Abstract
Background
Chronic obstructive pulmonary disease (COPD) can affect cognition. The effects of other less severe chronic airway disorders on cognition remain to be clarified. This study aimed to measure and compare cognitive deterioration in subjects with COPD, subjects with chronic non-obstructive bronchitis (CNOB), and asymptomatic smokers (AS), and to relate the corresponding prevalence to several demographic and clinical variables and to normal reference values.
Methods
Four hundred and two subjects (COPD n=229, CNOB n=127, and AS n=46) of comparable age were included in the study. Cognitive impairment was assessed using the Mini Mental Status test, the Clock Drawing test, and the Trail Making test A and B.
Results
The extent and prevalence of cognitive deterioration was greater in COPD subjects, followed by CNOB subjects and AS (P<0.001). The Medical Research Council and COPD Assessment test scores, forced expiratory volume in the first second predicted, and arterial partial pressure of O2 and of CO2 were related to the extent and the prevalence of cognitive deterioration. COPD subjects, CNOB subjects, and AS aged 40–69 years showed the greatest cognitive impairment (P<0.01 compared to normal values). This was particularly clear in COPD subjects.
Conclusion
Cognitive impairment may start at the early stages of chronic airway damage and progress with a worsening of the respiratory condition. Indeed, the greatest cognitive deterioration was seen in COPD subjects. Cognition impairment may contribute to explaining the insufficient adherence to therapeutic plans and strategies, and the increasing social costs in respiratory subjects.
Keywords: cognition, chronic airway flow limitation, COPD, chronic bronchitis, smoke
Introduction
Chronic obstructive pulmonary disease (COPD) is a pathological condition of the respiratory system commonly diagnosed in the fifth decade of life and characterized by a high socioeconomic impact.1–3 COPD is only partially reversible and can progressively affect the function of other organs (eg, heart, vasculature, muscles, kidney, liver, gastroenteric apparatus, and brain) causing different comorbidities of various severities,4,5 including cognitive impairment.6–9 COPD effects on cognition are still poorly understood; indeed, this comorbidity has not been investigated as extensively as other pathologies.10–13 Cognitive function impairment was found to be associated with severe pulmonary dysfunction long ago,14–19 although its prevalence varied from study to study20–23 and was dependent on the specific diagnostic criteria adopted, the methods used for assessing the impairment, and the number of subjects investigated.12,24,25 Several studies investigating cognition in subjects with chronic airflow limitation had methodological limitations, including lack of clinical assessment of airflow impairment;26 a specific focus on cognitive impairment in elderly subjects with severe respiratory conditions; a lack of interest in subjects without a defined respiratory disease;20,27–29 a lack of extensive comparison of cognitive impairment in COPD subjects versus subjects with other chronic airway disorders and/or healthy subjects;23,29 a lack of multiple psychometric tools;30 and small sample sizes.7,21,31,32
To better define the cognitive status in subjects with COPD and other airway disorders, this study aimed to: 1) measure and grade the extent of cognitive deterioration in subjects with COPD, subjects with chronic non-obstructive bronchitis (CNOB), and asymptomatic smokers (AS) versus normal reference values; and 2) to relate the corresponding prevalence to major demographic and clinical variables.
Materials and methods
Written informed consent was obtained from all subjects participating in the study. Participants were subjects with different grades of COPD as defined in the Global Initiative for COPD (GOLD) guidelines,4 subjects with CNOB, and AS of comparable age. Each subject was assessed for: personal and family history, lung function (complete spirometry using CPFS/D; Medical Graphics Co, Oak Grove Parkway, St Paul, MN, USA), health status (using the COPD Assessment test [CAT] questionnaire),33 and disability (using the Medical Research Council [MRC] dyspnea scale).34
Cognition impairment was evaluated using four validated psychometric questionnaires:30 1) the Mini Mental Status test (MMSE), which assesses spatial and time orientation, attention, and calculation (normal score: ≥27 points; moderate cognitive impairment: 24–18 points; severe cognitive impairment: <18 points);35 2) the Clock Drawing test, which assesses memory, attention, and symbolic representation (normal score: 7–10 points; cognitive impairment: ≤6 points);36 3) the Trail Making test (TMT) A, which assesses visual processing and reproduction of numeric sequences (cognitive impairment: ≥94 seconds);37 and 4) the TMT B, which assesses cognition flexibility and shifting capacity (cognitive impairment: ≥283 seconds).37
Psychometric data were compared to those of normal controls of comparable age obtained from the available scientific literature.
The study was approved by the Lung Division, Bussolengo General Hospital, Bussolengo, Italy (approval number: 3898, November 11, 2011).
