Abstract
Resveratrol (trans-3,4′,5-trihydroxystilbene), a natural polyphenolic compound detected in grapes, berries, and peanuts, possesses a wide spectrum of pharmacological properties, including anti-tumor metastasis activities. However, the underlying mechanisms through which resveratrol inhibits the metastasis of pancreatic cancer are still not fully elucidated. As epithelial-to-mesenchymal transition (EMT) is a key player for metastasis in tumor, the aim of this study is to determine whether resveratrol affects EMT in pancreatic cancer cells and the related mechanism. The results showed that resveratrol not only inhibited cell proliferation, migration, and invasion in a dose-dependent manner, but also mediated the expression of EMT-related genes (E-cadherin, N-cadherin, vimentin, MMP-2, and MMP-9) which are important for cancer cellular motility, invasiveness and metastasis during tumorigenesis. In addition, the levels of phospho-Akt and phospho-NF-κB in BxPC-3 and Panc-1 cells were reduced by both resveratrol and LY294002 (a PI3-K inhibitor). Furthermore, transforming growth factor-β (TGF-β)-induced alterations in cell morphology that are characteristic of EMT as well as increased cell invasive ability could also be reversed by resveratrol. Taken together, these data indicate that resveratrol suppresses pancreatic cancer migration and invasion through the inhibition of the PI-3K/Akt/NF-κB signaling pathway. This study suggests that resveratrol may be a potential anticancer agent for pancreatic cancer.
Keywords: Resveratrol, pancreatic cancer, invasion, metastasis, EMT, E-cadherin, PI-3K/Akt pathway, NF-κB pathway, TGF-β
INTRODUCTION
Pancreatic cancer, the fourth leading cause of cancer death worldwide, is an aggressive malignant disease due to the lack of early diagnosis and treatment options [1,2]. Approximately 80% of pancreatic cancer patients are diagnosed with the unresectable form as the metastasis occurs frequently in the early stages of tumor development [3,4]. Moreover, even patients with seemingly resectable pancreatic cancer is often not cured by surgery due to a microscopic systemic spread of the cancer that occurs prior to the procedure [5]. Current treatments for inoperable patients are still limited to chemotherapy, radiation, or both [6]. Thus, more comprehensive and effective therapeutic strategies are urgently needed.
Metastasis occurs through a complex, multistep process, which consists of the invasion of cells from primary tumors into the circulation, migration of these cells to distant organs, adhesion to endothelial cells and infiltration into tissues [3]. As a cellular process through which polarized epithelial cells transform into motile mesenchymal-appearing cells, epithelial-mesenchymal transition (EMT) plays an important role in tumor invasion and metastasis via the loss of expression of the cell-cell adhesion molecule E-cadherin and a gain of mesenchymal markers (e.g., vimentin, N-cadherin, alpha-smooth muscle actin) [7,8]. Transforming growth factor-β (TGF-β) is the first EMT inducer described in normal mammary epithelial cells [9]. TGF-β plays a crucial role in tumor metastasis, and inhibitors of TGF-β signaling have become attractive targets as strategies to prevent tumor progression.
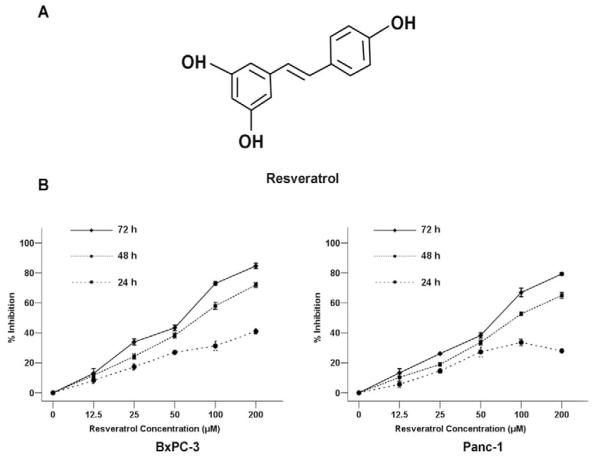
Resveratrol (trans-3,4′,5-trihydroxystilbene, Fig. 1A), a natural polyphenol, is famous for the story of the French Paradox: the French population suffers less from cardiovascular diseases than populations in other industrialized countries despite a diet rich in saturated fats [10]. Resveratrol has been found in various plants, including grapes, berries, peanuts and many types of traditional Chinese medicine (TCM) [11]. Recent studies have shown that resveratrol has many biological and pharmaceutical properties, including anti-inflammatory, antioxidant, anti-aging, neuroprotective, and anti-tumorigenic capabilities [12-18]. Its anti-tumorigenic property was found in more than 30 types of tumor cells, including those originating from lung, liver, breast, prostate, colon, and esophageal cancers [19,20]. Resveratrol is reported to be one of the most potent chemotherapeutic agents that are capable of inhibiting the cellular processes associated with tumor development, such as initiation, promotion, and progression [21,22].
