Abstract
Fish are exposed to many kinds of environmental stressors and the chances of succumbing to infectious diseases may be increased a result. For example, an acute increase in temperature can induce numerous physiological changes in the body. In the present study, we examined the redox state in response to a severe acute stress resulting from heat shock in teleost coho salmon (Oncorhynchus kisutch). The plasma lipid peroxides levels in fish gradually increased after heat shock treatment. By 2.5 h post-heat stress, plasma glutathione (GSH) levels had decreased, but they had returned to basal levels by 17.5 h post-stress. Plasma superoxide dismutase activities in stressed fish were significantly increased compared with those in control fish at 17.5 h post-stress, but had returned to basal levels by 48 h post-stress. Expression levels of hepatic GSH and heat shock protein 70 gradually increased after heat shock treatment. These results concerning the changing patterns of multiple important redox-related biomarkers suggest that severe thermal stressors can affect the redox state and induce oxidative stress in ectothermal animals, such as fish, in vivo. Hence, manipulation of appropriate thermal treatment may possibly be useful to control fish fitness.
Keywords: Redox state, Thermal stressor, Heat shock, Oxidative stress, Fish, Coho salmon
Graphical abstract
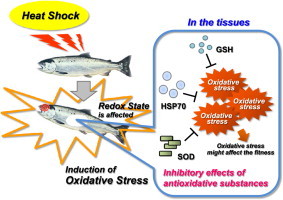
Highlights
-
•
The plasma lipid peroxides levels gradually increased after heat shock.
-
•
The plasma glutathione levels had decreased, but they had returned to basal levels.
-
•
The plasma superoxide dismutase activities were increased.
-
•
The hepatic glutathione and heat shock protein 70 levels gradually increased.
-
•
Severe thermal stressors can affect the redox state and might induce oxidative stress.
Introduction
Aquatic organisms are exposed to local and global environmental stressors, such as pollutants and acute changes in temperature [1–6]. Exposure of organisms to stressors may result in a series of biochemical and physiological changes. At the organismal level, these changes are mediated by the neuroendocrine system. In addition to this neuroendocrine stress response, there is a cellular stress response following exposure to stressful situations. These stress responses in organisms affect their general health, disease resistance, growth, and reproduction [3,5–7]. An acute increase in temperature is known as heat shock and can induce numerous changes in the body. The physiological states of fish depend on the environmental temperature. As a result, temperature is an important factor influencing their biological geographic distribution. Furthermore, daily and seasonal temperature changes have an impact during the lifetime of individual fish [8]. Unfortunately, studies on the heat shock response in fish have primarily focused on the expression and characterization of cellular molecular chaperons, heat shock proteins (HSPs) [1,3,8–10].
Recently, in the course of studies on the fish fitness in response to a stress, we found that mild stress caused by handling as an acute physiological stressor regulates the expression of growth-related genes, such as growth hormone receptor (ghr) and insulin-like growth factor-1 (igf1) genes, in fish [11]. The growth of fish is known to be genetically regulated and to be also influenced by cellular, endocrinological, and environmental factors. The responses of endocrine tissue are affected by the integration of external stimuli with internal signals according to the physiological state [1,3,5–7,12–16]. Fish growth can be enhanced by improved nutrition, husbandry conditions, elevated temperature, and changes in the endocrine system of the animal [5,15,17]. Accordingly, it is of interest to determine the effects of severe stressors on the expressions of important genes such as growth-related genes in fish. It is needed to reveal the features of and the resulting effects of stressors on fish fitness in order to improve their production and health. In addition, fish are thought to be an ideal and a convenient model to examine the effects of thermal and other complex stressors on the organism for both short and long periods. This is because fish are a typical ectothermic vertebrate. However, little is known about the effects of acute increases in temperature, which should be severe stressors for fish, on conditions such as the redox state in fish.
In the present study, we examined the redox state in response to a severe stress derived from heat shock in teleost coho salmon (Oncorhynchus kisutch). Coho salmon is one of the most valued species used in aquaculture worldwide and is known to be susceptible to increases in temperature [18]. Additionally, in Japan, coho salmon farming is one of the basic industries in the northeastern (Tohoku) Pacific coastal area, where the great earthquake and massive tsunami occurred in 2011. We discuss the relationships between the thermal stress responses and the redox states in fish in the context of our findings.
Materials and methods
Animal experiment
All experimental procedures were approved by the Animal Care Committee at Tohoku University (Sendai, Japan). Coho salmon O. kisutch were purchased from a local hatchery (Miyagi, Japan). After acclimatization for 2 weeks at the Aquarium Facility of Tohoku University, fish were exposed to heat shock (+11 °C for 2 h) and sampled at 2.5, 17.5, and 48 h after stress.