Statistics
A sample of ≥350 consecutive observational units was believed to provide a representative sample. Mean values ± standard deviation (SD) of all variables were calculated in each group of subjects. Comparisons between means were carried out by parametric (two-tailed t-test) and non-parametric tests (Welch’s t-test), while analysis of variance (ANOVA) and Duncan’s new multiple range test were used to compare mean values across the different groups of subjects (P<0.05 indicated statistical significance). Mean values of all the psychometric scores calculated by decades of age (ie, <40, 40–49, 50–59, 60–69, 70–79, and ≥80 years) were compared within groups, and to the corresponding normal reference values. Possible relationships between cognitive impairment and the following variables was evaluated within each group by multiple regression (P<0.005 indicated statistical significance): age, sex, body mass index (BMI), smoking habit, forced expiratory volume in the first second (FEV1), FEV1/forced vital capacity (FVC), arterial partial pressure of O2 (PaO2) and CO2 (PaCO2), CAT score, MRC score, and the presence of comorbidities.
Results
The study population consisted of 402 consecutive outpatient subjects (274 [68.1%] males and 128 [31.9%] females), recruited over 18 months (Table 1). Of these subjects, 229 had COPD, 127 had CNOB, and 46 were AS. Overall, subjects were well-matched for gender, and represented the expected characteristics of each condition well (COPD, CNOB, and AS) in terms of age, smoking habits, health status, and lung function. The overall mean age was 65.6±15.1 years (males: 67.4±15.3 years; females: 66.1±14.8 years); the corresponding percentage distribution of age is presented in Figure 1. AS were slightly younger than subjects with COPD and subjects with CNOB (50.6±11.3 years versus 70.5±12.9 years and 62.2±15.7 years, respectively). In contrast to the other two groups, COPD subjects were characterized by a FEV1/FVC ratio <70%. FEV1 reversibility from baseline after albuterol 400 mcg was +1.2%±1.8% in AS, +3.6%±2.3% in CNOB, and +3.4%±4.1% in COPD subjects (P= not significant). Overall, the whole population was characterized by a moderate impairment of respiratory function, both in terms of airway obstruction and blood gases deterioration; this profile was also confirmed by the subjects’ overall perception of dyspnea (MRC score) and by their claimed level of respiratory health status (CAT score). With the exception of mean FEV1% predicted and FEV1/FVC (significantly higher in females than males; P<0.008 and P<0.005, respectively) in COPD subjects, and of mean CAT scores (significantly higher in females than males; P<0.001) in CNOB subjects, no statistically significant differences were observed between sexes.
Table 1.
Main characteristics of the whole study population and of each group of subjects investigated
| Whole study population n=402 | AS n=46 | CNOB n=127 | COPD n=229 | |
|---|---|---|---|---|
| Males/females, n | 274/128 | 32/14 | 84/43 | 158/71 |
| Ratio | 2.1/1 | 2.2/1 | 2.0/1 | 2.2/1 |
| Age, mean (SD) | 65.6 (15.1) | 50.6 (11.3) | 62.2 (15.7) | 70.5 (12.9) |
| Active smokers, % | 22.6 | 100.0 | 36.3 | 16.6 |
| Ex-smokers, % | 63.7 | 0.0 | 40.9 | 65.5 |
| Non-smokers, % | 13.7 | 0.0 | 22.8 | 17.9 |
| BMI, mean (SD) | 27.7 (5.7) | 26.9 (4.5) | 28.8 (6.1) | 27.1 (5.6) |
| MRC score, mean (SD) | 2.0 (1.2) | 1.1 (0.8) | 1.7 (1.2) | 2.4 (1.1) |
| CAT score, mean (SD) | 15.3 (6.9) | 12.7 (7.1) | 14.3 (6.8) | 16.4 (6.7) |
| FEV1% predicted, mean (SD) | 68.4 (29.5) | 100.0 (15.2) | 87.6 (26.2) | 54.9 (23.6) |
| FEV1/FVC, mean (SD) | 65.8 (17.0) | 77.6 (6.0) | 79.8 (9.2) | 52.7 (11.9) |
| PaO2, mean (SD) | 71.6 (11.8) | 81.5 (6.1) | 73.4 (12.1) | 69.5 (11.3) |
| PaCO2, mean (SD) | 42.3 (9.7) | 38.3 (7.7) | 40.8 (7.5) | 43.5 (10.6) |
| Comorbidities, % | 64.7 | 63.0 | 64.6 | 65.1 |
Abbreviations: AS, asymptomatic smokers; BMI, body mass index; CAT, COPD assessment test; CNOB, chronic non-obstructive bronchitis; COPD, chronic obstructive pulmonary disease; FEV1, forced expiration volume in the first second; FVC, forced vital capacity; MRC, Medical Research Council; PaCO2, arterial partial pressure CO2; PaO2, arterial partial pressure O2; SD, standard deviation.