Fig. (1). Resveratrol inhibits the proliferation of pancreatic cancer cells.
A, The structure of resveratrol. B, BxPC-3 and Panc-1 pancreatic cancer cells were treated with increasing concentrations of resveratrol (0, 12.5, 25, 50, 100, 200 μM) for 24 h, 48 h, or 72 h to analyze the inhibition ratio for cancer cell proliferation. *P < 0.05 compared with the control (0 μM) group.
Previous studies have shown that resveratrol could inhibit the proliferation of pancreatic cancer [23,24]. Ding et al. [23] demonstrated that resveratrol might have a potent anti-proliferative effect on human pancreatic cancer through the induction of apoptosis in vitro. Using an orthotopic mouse model of human pancreatic cancer, a recent in vivo study proved that resveratrol could not only inhibit the growth and metastasis of the tumor by suppressing the expression of cyclin D1, COX-2, ICAM-1, MMP-9, and survivin, but that it could also potentiate the effects of gemcitabine in cancer treatment [24].
In this study, we hypothesized that resveratrol may have a repressive role in EMT, a key player for metastasis in pancreatic cancer. To test this hypothesis, we investigated the expression of EMT-related molecules in Panc-1 and BxPC-3 pancreatic cancer cells with or without resveratrol and the signaling pathways triggered by resveratrol.
MATERIALS AND METHODS
Cell Culture and Reagents
The human pancreatic cancer cell lines, BxPC-3 and Panc-1, were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM medium containing 10% dialyzed heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Resveratrol (>99% pure) was acquired from Xi’an Chongxin Natural Additive Company (Xi’an, China). Dimethylsulfoxide (DMSO) and 3-(4, 5-dimethylthiazol -2–yl)-2, 5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone (Logan, UT, USA). Millicell culture plate inserts were bought from Millipore (Bedford, MA, USA). Matrigel was purchased from BD (Biosciences, Bedford, MA, USA). The PI-3K inhibitor, LY294002 was obtained from Sigma Chemical Co.. Recombinant human TGF-β1 was purchased from Zhongshan Goldenbridge Biotechnology Co.. Primary antibodies against MMP-2, MMP-9, E-cadhein, N-cadherin, and vimentin were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-Akt, anti-phospho-Akt (Ser473), anti-NF-κB, and anti-phospho-NF-κB (Ser468) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Other reagents were purchased from common commercial sources. All drug solutions were freshly prepared on the day of testing.
MTT Assay
BxPC-3 and Panc-1 cells were seeded in 96-well plates at the density of 1×104 cells per well and incubated overnight in 10% FBS medium. The cells were then treated with increasing concentrations of resveratrol or 0.1% DMSO alone as the control. After incubation for 24, 48, and 72 h at 37°C, 15 μL of MTT solution (5 mg/ml in phosphate-buffered saline, PBS) was added to each well, and then the cells were incubated for 4 h at 37°C. 100 μL of DMSO was then added to each well. The optical density (OD) value at 490 nm was determined using a spectrophotometer (Bio-Rad, CA, USA). The results were presented as the percentages relative to the controls. The proliferation inhibition rate was calculated as = (1 – ODsample/ODcontrol) × 100%.
Cell Migration Assay
Cell migratory ability was detected by a wound-healing assay. Pancreatic cancer cells were seeded in 24-well plates (1.0×105 cells/500 μL). After the cells grew to 90-100% confluence, a sterile pipette tip was used to produce a wound line between the cells. Cellular debris was removed by washing with PBS. The wounded monolayers were then incubated with resveratrol or vehicle for 24 h and digitally photographed.
Cell Invasion Assay
The invasion of pancreatic cancer cells was performed in transwell chambers. The Millicell culture plate filter inserts (pore size, 8.0 μm) were coated with matrigel. The cells were suspended in DMEM containing 1% FBS. Then the cell suspensions (100 μL containing 2×104 cells) were added to the upper chambers. Simultaneously, 500 ml of DMEM containing 20% FBS was placed in the lower chambers. The cells were allowed to migrate for 48 h at 37°C. The non-migrated cells were removed from the upper surface by scraping with a wet cotton swab. After rinsing with PBS, the filter was fixed and stained with crystal violet. The number of cells in five random fields was counted under a microscope (×100). The mean values of the data were obtained from three separate chambers.