The fish (approximate body weight, 144 g) were reared in 60-L flow-through tanks at 8 °C (light/dark=12 h/12 h). Healthy, mixed sex, fish were divided into 4 groups (n=8). The fish in the first group were undisturbed (prestressed) fish used as a control, maintained under quiet and suitable conditions, and sampled at 13:30. The fish in the second group were subjected to heat shock from 9:00 to 11:00 and sampled at 2.5 h post-stress (at 13:30). The fish in the third group were subjected to the stressor from 18:00 to 20:00 and sampled at 17.5 h post-stress (at 13:30). The fish in the fourth group were also subjected to the stressor from 11:30 to 13:30 and sampled at 48 h post-stress (at 13:30). Accordingly, all tissues and blood for analysis were sampled at the same time, so that the effects of several factors, such as diurnal rhythm and photoperiod, on the expressions of redox state-related factors could be minimized.
Food was withheld for over 48 h before each sampling period. At each sampling period (2.5, 17.5, and 48 h post-stress), fish were sacrificed by an overdose of buffered MS222 (m-aminobenzoic acid ethyl ester methanesulfonate). Blood was withdrawn and plasma was separated by centrifugation. Fish were gutted, and the tissues were quickly removed. All plasma and tissue samples were frozen at −80 °C until analysis.
Measurements
Plasma cortisol and glucose levels
Plasma cortisol levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit from Oxford Biomedical Research, UK [19]. Plasma glucose was measured using an enzymatic assay method with a Glucose CII-Test Wako kit from Wako Pure Chemical Industries, Ltd., Japan.
Lipid peroxides, glutathione, superoxide dismutase, and heat shock protein 70 levels
Lipid peroxides (LPO) were determined as thiobarbituric acid reactive substances (TBARS) by a HPLC-fluorescence method [20]. TBARS concentrations were determined from a standard curve established with TBA-malondialdehyde (MDA, 1,1,3,3-tetramethoxypropane) adducts.
Glutathione (GSH) levels were determined by a glutathione reductase-recycling method with a Total Glutathione Quantification kit from Dojindo Laboratories, Japan. This kit can measure the total amount of reduced GSH and oxidized form of GSH (GSSG).
Superoxide dismutase (SOD) activity was assayed by the formazan-WST method (Total SOD Assay kit, Dojindo Laboratories, Japan).
Levels of HSP70 protein were determined by immunoblotting as described by Basu et al. (2001) [19]. Anti-HSP70 and anti-β-actin antibodies were purchased from Sigma-Aldrich, St. Louis, MO. The expressions of HSP70 were normalized by those of β-actin.
Protein contents were measured by a DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA) using bovine serum albumin as standard.
Statistical analysis
All samples were run in duplicate and results were expressed as means±SEM. All data were subjected to one-way analysis of variance (ANOVA). Means were compared with the Tukey–Kramer multiple comparison test. Differences were considered to be statistically significant at p<0.05.
Results
Plasma cortisol and glucose levels
At 2.5 h post-heat stress, plasma cortisol levels had increased compared with those in control fish, but had returned to basal levels at 17.5 h post-stress (Fig. 1). At 2.5 and 17.5 h post-stress, plasma glucose levels had increased as compared with those in control fish (the average plasma glucose concentration in control fish was 66.4 mg/dL). However, at 48 h post-stress, plasma glucose levels in stressed fish had decreased and were not significantly different from those in control fish (data not shown).
Fig. 1.
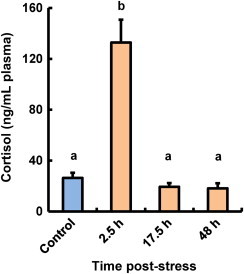
Effect of thermal stressors on cortisol levels in plasma of coho salmon O. kisutch. Data represent means±SEM (n=8). Statistical relationships between groups are indicated by letters where significant differences were detected (p<0.05).
Plasma LPO, GSH, and SOD levels
As shown in Fig. 2A, the plasma LPO levels in stressed fish gradually increased after heat shock treatment, and had significantly increased compared with those in control fish at 17.5 and 48 h post-stress.
Fig. 2.
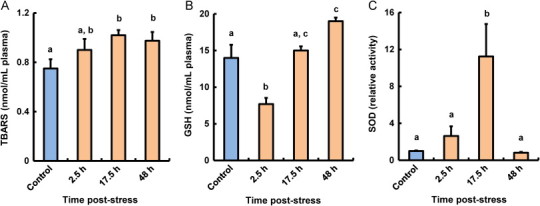
Effect of thermal stressors on LPO (A), GSH (B), and SOD (C) levels in plasma of coho salmon O. kisutch. Data represent means±SEM (n=5). Statistical relationships between groups are indicated by letters where significant differences were detected (p<0.05).
