Abstract
Purpose of Review
Emerging data demonstrates the potential of translational applications of antibodies directed against oxidation-specific epitopes (OSE). “Biotheranostics” in cardiovascular disease (CVD) describes targeting of OSE for biomarker, therapeutic and molecular imaging diagnostic applications.
Recent findings
Lipid oxidation collectively yields a large variety of oxidation-specific epitopes (OSE), such as oxidized phospholipids (OxPL) and malondialdehyde (MDA) epitopes. OSE are immunogenic, pro-inflammatory, pro-atherogenic and plaque destabilizing and represent danger associated molecular patterns (DAMPs). DAMPs are recognized by the innate immune system via pattern recognition receptors, including scavenger receptors IgM natural antibodies and complement factor H (CFH), that bind, neutralize and/or facilitate their clearance. Biomarker assays measuring OxPL present on apolipoprotein B-100 lipoproteins, and particularly on lipoprotein (a), predict the development of CVD events. In contrast, OxPL on plasminogen facilitate fibrinolysis and may reduce atherothrombosis. Oxidation-specific antibodies (OSA) attached to magnetic nanoparticles image lipid-rich, oxidation-rich plaques. Infusion or overexpression of OSA reduces the progression of atherosclerosis, suggesting that they may be used in similar applications in humans.
Summary
Using the accelerating knowledge base and improved understanding of the interplay of oxidation, inflammation and innate and adaptive immunity in atherogenesis, emerging clinical applications of OSA may identify, monitor and treat CVD in humans.
Keywords: biotheranostic, oxidation, innate immunity, atherogenesis, molecular imaging
INTRODUCTION
In their seminal 1989 review paper entitled “Beyond cholesterol: Modifications of low density lipoprotein that increase its atherogenicity,” [1] Steinberg, Witztum and colleagues provided a scientific rationale for the “oxidation hypothesis of atherosclerosis.” This hypothesis was strongly supported by in vitro data and animal experiments in which antioxidants reduced atherosclerosis. However, the results of human clinical trials with antioxidant vitamins were mainly negative, except in selected groups of patients with clearly increased systemic oxidative stress, such as patients on hemodialysis or diabetics with haptoglobin 2-2 genotypes associated with higher hemoglobin-mediated oxidative stress. Subsequently, Witztum and colleagues developed a deeper understanding of the biological effects of oxidized low-density lipoprotein (OxLDL), and particularly the role of the innate and adaptive immune system in the response to the generation of “oxidation-specific epitopes (OSE)” (Figure 1) [2] [3]. These observations led to the appreciation of the role of OSE in inflammatory and immune reactions that defined key pathways in the development and progression of atherosclerotic lesions [2, 4, 5]. Cloning and characterization of new monoclonal antibodies against OSE greatly facilitated mechanistic and translational research of atherosclerosis. These concepts defining the role of OSE in vascular inflammation and atherogenesis have matured to allow potential clinical translation in several areas, including biomarkers, diagnostic molecular imaging and therapy of cardiovascular disease. In this review, we unify these three concepts under the term “biotheranostics”, where the target is OSE in plasma or in the vessel wall and the targeting agents are oxidation-specific antibodies. A rationale is provided why targeting OSE may not only help to understand the transition of occult atherosclerosis to clinically relevant cardiovascular disease (CVD) but also in targeting OSE to develop clinical tools to define, monitor and treat CVD in humans.
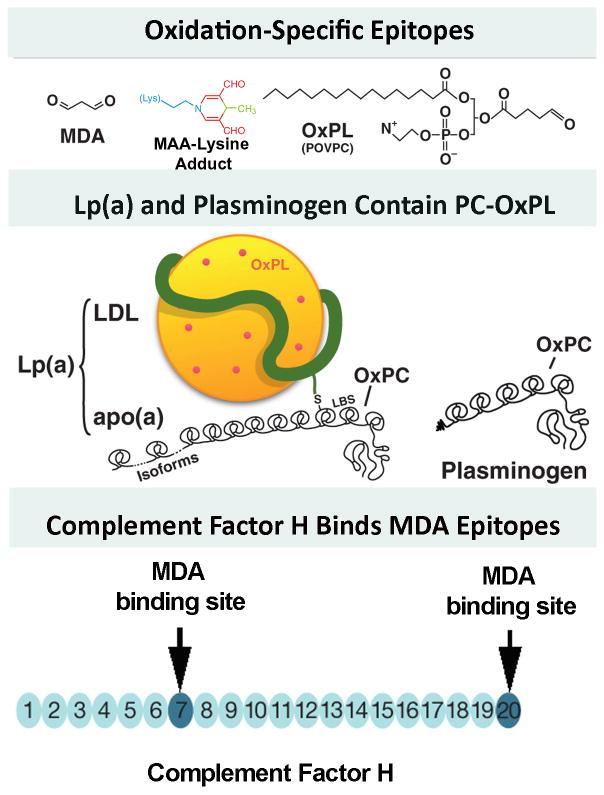
Figure 1. Well defined oxidation-specific epitopes (OSE).
Panel A- Oxidative modifications of lipoproteins and cell membranes creates a variety of OSE, of which the best characterized are MDA epitopes, advanced MDA epitopes such as malondialdehyde-acetaldehyde adducts (MAA) and the OxPL POVPC (1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine). Panel B- OxPL are present on Lp(a) are covalently bound to apo(a) and also dissolved in the lipid phase of Lp(a) [46]. OxPL are also covalently bound to plasminogen. Panel C- Complement factor H can bind MDA-protein adducts at sites of inflammation, such in the macula of the eye and in atherosclerotic lesions. Modified and reproduced with permission from references [2] (A and B) and [3] (C).