Figure 1.
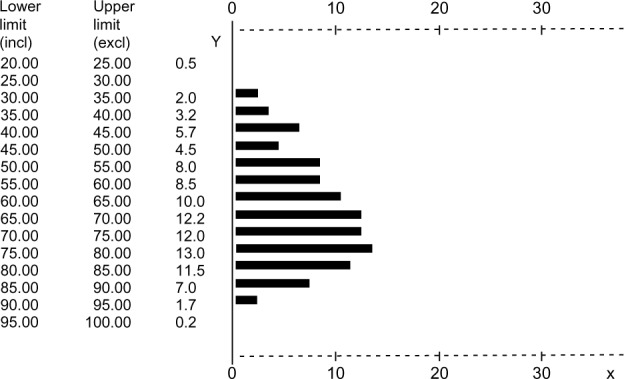
Percentage distribution of age, ranging 20–100 years (intervals of 5 years).
Abbreviations: Y, years; incl, included; excl, excluded.
In the whole study population, the cognitive level was sex-independent and the extent of cognitive impairment was significantly related, although only slightly, to some demographic and clinical variables (ie, BMI, MRC score, CAT score, FEV1% predicted, PaO2, and PaCO2), although none of these variables prevailed in affecting the regression results (Table 2). The percentage rate of comorbidities assessed is reported in Table 1, for the whole sample and for the different groups. In particular, cardiovascular/neurological comorbidities were 37.9% in AS, 51.2% in CNOB, and 56.4% in COPD subjects, while psychiatric comorbidities were 3.4% in AS, 3.6% in CNOB, and 4.0% in COPD subjects.
Table 2.
Correlation between demographic and clinical variables and psychometric scores in the whole study population (statistical significance and r values)
| Clock Drawing test | MMSE | TMT A | TMT B | |
|---|---|---|---|---|
| BMI | ||||
| P-value | ns | ns | 0.003 | 0.004 |
| r | – | – | 0.34 | 0.17 |
| MRC score | ||||
| P-value | 0.001 | 0.001 | 0.001 | 0.001 |
| r | −0.34 | 0.25 | 0.34 | 0.41 |
| CAT score | ||||
| P-value | 0.002 | 0.05 | 0.001 | 0.001 |
| r | 0.16 | −0.10 | 0.18 | 0.41 |
| FEV1% predicted | ||||
| P-value | 0.001 | 0.008 | 0.001 | 0.001 |
| r | 0.26 | 0.25 | −0.20 | −0.22 |
| PaO2, mmHg | ||||
| P-value | 0.001 | ns | 0.02 | 0.002 |
| r | 0.22 | – | 0.14 | −0.22 |
| PaCO2, mmHg | ||||
| P-value | 0.02 | 0.02 | 0.007 | 0.04 |
| r | −0.14 | −0.4 | 0.17 | 0.17 |
Abbreviations: BMI, body mass index; CAT, chronic obstructive pulmonary disease (COPD) assessment test; FEV1, forced expiration volume in the first second; MMSE, Mini Mental Status test; MRC, Medical Research Council; ns, not significant; PaCO2, arterial partial pressure CO2; PaO2, arterial partial pressure O2; TMT A, Trail Making test A; TMT B, Trail Making test B.
These comorbidities were equally represented in all groups investigated, and thus equally able to affect our data. No specific comorbidity was significantly related per se to the extent of cognitive dysfunction in the whole study population and in each group.
The mean score values calculated for each psychometric questionnaire in each group of subjects are reported in Table 3, together with the corresponding normal reference limits and the level of statistical significance for the corresponding multiple comparisons (ANOVA). The prevalence of pathological scores, assessed according to each psychometric test, is reported in the same table. Both the extent and the prevalence of cognitive impairment were significantly different in the three groups of subjects (P<0.001), with COPD subjects generally showing the highest extent and prevalence of deterioration (COPD > CNOB > AS, Table 3). This trend became much clearer when the subjects’ cognition was assessed with the TMT A and B, which were proved to be the most sensitive investigational tools (Table 3).
Table 3.