Real Time Quantitative PCR (QT-PCR)
Total RNA was extracted from the pancreatic cancer cells using the Fastgen200 RNA isolation system (Fastgen, Shanghai, China) according to the manufacturer’s protocol. Total RNA was reverse-transcribed into cDNA using the Fermentas RevertAidTM Kit (MBI Fermentas, Canada). The primer sequences were as follows
E-cadherin -F: 5′- ATTCTGATTCTGCTGCTCTTG - 3′
E-cadherin -R: 5′- AGTCCTGGTCCTCTTCTCC - 3′
N-cadherin -F: 5′- TGTTTGACTATGAAGGCAGTGG-3′
N-cadherin -R: 5′- TCAGTCATCACCTCCACCAT - 3′
vimentin -F: 5′- AATGACCGCTTCGCCAAC - 3′
vimentin -R: 5′- CCGCATCTCCTCCTCGTAG - 3′
MMP-2 -F: 5′-GATGATGCCTTTGCTCGTGC-3′
MMP-2 -R: 5′-CAAAGGGGTATCCATCGCCA-3′
MMP-9 -F: 5′- TGGTCCTGGTGCTCCTGGTG-3′
MMP-9 -R: 5′- GCTGCCTGTCGGTGAGATTGG-3′
β-actin -F: 5′-GACTTAGTTGCGTTACACCCTTTCT-3′
β-actin -R: 5′-GAACGGTGAAGGTGACAGCAGT-3′
The PCR amplification was performed using following condition: 30 s at 95°C followed by 40 cycles of at 95°C for 5 s, at 60 °C for 30 s and at 72 °C for 30 s. After each QT–PCR, a dissociation curve analysis was conducted. Relative gene expression was calculated using the 2-ΔΔCt method reported previously [25]. Each measurement was performed in triplicate.
Western Blotting
Proteins were electrophoretically resolved on a denaturing SDS-polyacrylamide gel and electrotransferred onto nitrocellulose membranes. The membranes were initially blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) for 2 h and then probed with antibodies against MMP-2, MMP-9, E-cadherin, N-cadherin, vimentin, p-Akt, Akt, p-NF-κB, NF-κB, or β-actin. After incubation with the primary antibodies at 4°C overnight, the membranes were hybridized with secondary goat anti-mouse or goat anti-rabbit antibodies (Sigma-Aldrich, St. Louis, MO, USA) for 2 h at room temperature. Immunopositive bands were developed using an enhanced chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ, USA). All analyses were conducted in triplicate.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 17.0, SPSS Inc., Chicago, USA). Data were presented as the means ± SEM of three replicate assays. Differences between the groups were analyzed by analysis of variance (ANOVA), followed by Bonferroni’s correction for multiple comparisons. P < 0.05 was considered statistically significant. All experiments were repeated independently at least three times.
RESULTS
Effect of Resveratrol on the Proliferation of Pancreatic Cancer Cells
The cytotoxicity of resveratrol was first determined using the MTT assay. BxPC-3 and Panc-1 cells were treated with resveratrol at various concentrations (12.5, 25, 50, 100, or 200 μM) for 24, 48, and 72 h. The results demonstrated that the proliferative abilities of both BxPC-3 and Panc-1 cells were decreased in response to the treatment of resveratrol in a time- and dose-dependent manner. The 50% inhibitory concentration (IC50) for both BxPC-3 and Panc-1 cells was approximately 50 μM of resveratrol, which exhibited no cytotoxic effects on the BxPC-3 and Panc-1 cells (Fig. 1B). Therefore, treatment concentrations of 12.5, 25, and 50 μM of resveratrol on the cells were used for the subsequent experiments.
Resveratrol Inhibited the Migration and Invasion of Pancreatic Cancer Cells
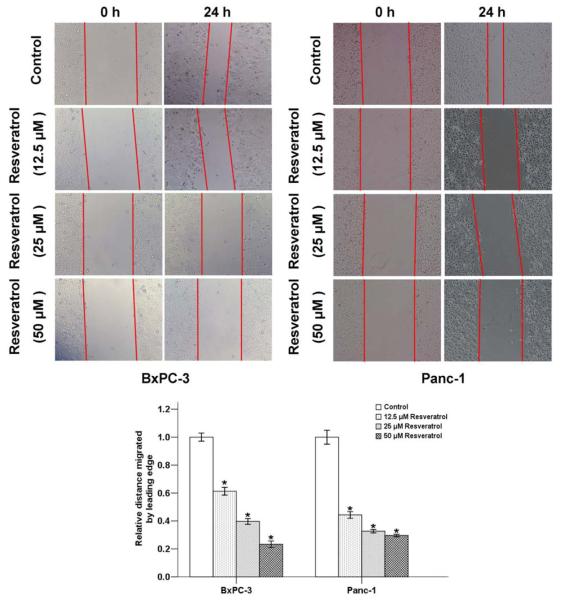
The effect of resveratrol on pancreatic cancer cell motility was determined by using a wound-healing assay. Serumstarved BxPC-3 and Panc-1 cells in the control group exhibited marked cell migration in the wound area after 24 h wounding, whereas the cells treated with different concentrations (12.5, 25, 50 μM) of resveratrol showed dose-related delays in wound closure (Fig. 2).
Fig. (2). The effects of resveratrol on the migration of pancreatic cancer cells.
BxPC-3 and Panc-1 cells were treated with the indicated concentrations of resveratrol for 24 h. Resveratrol significantly inhibited the migration of cells into the denuded areas. *P < 0.05 compared with the control group.