LDL OXIDATION
The LDL particle is exquisitely sensitive to oxidative damage due to its complex lipid-protein composition and a large number of polyunsaturated acyl chains. The mechanisms of LDL oxidation in vivo include reactions catalyzed by 12/15-lipoxygenase (12/15-LO), myeloperoxidase (MPO), nitric oxide synthases and NADPH oxidases, as well as those mediated by heme and hemoglobin (Hb) [6]. Small amounts of Hb are constantly leaking from damaged erythrocytes, particularly in the vascular regions with turbulent flow, such as arterial bifurcations and aortic curvatures, and in vasa vasorum of atherosclerotic lesions. The LDL oxidation by Hb is normally prevented by haptoglobin (Hp) binding to Hb to, but the Hp2 isoform is less effective than the Hp1 isoform [7]. Recent findings confirm that the Hp2-2 genotype is associated with an increased risk of coronary artery disease (CAD), and evidence of increased iron content, expression of oxidized phospholipids (OxPL) and malondialdehyde (MDA) OSE, apoptotic cells, and cytoplasmic blebs were found in human aortic atherosclerotic lesions [8]. Novel data was also recently published by van Dijk et al [9], showing that in human vulnerable plaques OSE become increasingly more prominent as lesions progress and rupture. OSE were particularly prominent in advanced coronary and carotid lesions in macrophage-rich areas, lipid pools, the necrotic core and in ruptured plaques. The presence of OSEs in clinically relevant human lesions provides a strong rationale to target such epitopes in plasma and in atherosclerotic plaques for clinical applications.
IMMUNE RECOGNITION OF OXIDATION-SPECIFIC EPITOPES
By analogy with microbial “pathogen associated molecular patterns” (PAMPs), OSE – the products of oxidation in lipoproteins and various cellular components – represent a class of “danger (or damage) associated molecular patterns” (DAMPs) (Figure 2) [4, 10]. The common feature of PAMPs and DAMPs is their recognition by the same “pattern-recognition receptors” (PRRs) of innate immunity. Cellular PRRs, such as scavenger receptors and toll-like receptors, are found on the cell surface and in intracellular domains of macrophages and in other cell types. In addition, there are important soluble PRRs including variants of some cellular PRRs, pentraxins, such as C-reactive protein, complement factor H [3] and natural antibodies (NAbs). NAbs can be considered immunoglobulin PRRs, having in common with cellular and soluble PRRs a limited repertoire and yet a wide range of pattern recognition. Remarkably, in normal mice and in newborn humans, as much as 15–30% of all IgM NAbs bind to OSE [11]. Among these, there is a high prevalence of IgM to MDA and related MDA- protein adducts. This suggests that removing pro-inflammatory OSE is important for host homeostasis and implies an evolutionary advantage in organisms that have high levels of OSE-specific NAbs [4].
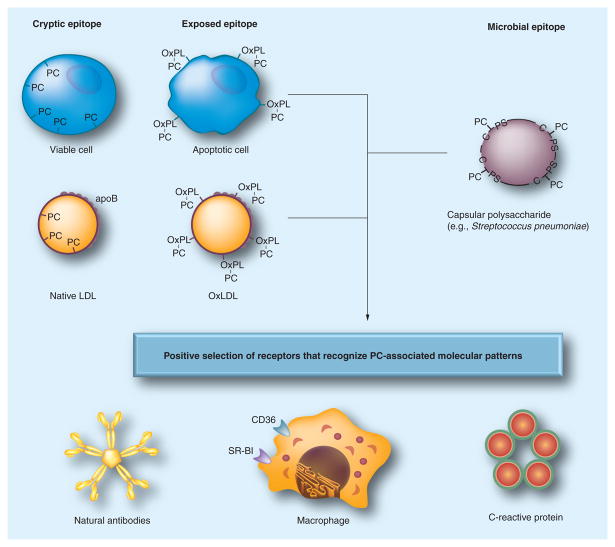
Figure 2. Pattern recognition of oxidation-specific DAMPs and microbial PAMPs.
Using the example of the PC epitope, this diagram illustrates the hypothesis of the emergence and positive selection of multiple PRRs that recognize common epitopes, shared by modified self and microbial pathogens. Oxidation of plasma membrane phospholipids in apoptotic cells alters the conformation of the PC head group, yielding an exposed epitope, accessible to recognition by macrophage scavenger receptors, NAbs and CRP. These PRRs were selected to clear apoptotic cells from developing or regenerating tissues. Recognition by the same receptors of the PC epitope of capsular polysaccharide in Gram-positive bacteria (e.g., S. pneumoniae), which is not part of a phospholipid, strengthened positive selection of these PRRs and probably helped to select additional strong proinflammatory components to PRR-dependent responses. Oxidized lipoproteins, prevalent in humans as a result of dyslipidemia and impact of environmental factors and in experimental animals, carry OxPLs with the PC epitope exposed in an analogous manner to that of apoptotic cells, which leads to recognition by PRRs and initiation of innate immune responses. The balance between proinflammatory responses of cellular PRRs and atheroprotective roles of NAbs plays an important role in the development of atherosclerosis. Modified and reproduced with permission from reference [4].
BIOTHERANOSTIC APPLICATIONS TARGETING OXIDATION-SPECIFIC EPITOPES
The concept of “biotheranostics” as related to cardiovascular disease is derived from the preposition that one can target biological processes in the plasma or vessel wall and develop biomarker assays, therapeutic agents and diagnostic molecular imaging probes to the target. In this case the target is OSE present in circulating lipoproteins or in the atherosclerotic plaque and the targeting agents are human and murine antibodies or peptide fragments validated to detect such OSE [2].