Psychometric test scores, prevalence of pathological scores, and statistical significance
| Clock Drawing test (score) Mean (SD) | % Subjects with score ≤6 | MMSE test (score) Mean (SD) | % Subjects with score <24 | TMT A (seconds) Mean (SD) | % Subjects with score ≥94 seconds | TMT B (seconds) Mean (SD) | % Subjects with score ≥283 seconds | |
|---|---|---|---|---|---|---|---|---|
| Normal reference values | 7–10 | >27 | <94 | <283 | ||||
| AS | 9.2 (0.5) | 0 | 28.6 (1.0) | 0 | 76.3 (19.0) | 0 | 164.3 (58.9) | 2.2 |
| CNOB | 8.2 (2.1) | 8.7 | 27.7 (2.4) | 15.7 | 92.3 (51.7) | 34.6 | 199.5 (109.9) | 28.3 |
| COPD | 7.2 (2.6)* | 16.6 | 26.9 (3.1)* | 32.8 | 115.1 (60.6)* | 49.3 | 236.2 (111.1)* | 40.2 |
| ANOVA | *P<0.001 | *P<0.001 | *P<0.001 | *P<0.001 |
Note:
Significance at P<0.001.
Abbreviations: ANOVA, analysis of variance; AS, asymptomatic smokers; CNOB, chronic non-obstructive bronchitis; COPD, chronic obstructive pulmonary disease; MMSE, Mini Mental Status test; SD, standard deviation; TMT A, Trail Making test A; TMT B, Trail Making test B.
Normal reference values of cognitive impairment calculated by decades of age ranging from 40 to 79 years are available in the literature only for the TMT A and B;38 therefore, only the scores obtained with these two tests in each group of subjects were compared to the corresponding normal reference values (Tables 4–6 and Figures 2 and 3). Since normal reference values for ages <40 and >79 years are still missing in the scientific literature, experimental values for these two ranges of age are reported in Tables 4–6 without any comparison.
Table 4.
TMT A and TMT B scores assessed by decades of age in COPD subjects: comparison versus corresponding normal reference values and significance levels
| Age (years) | Group | Numbers | TMT A Mean (SD) | P-value | TMT B Mean (SD) | P-value |
|---|---|---|---|---|---|---|
| <40 | Control | – | NA | NA | ||
| COPD | 4 | 66.5 (27.1) | – | 112.8 (33.4) | – | |
| 40–49 | Control | 52 | 48.9 (23.6) | 111.8 (53.9) | ||
| COPD | 13 | 73.2 (35.1) | 0.002 | 157.3 (82.8) | 0.01 | |
| 50–59 | Control | 52 | 53.8 (26.3) | 134.5 (80.1) | ||
| COPD | 24 | 96.0 (55.3) | 0.001 | 184.2 (101.9) | 0.01 | |
| 60–69 | Control | 39 | 67.3 (28.7) | 164.5 (97.4) | ||
| COPD | 56 | 99.9 (58.3) | 0.001 | 186.7 (100.3) | 0.1 | |
| 70–79 | Control | 10 | 84.6 (23.8) | 336.8 (197.8) | ||
| COPD | 68 | 110.1 (44.3) | 0.004 | 254.6 (106.4) | 0.1 | |
| >80 | Control | – | NA | NA | ||
| COPD | 64 | 155.6 (66.2) | – | 318.0 (77.9) | – |
Abbreviations: COPD, chronic obstructive pulmonary disease; NA, not available; SD, standard deviation; TMT A, Trail Making test A; TMT B, Trail Making test B.
Table 6.
TMT A and TMT B scores assessed by decades of age in AS: comparison versus corresponding normal reference values and significance levels
| Age (years) | Group | Numbers | TMT A Mean (SD) | P-value | TMT B Mean (SD) | P-value |
|---|---|---|---|---|---|---|
| <40 | Control | – | NA | NA | ||
| AS | 9 | 61.6 (15.3) | – | 107.9 (22.2) | – | |
| 40–49 | Control | 52 | 48.9 (23.6) | 111.8 (53.9) | ||
| AS | 12 | 70.8 (7.9) | 0.05 | 156.0 (40.0) | 0.001 | |
| 50–59 | Control | 52 | 53.8 (26.3) | 134.5 (80.1) | ||
| AS | 15 | 80.3 (21.9) | 0.001 | 170.3 (71.6) | 0.05 | |
| 60–69 | Control | 39 | 67.3 (28.7) | 164.5 (97.4) | ||
| AS | 9 | 81.3 (17.6) | 0.08 | 188.1 (59.6) | 0.25 | |
| 70–79 | Control | 10 | 84.6 (23.8) | 336.8 (197.8) | ||
| AS | 2 | 100.5 (16.3) | 0.3 | 163.0 (2.8) | 0.01 |
Abbreviations: AS, asymptomatic smokers; SD, standard deviation; TMT A, Trail Making test A; TMT B, Trail Making test B; NA, not available.