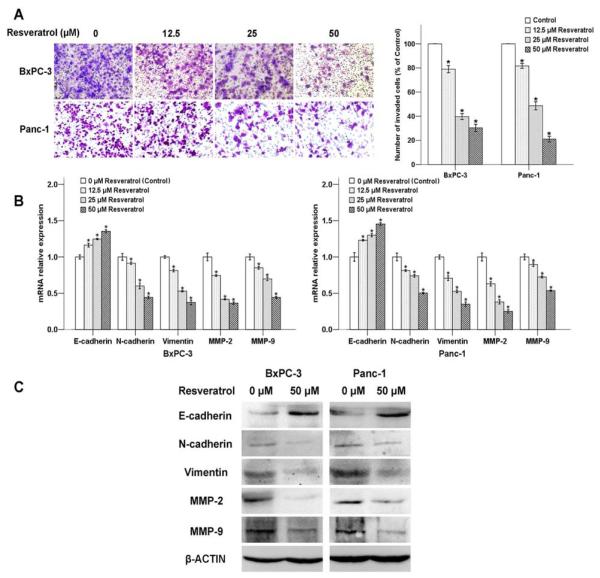
To examine potential anti-invasive effects, BxPC-3 and Panc-1 cells treated with resveratrol were analyzed for the ability of invasion. As shown in Fig. 3A, the average cell number that invaded into the lower chamber decreased as the resveratrol concentration increased from 0 μM to 50 μM. This finding revealed that resveratrol might be an effective inhibitor of the migration and invasion of pancreatic cancer cells.
Fig. (3). The effects of resveratrol on pancreatic cancer cell invasion and EMT.
The BxPC-3 and Panc-1 cells were treated with increasing concentrations of resveratrol (0, 12.5, 25, and 50 μM) for 48 h. A, Photos of the invasive BxPC-3 and Panc-1 cells were taken under a microscope with a 100× objective. B, mRNA; and C, Protein expression levels of EMT-related genes were detected. *P < 0.05 compared with the control group.
Effects of Resveratrol on the Expression of EMT-Related Markers in Pancreatic Cancer Cells
It is believed that acquiring the migratory characteristics of a mesenchymal-like state enhance the invasive capabilities of cancer cells. The EMT switch is associated with an unfavorable prognosis in many different types of tumors, including pancreatic ductal adenocarcinoma [26]. We therefore examined the expression of EMT-related genes in response to the treatment of resveratrol. The result showed that the mRNA level of the epithelial marker, E-cadherin, up-regulated with the increasing concentration of resveratrol (Fig. 3B). However, the mesenchymal markers, N-cadherin as well as vimentin decreased in a dose-dependent manner. Moreover, the expression of metastasis-related genes MMP-2 and MMP-9 were also influenced by resveratrol in the mRNA level.
The protein expression of MMP-2, MMP-9, E-cadherin, N-cadherin, and vimentin in the pancreatic cancer cells exposed to resveratrol were evaluated by Western blotting. As shown in Fig. 3C, resveratrol treatment also influenced the expression levels of EMT-related factors at their protein levels, and this trend was consistent with the mRNA results.
Resveratrol Down-Regulated the Activation of the Akt/NF-κB Pathway
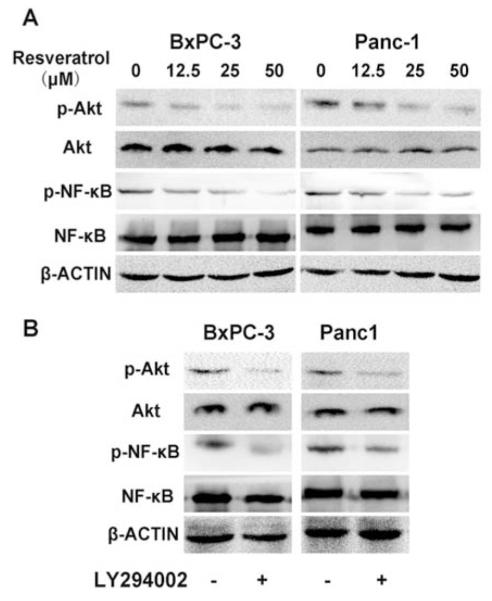
The serine/threonine kinase Akt, a downstream effector of PI-3K, is involved in cell survival and anti-apoptotic signaling. A recent study demonstrated that cell signaling is mediated by PI-3K/Akt via induction of the NF-κB transcription factor, which is associated with cell proliferation, cell migration and angiogenesis [27].
In this study, we observed that both Akt and NF-κB phosphorylation were strongly decreased with the addition of resveratrol in a dose-dependent manner (Fig. 4A). Moreover, a PI-3K inhibitor, LY294002, could also inhibit the expression of p-Akt and p-NF-κB, indicating that the NF-κB transcription factor is modulated by the PI-3K/Akt pathway (Fig. 4B).