Biomarkers
The ideal biomarker would be involved in causal pathways of atherogenesis and allow changes in clinical management when levels are measured.
A. Oxidized Phospholipid Biomarkers
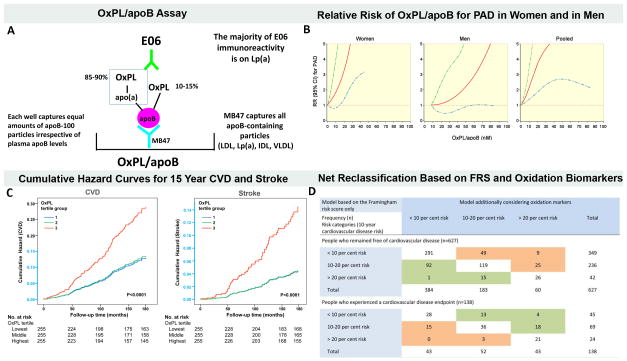
A large body of work shows that measuring oxidized phospholipids on apolipoprotein B-100 particles (OxPL/apoB) fulfills many of the criteria for a clinically useful biomarker (Figure 3A, reviewed in ref [12]). Levels of OxPL/apoB, as measured with the monoclonal antibody E06, reflect vascular dysfunction and coronary calcification [13, 14], predict the presence and progression of ultrasound-measured carotid and femoral disease and angiographically-determined coronary artery disease and are elevated in acute coronary syndromes and following percutaneous coronary interventions [12]. Recently, in two parallel nested case-control studies within the Health Professionals Follow-up Study and the Nurses’ Health Study, OxPL/apoB were positively associated with risk of peripheral arterial disease (PAD) in men and women with a relative risk (RR) 1.37, 95% CI, 1.19–1.58 for each 1-standard deviation increase after adjusting for traditional risk factors (Figure 3B) [15]. Furthermore, OxPL/apoB levels, measured at baseline in a community dwelling cohort followed prospectively for 15 years, predict death, MI and stroke with hazard ratios 2.1–3.6 (Figure 3C) [16, 17]. Importantly for clinical applications, measurement of OxPL/apoB along with IgG and IgM autoantibodies to MDA-LDL, allowed reclassification of approximately 30% of patients initially in intermediate Framingham risk categories into either lower or higher risk categories (Figure 3D) [16]. Approximately one half of these patients were placed in a higher risk category and one half in a lower risk category. Additional assays to measure OxPL and assays to measure modified apoB have been described in the literature. However, a thorough comparison of the clinical predictability of cardiovascular risk by these assays has not been performed to date [18]. Due to lack of space, the reader is referred to several papers in this area for further details [19, 20].
Figure 3. Oxidized phospholipid (OxPL) biomarkers.
Panel A describes the OxPL/apoB assay that reflects the content of OxPL on apoB particles captured on microtiter well plates. The assay is set up so that all plates capture a similar amount of apoB and therefore the assay is normalized to and independent of plasma apoB and LDL-C levels. The assay is highly sensitive to the number of moles of OxPL on apoB and primarily reflects the OxPL content on the most atherogenic Lp(a) particles (high Lp(a) levels mediated by small apo(a) isoforms). Panel B shows the relationship between plasma levels of OxPL/apoB and relative risk (RR) of peripheral arterial disease (PAD) after full adjustment of other risk factors. The 95% confidence interval (CI) is indicated by the dashed lines. Panel C shows the cumulative hazard curves for CVD incidence and stroke Incidence by OxPL/apoB tertile groups followed in the Bruneck population representing a cross-section of the general community. Y-axis shown in light blue indicates range from 0 to 0.15. Panel D shows risk reclassification based on oxidation markers (OxPL/ApoB, Cu-OxLDL IgG, and MDA IgM) in patients who experienced a CVD endpoint (n = 138) and in those who remained free of CVD during follow-up (n = 627; 1995 to 2010). This reclassification table compares a model based on the Framingham Risk Score (FRS) only with a model considering the FRS and levels of oxidation markers (OxPL/apoB, Cu-OxLDL IgG, and MDA IgM). The shaded values reflect subjects who were reclassified. Reprinted with permission from references [15](panel B) and [16] (panels C and D).
Interestingly, lipoprotein (a) [Lp(a)], which is composed of apoB and apo(a), strongly binds OxPL [21, 22]. Lp(a) is now generally recognized as a causal, independent, genetic risk factor for CVD and myocardial infarction (MI) and has become a target of therapy for reducing cardiovascular disease [23–25]. Measurement of OxPL/apoB primarily reflects the content of OxPL on Lp(a)-associated apoB particles and may account in part for the pro-atherogenic and pro-inflammatory properties of Lp(a). In fact, OxPL, OxLDL and Lp(a) trigger apoptosis in endoplasmic reticulum-stressed macrophages through a mechanism requiring both CD36 and TLR2 [26]. Macrophage apoptosis is a key process in plaque necrosis in advanced atheromata and likely contributes to plaque progression, destabilization and clinical events. Because small apo(a) isoforms are associated with high Lp(a) plasma levels, and because these lipoproteins contain the most OxPL content, the OxPL/apoB measurement is primarily a reflection of the most atherogenic Lp(a) particles [27]. Since small apo(a) isoforms are generally accepted as highly atherogenic than large isoforms but are not easily measured and not performed clinically, measuring OxPL/apoB may reflect a key biological activity of Lp(a) in predicting CVD risk. In all studies reported to date, OxPL/apoB is either a superior or equal to Lp(a) in predicting CVD risk (reviewed in ref [12]).