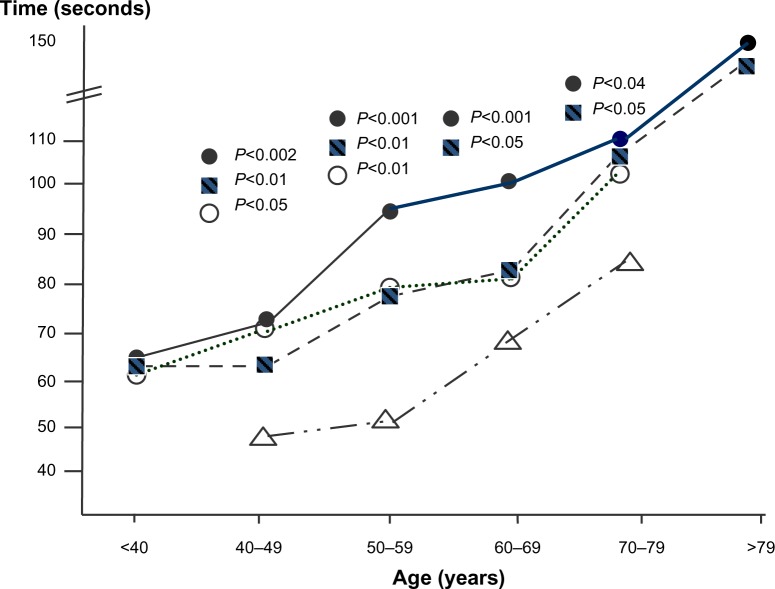
Figure 2.
TMT A test: comparison among the trends of cognitive impairment measured by decades of age (ANOVA) in AS (○), CNOB (■), and COPD subjects (•) versus the corresponding normal reference values (△).
Abbreviations: ANOVA, analysis of variance; AS, asymptomatic smokers; CNOB, chronic non-obstructive bronchitis; COPD, chronic obstructive pulmonary disease; TMT A, Trail Making test A.
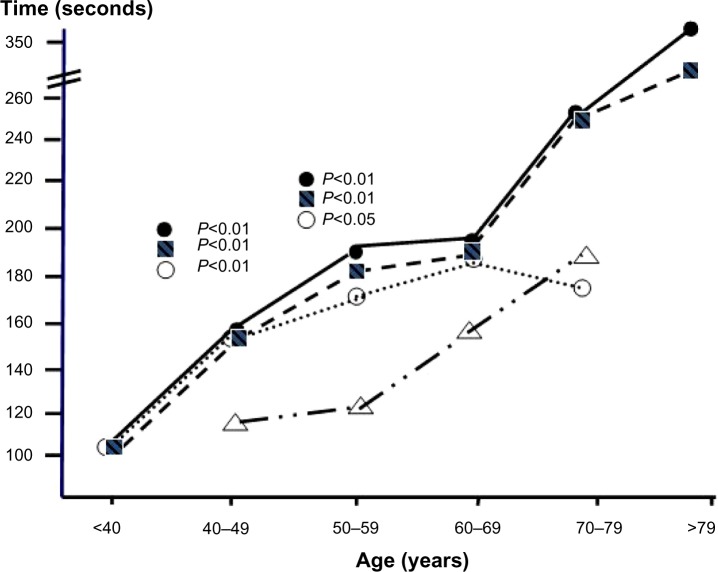
Figure 3.
TMT B test: comparison among the trends of cognitive impairment measured by decades of age (ANOVA) in AS (○), CNOB (■), and in COPD subjects (•) versus the corresponding normal reference values (△).
Abbreviations: ANOVA, analysis of variance; AS, asymptomatic smokers; CNOB, chronic non-obstructive bronchitis; COPD, chronic obstructive pulmonary disease; TMT B, Trail Making test B.
With the TMT A, the extent of cognitive impairment was significantly greater in COPD and CNOB subjects aged 40–79 years and in AS aged 40–59 years than in the corresponding normal controls (Figure 2 and Tables 4–6). As mentioned earlier, since normal reference values for subjects aged <40 and >79 years are missing in the literature, experimental data obtained in the present study could not be compared to normal controls, and no reliable trend was assessed for subjects belonging to these two extreme classes of age.
With the TMT B, the extent of cognitive impairment was significantly greater in COPD subjects, CNOB subjects, and AS aged 40–59 years than in the corresponding normal controls (Figure 3 and Tables 4–6). Further comparisons were not possible due to the lack of normal reference values in the literature for the TMT B in subjects aged <40 and >79 years, and due to the small number of AS aged 70–79 years.