Fig. (4). Roles of resveratrol and LY294002 in the phosphorylation of Akt and NF-κB.
A, Resveratrol inhibits Akt and NF-κB phosphorylation in a dose-dependent manner. BxPC-3 and Panc-1 cells were exposed to escalating doses of resveratrol (0-50 μM) for 24 h and whole cell lysates were prepared for Western blotting. B, Pancreatic cancer cells were treated with LY294002 (10 μM), a PI-3K inhibitor, for 24 h and used for Western blotting to assess the activation of Akt and NF-κB.
The Effect of the PI-3K/Akt Signaling Pathway on Pancreatic Cancer Invasion and Migration
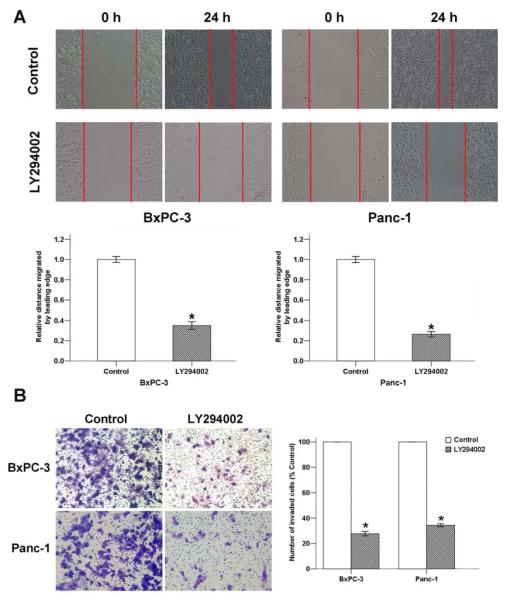
To assess whether the PI-3K/Akt signaling pathway plays an important role in pancreatic cancer metastasis, the effect of LY294002 on pancreatic cancer cell motility and invasion was determined by wound-healing and transwell invasion assays, respectively. The results showed that the cell migration (Fig. 5A) ability was significantly inhibited 24 h after the addition of LY294002. Additionally, the average cell number that invaded into the lower chamber decreased with LY294002 treatment (Fig. 5B). This finding suggested that the PI-3K/Akt signaling pathway is involved in pancreatic cancer migration and invasion.
Fig. (5). The effect of LY294002 on pancreatic cancer progression.
A, LY294002 (10 μM) exposure for 24 h reduced the migration of pancreatic cancer cells, as determined by a wound-healing assay. B, LY294002 (10 μM) exposure for 48 h inhibited the invasion of BxPC-3 and Panc-1 cancer cells. *P < 0.05 compared with the control group.
Resveratrol Suppressed TGF-β-Induced Migration and Invasion of Pancreatic Cancer Cells
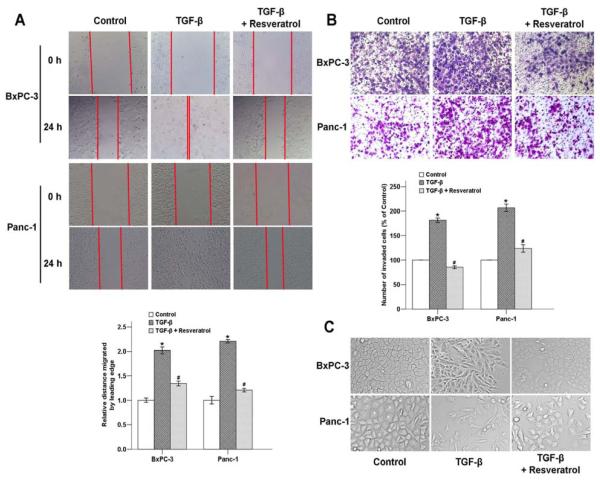
Previous studies have revealed that TGF-β plays an important role in driving EMT in the pathogenesis of pancreatic cancer [28,29]. We next examined whether resveratrol could inhibit TGF-β-induced EMT, which is associates with cellular invasive ability. As shown in Fig. 6C, after treated with 5 ng/ml TGF-β for 48 h, the cellular morphologies of both BxPC-3 and Panc-1 cells were changed from an epithelial phenotype to a classical mesenchymal phenotype. Cells treated with 5 ng/ml TGF-β and 50 μM resveratrol displayed classical epithelial morphology.
Fig. (6). The effects of resveratrol on the TGF-β-induced migration and invasion of pancreatic cancer cells.
A, BxPC-3 and Panc-1 cells were treated with 5 ng/ml TGF-β with or without 50 μM resveratrol for 24 h. Resveratrol significantly inhibited TGF-β-induced migration of cells into the denuded areas. (B) The effects of resveratrol in pancreatic cancer cell invasion. The number of migrated cells was quantified by counting the number of cells from 5 random fields at 100× magnification. (C) Cells were treated with 5 ng/ml TGF-β for 48 h, and the phenomenon of EMT was observed based on morphological changes of the cells. *P < 0.05 compared with the control group; #P < 0.05 compared with the TGF-β group.