B. OxPL and Plasminogen
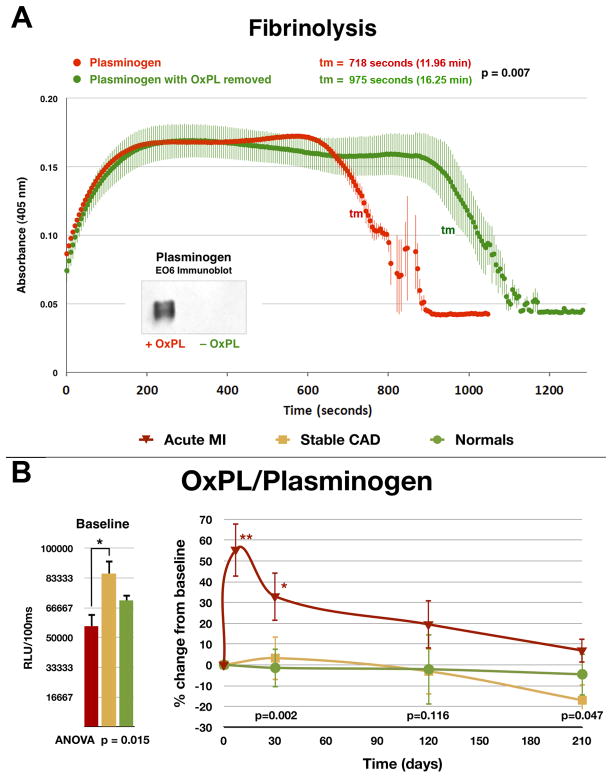
Apo(a) is highly homologous to plasminogen, duplicated itself from the plasminogen gene, sits in the opposite direction to plasminogen on chromosome 6, and has changed and remodeled by losing its protease activity, losing kringles I–III while retaining kringles IV–V, developing multiple isoforms of kringle IV-2 and binding to apoB to become the lipoprotein Lp(a). Recent studies have shown that plasminogen also binds OxPL and represents a second major plasma pool of OxPL in addition to those present on Lp(a) [28, 29]. Importantly, as opposed to OxPL on Lp(a), which predict increased cardiovascular risk, OxPL on plasminogen are associated with enhanced potential for fibrinolysis and thus may be associated with reduced atherothrombotic risk (Figure 4A) [29]. Enzymatic removal of OxPL from plasminogen resulted in a longer lysis time for fibrin clots as measured in in vitro assays. OxPL/plasminogen levels increased following acute myocardial infarction, implying that OxPL carried by plasminogen have a role in atherothrombosis (Figure 4B). Measurement of OxPL on plasminogen may provide insights into both risk of thrombosis in patients at risk of thrombotic disorders, such as MI, stroke, atrial fibrillation, pulmonary emboli and deep venous thrombosis. It may also reflect bleeding risk for patients treated with anti-coagulants and anti-platelet agents. Studies are underway to assess the potential clinical value of OxPL/plasminogen as a biomarker of thrombosis and bleeding risk.
Figure 4. In vitro clot lysis assay assessing the ability of plasminogen to degrade fibrin clots.
Panel A- Native plasminogen containing OxPL and plasminogen with OxPL enzymatically removed (inset) with phospholipase A2 were used. Thrombin-induced clot formation occurs within the first 2 min and is marked by an initial rapid increase in turbidity, as measured by absorbance at 405 nm. Subsequent clot lysis is indicated by a rapid return of the turbidity signal to baseline levels. The parameter tm (transition midpoint) is taken as the standard measure of lysis time and is defined as the time point on the lysis curve that is halfway between the minimum and maximum excursions. The curves represent the mean±SEM of 3 separate experiments with measurement of absorbance at 405 nm every 5 seconds. Panel B- Baseline and change in plasma levels of plasminogen and OxPL/plasminogen in normal healthy subjects, patients with stable CAD and acute myocardial infarction (AMI) over 7 months. The p values at the bottom of the figures represent the hospital discharge (average of 4 days for the AMI group) and 30-, 120-, and 210-day differences between groups at each time point. *p < 0.05 and **p < 0.01 represent Bonferroni post-test for changes within groups over time. Reproduced with permission from reference [29].