Discussion
A substantial deterioration in cognition was assessed independently of sex in the three groups of subjects suffering from chronic airway impairment, although to different extents and prevalence. In contrast to what has been previously reported,29,37,39 our results suggest that significant cognitive impairment may occur starting from the early stages of chronic airway damage, even in relatively younger AS. The impact on cognition tends to increase with the worsening of the airway impairment, and was progressively greater in AS, CNOB, and COPD subjects. Our data show that the occurrence of a cognitive deterioration of true pathological relevance is more frequent than previously thought in subjects not characterized by severe airway flow limitation, or severe hypoxemia, and/or hypercapnia. This is in agreement with the report by Liensker et al, which showed that nonhypoxemic subjects with COPD exhibited significant impairment in their cognitive performance.40 In the case of AS who were mainly defined asymptomatic in terms of their lung function, they were nevertheless continually exposed to very complex and dangerous substances (such as cigarette smoke) that are also known to induce a significant vascular insult when chronically consumed. Therefore, the cognitive impairment we assessed in these subjects is likely only partially related to the sole airway involvement during their respiratory asymptomatic phase.
In agreement with previous studies,20,22,23 the extent and prevalence of cognitive impairment were directly related to the specific methodology used for the psychometric investigation. The presence of a substantial deterioration is more clearly observed using multiparametric psychometric measures (ie, the four different questionnaires in the present study) that are able to inform on several domains of cognition. In particular, when the Clock Drawing Test and both the TMT A and B are added to the commonly used MMSE, the subjects’ cognitive dysfunction can be measured more clearly. To our knowledge, this is the first study in which four questionnaires, characterized by different sensitivities, were administered sequentially to the same sample of respiratory subjects to measure their cognitive status. In this study, the TMT A and B were the most sensitive tools, and their use significantly affected the data on the prevalence of subjects characterized by a clear pathological cognitive deterioration. This prevalence was significantly higher than in healthy subjects, and was strictly related to the severity of the respiratory condition (highest in COPD subjects, followed by CNOB subjects and AS).
Based on the results of the TMT A and B, substantial cognitive dysfunction was slightly related to BMI, in COPD subjects with a BMI value <23 (ie, sarcopenic subjects), and in CNOB subjects with a BMI value >39 (ie, overweight subjects).
The TMT A and B were also the most sensitive tools in assessing any possible relationships within other demographic, clinical, and lung function variables. In particular, the MRC and the CAT score, the FEV1% predicted, PaO2, and PaCO2 were found to be slightly related to cognitive impairment, particularly in COPD subjects. Nevertheless, no variable per se was documented as specifically responsible for the onset of any deterioration in cognition.
It has been demonstrated that hypoxia per se has a significant effect on memory but not on other mental functions.38 Thus, it can be further speculated that several factors, variably interacting with each other, might contribute to the onset and the progressive worsening of cognition in subjects suffering from, or exposed to, chronic airway damage.
Our results did not highlight any relationship between cardiovascular and/or metabolic comorbidities and significant cognitive dysfunction. This is in agreement with the report by Dodd et al, which showed that hypoxemia, hypercapnia, smoking, and vascular comorbidities are unlikely to account per se for the cognitive dysfunction observed in COPD.9
It is important to note that, independently of the role of age in determining the physiological decline of cognition, in all subjects investigated and characterized by respiratory conditions of different severity levels, the greatest difference from normal cognition was observed in subjects aged 40–69 years. It is remarkable that subjects who are still in the active and productive phase of their life may be handicapped by a substantial deterioration of their cognitive performance due to their respiratory condition. It can then be argued that COPD, but also other persistent airway disorders, may act as independent risk factors in the impairment of cognitive function in this age range.
Our results clearly demonstrate that the substantial cognitive deterioration assessed in respiratory subjects aged 40–69 years is equivalent to that reported in healthy subjects aged 70–79 years. This observation suggests that chronic airway involvement, in particular when obstructive in nature, may act as a strong aging factor leading to an early deterioration of cognition.
Although all the domains of cognition were variably affected, memory, attention, symbolic representation and visual processing, reproduction of numeric sequences, cognition flexibility, and shifting capacity were the most affected cognitive functions. This assumption can be of strategic value, particularly for COPD subjects who need to have a good awareness of their disease, and who are increasingly encouraged to self-manage in order to obtain maximal adherence to their long-term therapeutic strategies. The evidence that COPD subjects may be significantly limited in their cognition from a younger age can contribute to the explanation of why these subjects frequently demonstrate insufficient interaction with their caregivers and general practitioners, and why their self-management is frequently inadequate even in the presence of persistent mild-to-moderate obstructive airway involvement. The psychometric profile of COPD subjects, as shown in this study, could also contribute to explaining their frequent requests for admission to health institutions and the consequent high economic impact.