In order to determine the potential role of resveratrol in pancreatic cancer metastasis by inhibiting TGF-β-induced EMT, wound-healing and transwell assays were performed to examine the effects of resveratrol on cell migration and invasion. The results showed that TGF-β not only led to significant wound closure (Fig. 6A) but also markedly enhanced the invasive ability of the cells (Fig. 6B), and resveratrol reversed these effects. Our findings provide strong evidence that resveratrol can inhibit both basal and exogenous TGF-β-induced migration and invasion in pancreatic cancer cells.
Resveratrol Regulated the TGF-β-Induced Expression of EMT Markers
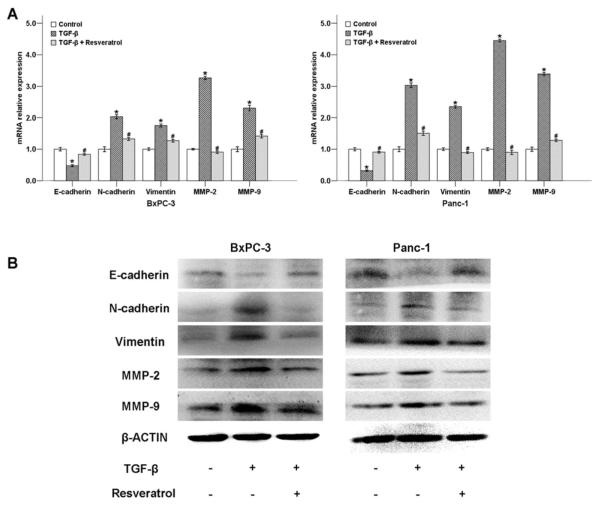
To further confirm the effect of resveratrol on TGF-β-induced EMT, we determined the expression levels of EMT-related genes after the cells were treated with 5 ng/ml TGF-β with or without 50 μM of resveratrol. As shown in Fig. 7A, TGF-β down-regulated the mRNA level of the epithelial marker E-cadherin, while the expression of mesenchymal markers N-cadherin, vimentin, MMP-2, and MMP-9 were strongly increased. Resveratrol significantly reversed all of these TGF-β-induced effects.
Fig. (7). The effects of resveratrol on TGF-β-modulated EMT-related genes of pancreatic cancer cells.
A, Treatment with resveratrol for 48 h diminished the effects of TGF-β on the expression of E-cadherin, N-cadherin, vimentin, MMP-2, and MMP-9 at the mRNA level in BxPC-3 and Panc-1 cells, as determined by QT-PCR. (B) Treatment with resveratrol for 48 h diminished the effects of TGF-β on the expression of EMT-related genes at the protein level in BxPC-3 and Panc-1 cells, as determined by Western blotting. *P < 0.05 compared with the control group; #P < 0.05 compared with the TGF-β group.
To evaluate the effects of resveratrol on the expression of E-cadherin, N-cadherin, vimentin, MMP2, and MMP9 at protein level, we determined these proteins in BxPC-3 and Panc-1 cells with or without resveratrol using Western blotting. As shown in Fig. 7B, resveratrol counterbalanced the TGF-β-induced, EMT-related factors at the protein level, and the trend was consistent with the mRNA results. Taken together, our results demonstrate that resveratrol suppresses TGF-β-induced EMT in both BxPC-3 and Panc-1 cells.
DISCUSSION
Cancer metastasis refers to the spread of cancer cells from the primary neoplasm to distant sites and the growth of secondary tumors at the new sites [30]. Pancreatic cancer is a highly metastatic tumor [4]. Blocking its invasion might lead to new, effective treatments for pancreatic cancer. Active compounds with anti-invasive and anti-metastatic properties, such as curcumin, pterostilbene, and resveratrol, have been defined as a new catalog of chemotherapeutic agents [31,30].
Resveratrol and its analogues have been proven to inhibit the invasion of lung, breast, and liver cancers [14,20]. Greater anti-invasive effects were demonstrated on highly invasive carcinoma cells and were independent of its anti-proliferative action [32,33]. Wu et al. [34] demonstrated that the anti-metastatic effect of resveratrol was associated with the restriction of invasion, mobility, adhesion, and MMP expression under both normoxic and hypoxic conditions in colon carcinoma. In this study, we found that the expression of MMP-9 and MMP-2 decreased in a dose-dependent manner after treatment with resveratrol for 48 h, indicating inhibition of pancreatic cancer cell invasion by resveratrol may be partly mediated by the down-regulation of MMP-9 and MMP-2 expression. Our study demonstrated that resveratrol could effectively inhibit basal invasive activity and modulate EMT-related factors of pancreatic cells.