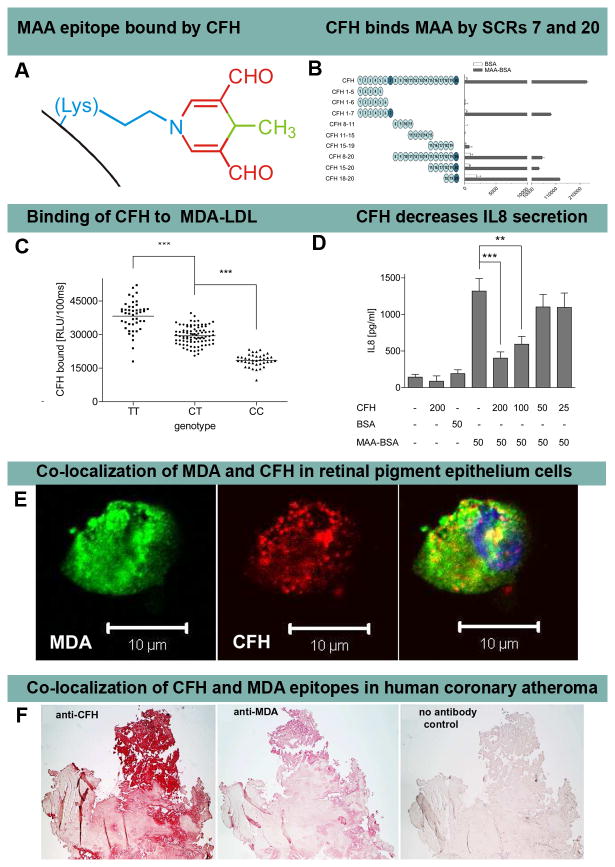
C. Malondialdehyde-acetaldehyde (MAA) and Complement Factor H (CFH)
CFH is a very abundant plasma protein that regulates complement activation by mediating anti-inflammatory properties and protecting cells from excessive complement activation. The single nucleotide polymorphism (SNP) Y402H (rs1061170) in the short consensus repeat 7 (SCR) of CFH, present in ~35% of patients, has been previously associated with age related macular degeneration (AMD), but the underlying pathophysiology until now was unknown. The molecular defect has been defined by Weismann et al [3] by demonstrating that SCR7 as well as SCR20 of CFH are MDA and MAA adduct-binding sites (Figure 5A and 5B). Compared to individuals with wild-type CFH, plasma from patients with AMD and the rs1061170 SNP have significantly diminished ability to bind to MDA-LDL, with f heterozygous subjects having a 23% reduction in binding and homozygous subjects 52%, irrespective of the total plasma CFH levels (Figure 5C). Furthermore, CFH was shown to block the uptake of MDA-modified proteins by macrophages and mitigated MDA-induced pro-inflammatory effects, such as IL-8 secretion, in vitro and in vivo in mice (Figure 5D). In retinal pigment epithelial (RPE) cells, CFH was shown to co-localize with MDA (Figure 5E). Overall, these data suggest that the CFH polymorphism H402, which is strongly associated with AMD, markedly reduces the ability of CFH to bind pro-inflammatory MDA/MAA-protein adducts, indicating a causal link to AMD.
Figure 5. Complement factor H binding to MDA and MAA epitopes.
Panel A shows an MAA-lysine adduct formed by the condensation of two MDA (red) and one acetaldehyde (derived from breakdown of MDA, green) molecules reacting with the ε-amino group of lysine (blue). Panel B shows an ELISA for binding of CFH and recombinant CFH fragments to coated bovine serum albumin (BSA) (white bars) or MAA-BSA (black bars). The length of CFH fragments is indicated by schematic representations with each circle depicting one short consensus repeats (SCR). SCRs 7 and 20 bind MAA epitopes. Panel C shows an ELISA for binding of plasma CFH to coated MDA-LDL in plasma of subjects homozygous for the H402 risk allele (CC), heterozygous for the H402 risk allele (CT) or homozygous for the Y402 allele (TT). Symbols represent individual subject samples with horizontal bars indicating the mean of each group. Values are mean ± SD relative light units (RLU) per 100 ms of triplicate determinations (***P<0.001). Panel D shows secretion of IL-8 by THP-1 cells stimulated for 12 hours with BSA or MAA-BSA in the absence or presence of CFH. Numbers below indicate concentrations of CFH, BSA and MAA-BSA in μg/ml. Error bars represent mean± SEM of three independent experiments. Panel E shows confocal immunofluorescent photograph of necrotic retinal pigment epithelial (RPE) cells stained with the MDA-specific IgM natural antibody EO14 (green) and CFH (red), and the merged picture indicating co-localization of CFH and MDA epitopes (yellow). Panel F shows that CFH and MDA are present in human coronary atherosclerotic lesion obtained from a patient with cardiogenic shock undergoing thrombectomy and percutaneous coronary intervention. Parallel sections stained for the presence of CFH with a guinea pig antiserum to CFH (A) and for MDA epitopes with monoclonal antibody MDA2 (B) and with secondary antibody only as control (C). Positive staining is indicated by the red color. Reproduced with permission for Weismann et al [3].
MDA and MAA adducts are also present in atherosclerotic lesions from patients with cardiovascular disease, and are particularly prevalent in vulnerable plaques and necrotic cores [9]. It was also demonstrated that CFH and MDA co-localize in human atheroma from patients with acute coronary syndromes (Figure 5F), suggesting that it may also play a role in mitigating the effects of MDA/MAA in plaque destabilization. Clinical studies are now being carried out to address this hypothesis.
Molecular Imaging
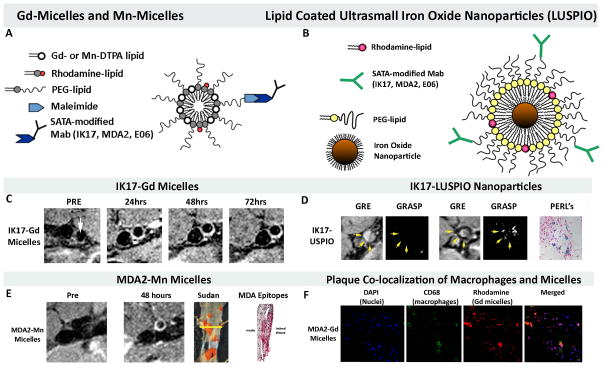
The presence of OSE in the vessel wall, and in particular in high-risk human lesions, provides the rationale to develop imaging approaches to detect such OSE [9]. Molecular imaging of OSE may provide a means to detect and monitor such lesions and assess the efficacy of established or experimental therapies. Three unique MRI based approaches have been developed to non-invasively image OSE, using gadolinium, manganese or specifically-targeted, lipid-coated, iron oxide nanoparticles (as opposed to dextran coated nanoparticles which are taken up non-specifically by macrophages) with attached murine (MDA2, E06) or human (IK17) oxidation-specific antibodies (Figure 6) [30–32]. MDA2 is a murine IgG monoclonal antibody that binds to MDA-lysine epitopes present on modified LDL or other MDA-modified proteins. IK17 is a fully human Fab or single-chain Fv (scFv) fragment that binds advanced MDA and MAA epitopes. E06 is a natural murine IgM monoclonal antibody cloned from apolipoprotein E–deficient (apoE−/−) mice that binds to the phosphocholine head group of oxidized, but not native phospholipids. These antibody targeted nanoparticles bind OSE in plasma or in the vessel wall, are then internalized by macrophages and provide excellent images of experimental atherosclerotic lesions in vivo (Figure 6). For the Mn-micelles, when this nanoparticle binds to extracellular OxLDL and is taken up by macrophages, free Mn is released intracellularly resulting in ~10X increase in relaxivity and visualization of macrophages, thus becoming an indirect macrophage-targeting agent. Because these nanoparticles accumulate in macrophages, they may not only provide a means to quantify plaque burden, but also to potentially predict plaque instability.