The present study has some limitations. Lung function was checked by complete spirometry; however, measurements of total lung capacity, residual volume, and diffusion capacity were not available for all subjects. A large proportion of COPD and CNOB subjects, in particular those aged >70 years, were insufficiently compliant to these respiratory tests. Thus, lung function measurements were available for a small proportion of subjects that was not representative of the whole group. Also, lung imaging was not systematically performed in the whole study population, mainly due to economic restrictions. These limitations did not allow any speculation on the specific role of emphysema in affecting subjects’ cognition. Another limitation was related to the cross-sectional approach of this study. Estimation of the economic impact of cognitive deterioration in these patients is in progress, together with the longitudinal phase of the study, which aims to assess the role of long-term respiratory treatments in changing the pattern of cognitive dysfunction.
Conclusion
In conclusion, cognition can deteriorate substantially in subjects with chronic airway flow limitation (ie, COPD subjects), but also in subjects with other milder persistent airway disorders (ie, CNOB subjects and AS). The extent and the prevalence of this deterioration were directly related to the severity of the respiratory impairment. MRC, CAT, BMI, FEV1, PaO2, and PaCO2 were the variables contributing to, although not individually, the onset and the progression of the cognitive impairment.
We believe that the assessment of cognition in subjects suffering from chronic airway disorders, in particular of the obstructive nature, should enter the routine of diagnostic procedures to grade the overall impact of patients’ respiratory condition, and to decide the most effective therapeutic actions and strategies. Further studies in this field are needed, especially studies carried out on subjects suffering from chronic airway disorders of different severities, and following protocols designed to match multiple respiratory functions to multiple domains of cognition.
Table 5.
TMT A and TMT B scores assessed by decades of age in CNOB subjects: comparison versus corresponding normal reference values and significance levels
| Age (years) | Group | Numbers | TMT A Mean (SD) | P-value | TMT B Mean (SD) | P-value |
|---|---|---|---|---|---|---|
| <40 | Control | – | NA | NA | ||
| CNOB | 11 | 66.0 (16.1) | – | 112.8 (33.4) | – | |
| 40–49 | Control | 52 | 48.9 (23.6) | 111.8 (53.9) | ||
| CNOB | 16 | 62.8 (15.3) | 0.01 | 157.3 (82.8) | 0.01 | |
| 50–59 | Control | 52 | 53.8 (26.3) | 134.5 (80.1) | ||
| CNOB | 27 | 76.6 (26.9) | 0.001 | 184.2 (101.9) | 0.01 | |
| 60–69 | Control | 39 | 67.3 (28.7) | 164.5 (97.4) | ||
| CNOB | 25 | 81.9 (44.8) | 0.05 | 186.7 (100.3) | 0.14 | |
| 70–79 | Control | 10 | 84.6 (23.8) | 336.8 (197.8) | ||
| CNOB | 30 | 105.5 (53.8) | 0.05 | 254.6 (106.4) | 0.11 | |
| >80 | Control | – | NA | NA | ||
| CNOB | 18 | 148.9 (72.9) | – | 318.0 (77.9) | – |
Abbreviations: CNOB, chronic non-obstructive bronchitis; NA, not available; SD, standard deviation; TMT A, Trail Making test A; TMT B, Trail Making test B.
Acknowledgments
The authors thank the Chiesi Foundation for the unrestricted support to the present investigation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(Suppl 2):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 3.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 4.Aït-Khaled N, Enarson DA, Ottmani S, El Sony A, Eltigani M, Sepulveda R. Chronic airflow limitation in developing countries: burden and priorities. Int J Chron Obstruct Pulmon Dis. 2007;2(2):141–150. [PMC free article] [PubMed] [Google Scholar]
- 5.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121(9):789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calverley PM. Neuropsycological deficits in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis. 1996;51(1):5–6. [PubMed] [Google Scholar]
- 7.Ambrosino N, Bruletti G, Scala V, Porta R, Vitacca M. Cognitive and perceived health status in patient with chronic obstructive pulmonary disease surviving acute on chronic respiratory failure: a controlled study. Intensive Care Med. 2002;28(2):170–177. doi: 10.1007/s00134-001-1165-6. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli-Incalzi C, Corsonello A, Troiano L, et al. Screening of cognitive impairment in chronic obstructive pulmonary disease. Dement Geriatr Cogn Disord. 2007;23(4):264–270. doi: 10.1159/000100773. [DOI] [PubMed] [Google Scholar]
- 9.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 10.Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86:257–260. [PMC free article] [PubMed] [Google Scholar]
- 11.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age- associated memory impairment: proposed diagnostic criteria and measures of clinical change: report of a National Institute of Mental Health Work Group. Dev Neuropsychol. 1986;2(4):261–276. [Google Scholar]