At 2.5 h post-heat stress, plasma GSH levels had decreased, but had returned to basal levels at 17.5 h post-stress (Fig. 2B). At 48 h post-stress, plasma GSH levels in stressed fish had increased significantly as compared with those in control fish.
Plasma SOD activities in stressed fish had increased significantly compared with those in control fish at 17.5 h post-stress, but had returned to basal levels at 48 h post-stress (Fig. 2C).
Hepatic GSH and HSP70 levels
Hepatic GSH levels in stressed fish at 17.5 and 48 h post-stress had significantly increased compared with those in control fish (Fig. 3A). The changing patterns of hepatic GSH levels in stressed fish were similar to those in the plasma of stressed fish (Figs. 2B and 3A).
Fig. 3.
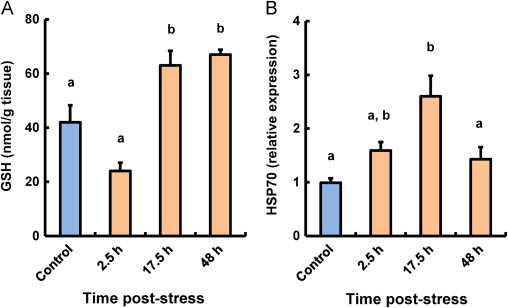
Effect of thermal stressors on GSH (A) and HSP70 (B) levels in the liver of coho salmon O. kisutch. Data represent means±SEM (n=5). Statistical relationships between groups are indicated by letters where significant differences were detected (p<0.05).
Hepatic HSP70 expression levels are shown in Fig. 3B. Hepatic HSP70 expression levels gradually increased after heat shock treatment and reached their maximum values at 17.5 h post-stress.
Discussion
The environmental temperature can induce numerous physiological changes in the biological functions of organisms. In particular, increased temperature can result in serious damage to cold-blooded fish species and cause metabolic stress in the body. Increased environmental temperature results in increased oxygen consumption and stimulates various metabolic processes on the basis of known thermodynamic principles [21,22]. In the present study, it was shown that heat shock-induced severe thermal stressors can influence several redox-related biomarkers and the redox homeostasis in teleost coho salmon.
LPO levels in fish tissues change under various stressful conditions and are known to be a sensitive indicator of damage to various tissues under different environmental conditions [4,15,23–29]. In this study, the plasma LPO levels in fish exposed to heat shock were observed to increase. These LPO in stressed fish plasma are considered to be metabolites derived from various damaged tissues. Accordingly, severe stressors caused by heat shock should result in damage to various tissues of coho salmon.
Fish have both enzymatic and non-enzymatic antioxidative defense systems against reactive oxygen species (ROS)-related damage [4,21]. Reduced GSH, the major nonprotein cellular thiol, is a cysteine-containing tripeptide (γ-glutamylcysteinylglycine) with reducing and nucleophilic properties that is one of the major regulators of the intracellular redox state and plays an important role in the non-enzymatic defense system [4,21,30–32]. GSH is also known to be the substrate for glutathione peroxidase, an antioxidative enzyme that scavenges ROS and LPO generated within cells [4,21,33,34]. The changing patterns of GSH levels in both plasma and liver observed in this study were similar to those in the livers of fish that were administered with an oxidant [25,35,36]. In the muscle and gill, GSH levels increased in response to thermal stress (data not shown). At the initial post-heat stress stage, plasma GSH may be consumed to eliminate ROS generated in blood. After 17.5 and 48 h of recovery, GSH plasma levels gradually increased, which suggested enhanced synthesis and transport of GSH from the liver. The liver is known to be the major source of GSH in vertebrates [32,37].
Antioxidative enzymes, such as SOD, glutathione peroxidase, and catalase, can scavenge radicals and contribute to the body’s enzymatic antioxidative defenses. The changes in the expression of antioxidative enzymes in fish have been observed with regard to stress [22,36,38,39]. In particular, SODs catalyze the reaction of dismutation of superoxide (O2•−) and H2O2, and are considered to play key roles in the first step of the enzymatic antioxidative defense system [4,21,22,40-42]. In this study, plasma SOD activities in stressed fish showed a transient increase at 17.5 h post-stress. Hence, increased SOD expression might neutralize the harmful effects of superoxides for the initial period of stressful conditions in tissues.