Figure 6. Magnetic resonance molecular imaging approaches targeting OSE.
Panel A shows a schematic of Gd-micelle and Mn-micelle composition using S-acetythioglycolic acid N-hydroxysuccinimide ester (SATA) to attach the antibodies to the micelles. The micelles are ~10–15 nm in diameter and contain approximately 50 Gd or Mn ions on each micelle. Panel B shows a schematic diagram of the lipid-coated, ultrasmall, iron oxide nanoparticles (LUSPIO). Panel C demonstrates non-invasive MR imaging of the atherosclerotic abdominal aorta of cholesterol-fed LDLR−/− mice using IK17-Gd-micelles. Note lack of signal at the pre-injection scan and strong signal (white contrast) in the 24–72 hour scans. Panel D shows imaging with IK17-LUSPIO. Iron oxide causes signal loss and it can be seen that the abdominal aorta plaque becomes darker following injection. The gradient echo acquisition for superparamagnetic particles with positive contrast (GRASP) sequence differentiates between iron oxide deposition (now shown as white signal) and artifacts that are often present when imaging the arterial wall. Panel E shows imaging with Mn-MDA2-micelles with the accompanying panels showing the Sudan (lipid) stained aorta where the imaging occurred and the presence of MDA OSE using immunostaining techniques. Panel F demonstrates in vivo plaque co-localization of the MDA-micelles and macrophages. PEG=polyethylene glycol. Reprinted with permission from references: panel A, C, F [42], Panel B, D [33] and panel E [33].
In an extension of these studies, transgenic zebrafish were generated to express a temperature-inducible, enhanced green fluorescent protein (EGFP)-labeled, IK17-scFc-EGFP construct. Feeding a high-cholesterol diet supplemented with a red fluorescent lipid marker to transgenic zebrafish larvae resulted in vascular lipid accumulation. After heat shock–induced expression of IK17-scFv-EGFP, time-dependent vascular accumulation of IK17-specific MDA epitopes could be observed [33].
Overall, these studies suggest that very early lesions, as in the zebrafish, as well as moderately advanced lesions containing macrophages, as in LDLR−/− and apoE−/− mice, can be visualized non-invasively. Ultimately, molecular imaging approaches targeting OSE may be useful in the diagnosis of high risk patients, surveillance of plaque progression and plaque instability, testing of novel therapeutic agents, assessing effect of established therapies and perhaps providing guidance on more aggressive therapy.
C. Therapeutic Approaches
The potential therapeutic use of oxidation-specific antibodies for CVD is being evaluated in pre-clinical and early phase studies. For example, immunization of LDLR−/− mice with pneumococcal vaccine (containing phosphocholine epitopes) caused an increase in circulating E06 levels that is associated with a reduction in atherosclerosis progression [34], potentially by inactivating or clearing relevant OSE. Deletion of IgM antibodies in mice is associated with higher risk of atherosclerosis progression [35, 36]. Several studies in experimental models have suggested that direct infusion of oxidation-specific antibodies results in lower rate of progression of atherosclerosis or enhanced regression of established lesions [37, 38]. Finally, high levels of IgM antibodies are associated with atheroprotection in epidemiological studies [16, 39, 40] although this is not always independent of traditional risk factors.
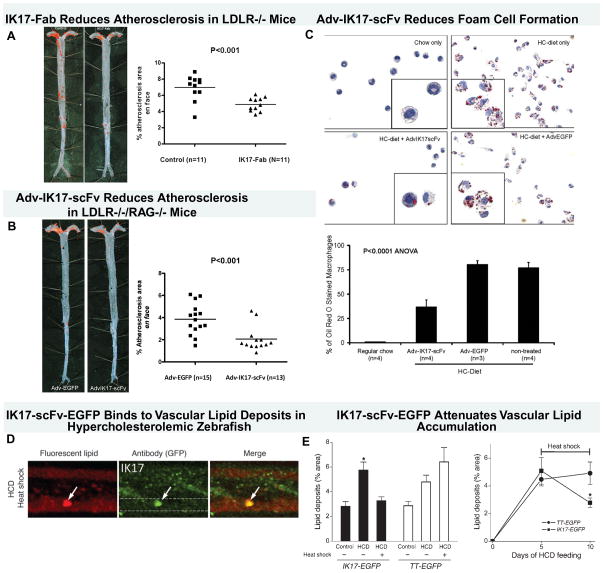
In recent work, the human antibody IK17 was either infused intraperitoneally as a Fab or overexpressed with an adenoviral vector as single-chain Fv fragment, resulting in significantly reduced atherosclerosis progression (Figure 7A–B) [41, 42]. Importantly, mechanistic information from these studies demonstrated reduced binding of OxLDL/MDA-LDL to macrophages and significant reduction in foam cell formation (Figure 7C). Furthermore, sustained overexpression of IK17 in a zebrafish model of hypercholesterolemia, induced regression of oxidized lipid deposits in the vascular wall (Figure 7D–E)[33].