Invasion and metastasis is a highly complicated process that requires the interaction of many different cell types, including connective tissue and blood vessel components within various organs. EMT is primarily known as an important biological program for multiple aspects of normal embryonic morphogenesis [22]. In this program, epithelial cells undergo a transition from an immotile, polarized epithelial phenotype to a highly motile, apolar, fibroblastoid-like mesenchymal phenotype with reduced epithelial markers (E-cadherin) and acquired mesenchymal markers (N-cadherin, vimentin). Currently, increasing evidence indicates that tumor cells achieve heightened invasiveness through this EMT process. The invasion of tumor cells into tumor-associated stroma and subsequent metastasis are central events in tumor progression. Metastasis of cancer cells involves changes in cell adhesion, rearrangement of the extracellular matrix (ECM), anoikis suppression and reorganization of the cytoskeleton [3].
TGF-β was the first inducer of EMT described in normal mammary epithelial cells. As a major inducer of the EMT phenotype in a variety of biological and pathophysiological conditions, it was shown to act by signaling through its receptor serine/threonine kinase complex [9]. The TGF-β growth factor plays a complex and important role in EMT induction via canonical and noncanonical TGF-β signaling systems [35]. A recent study showed that resveratrol could inhibit TGF-β-induced lung cancer invasion and metastasis [36]. In the current study, we found that resveratrol inhibited both basal and exogenous TGF-β-induced migration and invasion as well as EMT-related gene expression in pancreatic cancer cells.
The PI-3K/Akt signaling pathway has long been related with pancreatic cancer metastasis. Tanno et al. [37] showed that increased insulin-like growth factor I receptor expression induced by active Akt markedly enhances the invasiveness of human pancreatic cancer cells. Liau et al. [38] also demonstrated that HMGA1-induced cellular invasiveness and MMP-9 activity is PI-3K/Akt-dependent in pancreatic cancer. As a PI-3K inhibitor, wortmannin not only down-regulated hyaluronan-induced SW1990 cell motility but also inhibited hyaluronan-induced peritoneal metastasis in nude mice [39]. Recent studies proved that the metastasis-mediated effect of the PI-3K/Akt signaling pathway might be modulated via the NF-κB transcription factor, which is associated with cell proliferation, cell migration and angiogenesis [27]. Our results indicated that resveratrol could inhibit the activation of p-Akt and p-NF-κB. After suppressing the PI-3K/Akt signaling pathway by LY249002, the expression of p-Akt and p-NF-κB as well as the invasion and migration ability of both BxPC-3 and Panc-1 pancreatic cancer cells were decreased.
In conclusion, the current study demonstrates that resveratrol plays an important role in suppressing the proliferation, migration and invasion of pancreatic cancer cells in vitro by modulating EMT-related factors via the PI-3K/Akt/NF-κB signaling pathway. Resveratrol was also able to suppress the migration and invasion of pancreatic cancer cells by inhibiting TGF-β-mediated EMT. These results suggest that resveratrol might be a potential anticancer agent for the treatment of pancreatic cancer.
ACKNOWLEDGEMENTS
This study was supported by grant from China National Natural Science Foundation (Grant serial No. 81172360, 81201824), the Clinical Innovation Funds of the 1st Affiliated Hospital of XJTU (10ZD07), and pilot project grants from Program Project Grants from the NCRR (P20 RR020151) and the NIGMS (P20 GM103505 and P30 GM103332-01 ) from the NIH. The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of China National Natural Science Foundation, the US NIH, National Center for Research Resources (NCRR), or National Institute of Resources (NCRR), or National Institute of General Medical Sciences (NIGMS).