- 12.Ward T, Dawe B, Procter A, Murphy E, Weinman J. Assessment in severe dementia: the Guy’s Advanced Dementia Schedule. Age Ageing. 1993;22(3):183–189. doi: 10.1093/ageing/22.3.183. [DOI] [PubMed] [Google Scholar]
- 13.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6(1):63–68. [PubMed] [Google Scholar]
- 14.Semenza C, Mondini S, Borgo F, Pasini M, Sgaramella MT. Proper names in patients with early Alzheimer’s disease. Neurocase. 2003;9(1):63–69. doi: 10.1076/neur.9.1.63.14370. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie K, Touchon J. Mild cognitive impairment: conceptual basis and current nosological status. Lancet. 2000;355(9199):225–228. doi: 10.1016/S0140-6736(99)06155-3. [DOI] [PubMed] [Google Scholar]
- 16.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neurophysiological findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476. [PubMed] [Google Scholar]
- 17.Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow study. Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134(5):1011–1019. doi: 10.1164/arrd.1986.134.5.1011. [DOI] [PubMed] [Google Scholar]
- 18.Kuller LH, Ockene JK, Townsend M, Browner W, Meilahn E, Wentworth DN. The epidemiology of pulmonary function and COPD mortality in the multiple risk factor intervention trial. Am Rev Respir Dis. 1989;140(3 Pt 2):S76–S81. doi: 10.1164/ajrccm/140.3_Pt_2.S76. [DOI] [PubMed] [Google Scholar]
- 19.Chyou PH, White LR, Yano K, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143(8):750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 20.Incalzil RA, Bellia V, Maggi S, et al. Salute Respiratoria nell’Anziano Study Group Mild to moderate chronic airways disease does not carry an excess risk of cognitive dysfunction. Aging Clin Exp Res. 2002;14(5):395–401. doi: 10.1007/BF03324468. [DOI] [PubMed] [Google Scholar]
- 21.Isoaho R, Puolijoki H, Huhti E, Laippala P, Kivelä SL. Chronic obstructive pulmonary disease and cognitive impairment in the elderly. Int Psychogeriatr. 1996;8(1):113–125. doi: 10.1017/s1041610296002517. [DOI] [PubMed] [Google Scholar]
- 22.Pleis JR, Leithbridge-Cejku M. Summary Health Statistics for US adults: National Health Interview Survey, 2006. Hyattsville, MD: Vital and Health Statistics, US Department of Health and Human Services; 2007. pp. 20–23. [Google Scholar]
- 23.Anstey KJ, Windsor TD, Jorm AF, Christensen H, Rodgers B. Association of pulmonary function with cognitive performance in early, middle and late adulthood. Gerontology. 2004;50(4):230–234. doi: 10.1159/000078352. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence- based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 26.Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(2):134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 27.Kozora E, Emery CF, Zhang L, Make B. Improved neurobehavioral functioning in emphysema patients following medical therapy. J Cardiopulm Rehabil Prev. 2010;30(4):251–259. doi: 10.1097/HCR.0b013e3181d0c47c. [DOI] [PubMed] [Google Scholar]
- 28.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 29.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 30.Maruta C, Guerreiro M, de Mendonça A, Hort J, Scheltens P. The use of neuropsycological tests across Europe: the need for a consensus in the use of assessment tools for dementia. Eur J Neurol. 2011;18(2):279–285. doi: 10.1111/j.1468-1331.2010.03134.x. [DOI] [PubMed] [Google Scholar]
- 31.Kozora E, Filley CM, Julian LJ, Cullum CM. Cognitive functioning in patients with chronic obstructive pulmonary disease and mild hypoxemia compared with patients with mild Alzheimer disease and normal controls. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(3):178–183. [PubMed] [Google Scholar]
- 32.Antonelli Incalzi R, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease – a neuropsychological and spect study. J Neurol. 2003;250(3):325–332. doi: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 33.Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 34.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 37.Brown EC, Casey A, Fisch RI, Neuringer C. Trail making test as a screening device for the detection of brain damage. J Consult Psychol. 1958;22(6):469–474. doi: 10.1037/h0039980. [DOI] [PubMed] [Google Scholar]
- 38.Huppert FA. Memory impairment associated with chronic hypoxia. Thorax. 1982;37(11):858–860. doi: 10.1136/thx.37.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Incalzi RA, Gemma A, Marra C, Muzzolon R, Capparella O, Carbonin P. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis. 1993;148(2):418–424. doi: 10.1164/ajrccm/148.2.418. [DOI] [PubMed] [Google Scholar]
- 40.Liensker JJ, Postma DS, Beukema RJ, et al. Cognitive performance in patients with COPD. Respir Med. 2004;98(4):351–356. doi: 10.1016/j.rmed.2003.11.004. [DOI] [PubMed] [Google Scholar]