Figure 7. Therapeutic properties of human oxidation-specific antibody IK17.
Panels A shows representative aortic images from a phosphate-buffered saline control or IK17-Fab–injected LDLR−/− mouse stained with Sudan IV to accentuate lipid. Next to the Sudan stained aorta is displayed quantitative analysis of en face atherosclerotic area in the entire aorta. Data are expressed as the percentage of Sudan IV stained area of entire aorta examined. Panel B shows representative Sudan IV stained en face preparations and atherosclerosis quantitation of aortas from mice injected with Adv-IK17-scFv or Adv-EGFP as a control. Adenovirus IK17-scFv–mediated hepatic expression was achieved in LDLR/Rag1 double-knockout mice, leading to a 46% reduction in en face atherosclerosis compared with control mice treated with a Adv-EGFP. Panel C shows that peritoneal macrophages isolated from Adv-IK17-scFv treated mice had decreased lipid accumulation compared with adv-GFP. Panel D shows images of vascular accumulation of hsp70:IK17-EGFP in zebrafish larvae were fed a HCD for 3 days, followed by heat shock and 2 more days of feeding with a high cholesterol diet (HCD) supplemented with cholesteryl BODIPY 576/589. Colocalization of green EGFP and red lipid marker signals was observed. Dashed lines trace the caudal vein in GFP-only images. Panel E (left) shows that expression of IK17-EGFP attenuates vascular lipid accumulation. Hsp70:IK17-EGFP and hsp70:(tetanus toxoid) TT-EGFP control zebrafish larvae were fed a HCD or control diet for 10 days. One group of HCD-fed zebrafish was subjected to heat shock 2 days before the start of feeding and then every 4–5 days to sustain IK17-EGFP or TT-EGFP expression levels. The other group was not subjected to heat shock at any time and, thus, did not express the transgene. Two days before imaging, the diet was switched to a diet supplemented with 10 μg/g cholesteryl BODIPY 576/589 C11, and then fluorescent lipid deposits were quantified. *P < 0.001 for IK17/HCD versus either IK17/control or IK17/HCD/heat shock. On the right panel, zebrafish larvae were fed a HCD for 5 days, and lipid deposits were imaged and quantified. The animals were subjected to heat shock after the imaging session on the fifth day and then again on the eighth day. The animals were imaged again on the tenth day, and lipid deposits were quantified. The results are expressed as the percentage area of lipid deposits per caudal vein segment. *P < 0.05. Reprinted with permission from references: panel A–C [42], Panel D–E [33].
This suggests that if these antibodies were to be used clinically, they would have the potential to acutely decrease OxLDL uptake and cholesterol accumulation in macrophages and potentially result in rapid plaque stabilization by preventing pro-inflammatory effects of foam cells. This is consistent with data from animal models showing that during dietary-induced regression, removal of OSE, such as OxPL and MDA epitopes, from the vessel wall is one of the first events that occurs, even before physical regression of the atheroma as a whole [43, 44]. Along with this loss of OSE, there is the concomitant presence of features of plaque stabilization, such as gain of smooth muscle cells and collagen and loss of macrophages, and reduced oxidative stress [45]. A clinical Phase II trial with an humanized IgG antibody that presumably bound modified apoB was reported to show no difference in FDG-PET uptake of the carotid arteries, but the details have not been published to date. Studies of such antibodies with clinical endpoints have not been performed to date.
V. CONCLUSIONS
Novel paradigms are now emerging to translate the advances in fundamental knowledge of the interaction of oxidative pathways, the immune system and the resulting inflammatory responses to OSE into clinical practice. Encompassed by the concept of biotheranostics, OSE biomarkers show strong associations with both progression of CAD/PAD and in predicting future events, suggesting that they may be used to complement the existing clinical armamentarium. Emerging diagnostic molecular imaging approaches targeting OSE, if translated to humans, may provide unique information about plaque burden as well as plaque activity, and therapeutic use of oxidation-specific antibodies may allow more refined approaches targeting bioactive, pro-inflammatory OSE to treat active CVD or diminish future risk of events.
KEY POINTS.
Oxidation of lipoproteins generates a variety of oxidation-specific epitopes that are immunogenic, pro-inflammatory, pro-atherogenic and plaque destabilizing.
The innate immune system recognizes oxidation-specific epitopes as danger associated molecular patterns (DAMPs) and uses innate pattern recognition receptors, including soluble receptors, such as IgM natural antibodies and complement factor H, to bind, neutralize and/or clear such DAMPs.
Oxidation-specific epitopes can be targeted in plasma or in the vessel wall for “biotheranostics”-, i.e. biomarker, therapeutic and molecular imaging diagnostic applications.
Development of assays to measure oxidized phospholipids on lipoproteins and plasminogen provide insights into atherothrombosis and can be used clinically to predict new cardiovascular events
Oxidation-specific antibodies attached to nanoparticles can be used for magnetic imaging of lipid-rich, oxidation-rich plaques and be directly infused to reduce the progression of atherosclerosis.
Acknowledgments
This study was supported by NIH grants HL055798, HL093767 and HL088093.
