg Med J. 2003;20(6):521–523. doi: 10.1136/emj.20.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie TJ. Blood cultures in ambulatory patients who are discharged from emergency with community-acquired pneumonia. Can J Infect Dis. 2004;15(1):21–24. doi: 10.1155/2004/530645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 5.Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133(3):618–624. doi: 10.1378/chest.07-1322. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niederman MS. Use of broad-spectrum antimicrobials for the treatment of pneumonia in seriously ill patients: maximizing clinical outcomes and minimizing selection of resistant organisms. Clin Infect Dis. 2006;42(Suppl 2):S72–S81. doi: 10.1086/499405. [DOI] [PubMed] [Google Scholar]
- 8.Niederman MS. De-escalation therapy in ventilator-associated pneumonia. Curr Opin Crit Care. 2006;12(5):452–457. doi: 10.1097/01.ccx.0000244126.84989.a2. [DOI] [PubMed] [Google Scholar]
- 9.Niederman MS. The importance of de-escalating antimicrobial therapy in patients with ventilator-associated pneumonia. Semin Respir Crit Care Med. 2006;27(1):45–50. doi: 10.1055/s-2006-933673. [DOI] [PubMed] [Google Scholar]
- 10.Schlueter M, James C, Dominguez A, Tsu L, Seymann G. Practice patterns for antibiotic de-escalation in culture-negative healthcare-associated pneumonia. Infection. 2010;38(5):357–362. doi: 10.1007/s15010-010-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. doi: 10.1186/cc11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederman MS, Soulountsi V. De-escalation therapy: is it valuable for the management of ventilator-associated pneumonia? Clin Chest Med. 2011;32(3):517–534. doi: 10.1016/j.ccm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Duchene E, Montassier E, Boutoille D, Caillon J, Potel G, Batard E. Why is antimicrobial de-escalation under-prescribed for urinary tract infections? Infection. 2013;41(1):211–214. doi: 10.1007/s15010-012-0359-x. [DOI] [PubMed] [Google Scholar]
- 14.Shime N, Satake S, Fujita N. De-escalation of antimicrobials in the treatment of bacteraemia due to antibiotic-sensitive pathogens in immunocompetent patients. Infection. 2011;39(4):319–325. doi: 10.1007/s15010-011-0116-6. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson AD, Barkham T. De-escalation for amoxicillin-susceptible Escherichia coli: easier said than done. J Hosp Infect. 2010;74(3):304–305. doi: 10.1016/j.jhin.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients? J Trauma. 2009;66(5):1343–1348. doi: 10.1097/TA.0b013e31819dca4e. [DOI] [PubMed] [Google Scholar]
- 17.Lin MY, Lyles-Banks RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57(9):1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JP, Cho EH, Lee SJ, Lee ST, Koo MS, Song YG. Influx of multidrug resistant, Gram-negative bacteria (MDRGNB) in a public hospital among elderly patients from long-term care facilities: a single-center pilot study. Arch Gerontol Geriatr. 2012;54(2):e19–e22. doi: 10.1016/j.archger.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation – a study of 521 patients in Germany. J Infect. 2009;59(4):232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–637. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328(7441):668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliziotis IA, Samonis G, Vardakas KZ, Chrysanthopoulou S, Falagas ME. Effect of aminoglycoside and beta-lactam combination therapy versus beta-lactam monotherapy on the Zemergence of antimicrobial resistance: a meta-analysis of randomized, controlled trials. Clin Infect Dis. 2005;41(2):149–158. doi: 10.1086/430912. [DOI] [PubMed] [Google Scholar]
- 23.Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med. 2001;110(6):451–457. doi: 10.1016/s0002-9343(00)00744-0. [DOI] [PubMed] [Google Scholar]
- 24.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131(2):466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 25.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35(8):1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 26.Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756–2764. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