ABBREVIATIONS
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- HIF
Hypoxia inducible factor
- MMP
Matrix metalloproteinase
- MTT
Methyl thiazolyl tetrazolium
- PI-3K
Phosphatidylinositol 3-kinase
- TCM
Traditional Chinese medicine
- TGF-β
Transforming growth factor-β
- VEGF
Vascular endothelial growth factor
Footnotes
CONFLICT OF INTEREST The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Loos M, Kleeff J, Friess H, Buchler MW. Surgical treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 2008;1138:169–180. doi: 10.1196/annals.1414.024. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA. Cancer J. Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [3].Li W, Ma Q, Liu J, Han L, Ma G, Liu H, Shan T, Xie K, Wu E. Hyperglycemia as a mechanism of pancreatic cancer metastasis. Front. Biosci. 2012;17:1761–1774. doi: 10.2741/4017. [DOI] [PubMed] [Google Scholar]
- [4].Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: pathogenesis, prevention and treatment. Toxicol Appl. Pharmacol. 2007;224(3):326–336. doi: 10.1016/j.taap.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castellanos EH, Cardin DB, Berlin JD. Treatment of early-stage pancreatic cancer. Oncology (Williston Park) 2011;25(2):182–189. [PubMed] [Google Scholar]
- [6].Campen CJ, Dragovich T, Baker AF. Management strategies in pancreatic cancer. Am. J. Health Syst. Pharm. 2011;68(7):573–584. doi: 10.2146/ajhp100254. [DOI] [PubMed] [Google Scholar]
- [7].Cheng ZX, Sun B, Wang SJ, Gao Y, Zhang YM, Zhou HX, Jia G, Wang YW, Kong R, Pan SH. Nuclear Factor-κB–Dependent Epithelial to Mesenchymal Transition Induced by HIF-1α Activation in Pancreatic Cancer Cells under Hypoxic Conditions. PLoS One. 2011;6(8):e23752. doi: 10.1371/journal.pone.0023752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol. Cancer Res. 2010;8(5):629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- [9].Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69(18):7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998;138(6):619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- [11].Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60(5):323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- [12].Albani D, Polito L, Signorini A, Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. 2010;36(5):370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- [13].Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 2010;131(4):261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today. 2010;15(17-18):757–765. doi: 10.1016/j.drudis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- [15].Jha RK, Ma Q, Sha H, Palikhe M. Emerging role of resveratrol in the treatment of severe acute pancreatitis. Front. Biosci. (Schol Ed) 2010;2:168–175. doi: 10.2741/s54. [DOI] [PubMed] [Google Scholar]
- [16].Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid. Redox. Sign. 2009;11(11):2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- [17].Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid. Redox. Sign. 2010;13(10):1535–1548. doi: 10.1089/ars.2009.3064. [DOI] [PubMed] [Google Scholar]
- [18].Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann. N. Y. Acad. Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- [19].Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev. Res. (Phila) 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- [20].Tang FY, Su YC, Chen NC, Hsieh HS, Chen KS. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol. Nutr. Food Res. 2008;52(6):683–691. doi: 10.1002/mnfr.200700325. [DOI] [PubMed] [Google Scholar]
- [21].Brisdelli F, D’Andrea G, Bozzi A. Resveratrol: a natural polyphenol with multiple chemopreventive properties. Curr. Drug Metab. 2009;10(6):530–546. doi: 10.2174/138920009789375423. [DOI] [PubMed] [Google Scholar]
- [22].Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269(2):243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- [23].Ding XZ, Adrian TE. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas. 2002;25(4):e71–76. doi: 10.1097/00006676-200211000-00024. [DOI] [PubMed] [Google Scholar]
- [24].Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer. 2010;127(2):257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [26].Cates JMM, Byrd RH, Fohn LE, Tatsas AD, Washington MK, Black CC. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas. 2009;38(1):e1. doi: 10.1097/MPA.0b013e3181878b7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gilmore T. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- [28].Lou C, Zhang F, Yang M, Zhao J, Zeng W, Fang X, Zhang Y, Zhang C, Liang W. Naringenin decreases invasiveness and metastasis by inhibiting TGF-beta-induced epithelial to mesenchymal transition in pancreatic cancer cells. PLoS One. 2012;7(12):e50956. doi: 10.1371/journal.pone.0050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yin T, Wang C, Liu T, Zhao G, Zhou F. Implication of EMT induced by TGF-beta1 in pancreatic cancer. J Huazhong Univ. Sci. Technolog. Med. Sci. 2006;26(6):700–702. doi: 10.1007/s11596-006-0619-z. [DOI] [PubMed] [Google Scholar]
- [30].Renouf D, Moore M. Evolution of systemic therapy for advanced pancreatic cancer. Expert Rev. Anticancer Ther. 2010;10(4):529–540. doi: 10.1586/era.10.21. [DOI] [PubMed] [Google Scholar]
- [31].Middleton G, Ghaneh P, Costello E, Greenhalf W, Neoptolemos JP. New treatment options for advanced pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2008;2(5):673–696. doi: 10.1586/17474124.2.5.673. [DOI] [PubMed] [Google Scholar]
- [32].Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7(2):128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001;167(2):151–156. doi: 10.1016/s0304-3835(01)00476-1. [DOI] [PubMed] [Google Scholar]
- [34].Wu H, Liang X, Fang Y, Qin X, Zhang Y, Liu J. Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomed. Pharmacother. 2008;62(9):613–621. doi: 10.1016/j.biopha.2008.06.036. [DOI] [PubMed] [Google Scholar]
- [35].Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009;5(8):1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, Yang Y, Chen X, Liu G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2012;303(7):139–146. doi: 10.1016/j.tox.2012.09.017. [DOI] [PubMed] [Google Scholar]
- [37].Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61(2):589–593. [PubMed] [Google Scholar]
- [38].Liau SS, Jazag A, Whang EE. HMGA1 is a determinant of cellular invasiveness and in vivo metastatic potential in pancreatic adenocarcinoma. Cancer Res. 2006;66(24):11613–11622. doi: 10.1158/0008-5472.CAN-06-1460. [DOI] [PubMed] [Google Scholar]
- [39].Teranishi F, Takahashi N, Gao N, Akamo Y, Takeyama H, Manabe T, Okamoto T. Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci. 2009;100(4):770–777. doi: 10.1111/j.1349-7006.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]