Footnotes
Disclosures
Dr. Tsimikas is a co-inventor and receives royalties from patents owned by the University of California for the commercial use of oxidation-specific antibodies, is a consultant to Quest, Sanofi, Genzyme, Regeneron and ISIS and has received investigator-initiated grants from Pfizer and Merck. Dr. Miller is a co-inventor of a patent owned by the University of California for the use of the hypercholesterolemic zebrafish model and has received an investigator-initiated grant from Merck.
References
- 1.Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.02.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weismann D, Hartvigsen K, Lauer N, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. The most up to date summary and discussion of the role of the adaptive immunity in atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem. 2010;51:229–251. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 7.Kalet-Litman S, Moreno PR, Levy AP. The haptoglobin 2–2 genotype is associated with increased redox active hemoglobin derived iron in the atherosclerotic plaque. Atherosclerosis. 2010;209:28–31. doi: 10.1016/j.atherosclerosis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Purushothaman KR, Purushothaman M, Levy AP, et al. Increased expression of oxidation-specific epitopes and apoptosis are associated with haptoglobin genotype: possible implications for plaque progression in human atherosclerosis. J Am Coll Cardiol. 2012;60:112–119. doi: 10.1016/j.jacc.2012.04.011. This study links genetic predisposition to oxidative stress through haptoglbin genetypes to clinical expression of OSE in human aortic atherosclerotic lesions. [DOI] [PubMed] [Google Scholar]
- 9*.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. This study comprehensively defines the expression of OSE in advancing and vulnerable himan coronary and carotid lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzinger P. The Danger Model: A Renewed Sense of Self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 11.Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Res. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi N, Tsimikas S, Hajsadeghi F, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105:459–466. doi: 10.1016/j.amjcard.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Bertoia ML, Pai JK, Lee J-H, et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. This study provides strong data for the potential clinical utility of OxPL/apoB in risk prediction and reclassification of risk in predicting future CVD. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51:1653–1662. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Holvoet P, Lee DH, Steffes M, et al. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Itabe H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: from atherosclerosis to periodontitis. J Clin Biochem Nutr. 2012;51:1–8. doi: 10.3164/jcbn.11-00020R1. A comprenesive review on OxLDL as a biomarker of CVD risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelstein C, Pfaffinger D, Hinman J, et al. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J Biol Chem. 2003;278:52841–52847. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 22.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merki E, Graham M, Taleb A, et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J Am Coll Cardiol. 2011;57:1611–1621. doi: 10.1016/j.jacc.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Kolski B, Tsimikas S. Emerging therapeutic agents to lower lipoprotein (a) levels. Curr Opin Lipidol. 2012;23:560–568. doi: 10.1097/MOL.0b013e3283598d81. [DOI] [PubMed] [Google Scholar]
- 26.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsimikas S, Clopton P, Brilakis ES, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelstein C, Pfaffinger D, Yang M, et al. Naturally occurring human plasminogen, like genetically related apolipoprotein(a), contains oxidized phosphatidylcholine adducts. Biochim Biophys Acta. 2010;1801:738–745. doi: 10.1016/j.bbalip.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Leibundgut G, Arai K, Orsoni A, et al. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J Am Coll Cardiol. 2012;59:1426–1437. doi: 10.1016/j.jacc.2011.12.033. This study define sthe presence of OxPL on palsminogen as a key functional parameter that optimizes fibrinolysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briley-Saebo KC, Cho YS, Shaw PX, et al. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J Am Coll Cardiol. 2011;57:337–347. doi: 10.1016/j.jacc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briley-Saebo KC, Nguyen TH, Saeboe AM, et al. In vivo detection of oxidation-specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probes. J Am Coll Cardiol. 2012;59:616–626. doi: 10.1016/j.jacc.2011.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briley-Saebo KC, Shaw PX, Mulder WJ, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L, Green SR, Baek JS, et al. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J Clin Invest. 2011;121:4861–9. doi: 10.1172/JCI57755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 35.Lewis MJ, Malik TH, Ehrenstein MR, et al. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyaw T, Tay C, Khan A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 37.Faria-Neto JR, Chyu KY, Li X, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 38.Schiopu A, Frend‚us Br, Jansson B, et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in Apobec-1−/−/Low-Density Lipoprotein receptor−/− mice. J Am Coll Cardiol. 2007;50:2313–2318. doi: 10.1016/j.jacc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- 39.Ravandi A, Boekholdt SM, Mallat Z, et al. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsimikas S, Brilakis ES, Lennon RJ, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Shaw PX, Horkko S, Tsimikas S, et al. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 42.Tsimikas S, Miyanohara A, Hartvigsen K, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torzewski M, Shaw PX, Han KR, et al. Reduced in vivo aortic uptake of radiolabeled oxidation-specific antibodies reflects changes in plaque composition consistent with plaque stabilization. Arterioscler Thromb Vasc Biol. 2004;24:2307–2312. doi: 10.1161/01.ATV.0000149378.98458.fe. [DOI] [PubMed] [Google Scholar]
- 44.Tsimikas S, Aikawa M, Miller FJ, Jr, et al. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2007;27:175–181. doi: 10.1161/01.ATV.0000251501.86410.03. [DOI] [PubMed] [Google Scholar]
- 45.Aikawa M, Sugiyama S, Hill CC, et al. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- 46.Leibundgut G, Scipione C, Yin HY, et al. Oxidized phospholipids on apolipoprotein(a) are only present on human Lp(a): Implications for understanding Lp(a) atherogenicity. J Am Coll Cardiol. 2013;61:E2037–E2037. [Google Scholar]