Organisms maintain a balance between generation and neutralization of ROS under normal physiological conditions. However, heat exposure and enhanced oxygen consumption are considered to promote the generation of ROS, such as O2•−, H2O2, hydroxyl radical (•OH), and peroxyl radical (ROO•). The resulting ROS exceed organismal scavenging capacity and attack cell components, such as nucleic acids, proteins, lipids, and membranes [4,21,22,36,40,43]. ROS production in cells, especially in the mitochondria, has been found to be increased in exercised mammalian muscle, heat-stressed bivalve gills, chicken muscles, lugworm, and cultured cells as compared with non-stressed control tissue [21,26,44–48]. The induction of various HSP families regarding environmental stressors, such as heat shock, bacterial pathogens, and pollutants, has been reported in cell lines and various tissues of fish [1,8–10,19]. In this study, HSP70 was also induced in stressed fish liver. HSP70 is known to assist the folding of ascent polypeptide and mediate the repair of denatured proteins, the breakdown and replacement of the proteins that are not repairable [1,8]. Therefore, the induction of hepatic HSP70, which was observed in stressed fish in this study, indicates high level of protein damage was induced by heat shock. Thus, the present results regarding the expression patterns of multiple redox-related biomarkers, such as LPO, GSH, SOD, and HSP, in response to thermal stressors suggest that severe thermal stress due to heat shock induces oxidative stress in coho salmon, which may enhance oxidation in the body and result in damage to tissues. Under oxidative stress conditions, the levels of antioxidative substances, such as GSH and SOD, may increase due to their de novo synthesis to protect tissues against oxidative damage. In addition, a redox state, such as antioxidative state, has already been reported to modulate the synthesis of HSP in mammalian tissue [49].
In fish, pituitary-secreted growth hormone (GH) - liver-derived insulin-like growth factor (IGF)-1 axis plays a critical role in the regulation of both growth and development. Secretion of GH is known to be under hypothalamic regulation by means of many modulators [12,14,16,50,51]. Additionally, the GH-IGF-1 axis might be influenced by stressors, such as temperature and oxygen levels. In practice, muscular GH receptors (GHR) protein expression in fish has been observed to increase after heat shock treatment [13]. We recently found that hepatic igf1 expression rapidly increased at 1.5 h post-stress without a change in ghr, whereas both ghr and igf1 levels decreased at 16 h after mild-stress treatment [11]. Accordingly, the expression of igf1 gene seems to be independently affected by stress other than GH and its signal transduction through GHR. These results suggest that growth-related gene expressions could be affected differently by the types and strength of stress, and thermal and handling stress could have positive effects on the growth-related factor expressions in fish. Consequently, it is of interest to determine the transcriptomic features regarding growth-related gene expressions in fish in response to heat shock-induced oxidative stress.
Intercellular signaling is known to be often affected by ROS or a pro-oxidative shift in the redox state resulting in the up- or down-regulation of the expressions of several genes and proteins [3,21,22,26,30,36,43,46,48,52–54]. We have previously observed that an antioxidative supplement, such as astaxanthin, known as a non-enzymatic small molecular component of the antioxidative defense system in vivo, could dramatically reduce oxidative stress-induced damage in fish [2,3,27,55,56]. Antioxidative substances could play a critical role in the tolerance against oxidative stress by organisms. Thus, the possible beneficial effects of antioxidative supplements in oxidative stressed fish should be determined.
In conclusion, the results of this study provide information that may be useful for improving fish fitness. An oxidative stress recently became a common theme in relation to the impact of climate change, such as climate warming, on natural ecosystems [21]. It is known that severe oxidative stress due to ROS leads to oxidative damage in vivo. However, a moderate level of oxidative stress could modulate cellular functions and have positive effects on animal health [57–60]. Accordingly, manipulation of appropriate thermal treatment could be employed to control and improve the health and production of fish. Further studies are now in progress to reveal the relationships between the redox state, oxidative stress, growth-related factors, and the fitness in fish.
Acknowledgments
The authors wish to thank Mr. T. Mandeville at New Day English Language Services, Sendai, Japan for editing this manuscript. The authors are grateful to Drs. S. Hidema, M. Ikeda, N. Ito, H. Kitazawa, M. Osada, H. Shirakawa, and K. Takahashi at Tohoku University for valuable discussion and technical support. The authors acknowledge Mr. M. Hidaka and Mr. T. Shiraishi at Dojindo Laboratories Co., Ltd., Japan for valuable suggestions concerning GSH analysis. The authors also acknowledge Dr. S. Minami at Shirako Co., Ltd., Japan and Miss. A. Yamauchi at Tohoku University for assistance in laboratory work. This study was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI and #23580277) from the Japan Society for the Promotion of Science (JSPS) to TN.
Each author of this study further declares no relationships with the companies or manufacturers who may benefit from the results of the present study. The authors have declared that no competing interests exist.
References
- 1.Iwama G.K., Afonso L.O.B., Vijayan M.M. Stress in fishes. In: Evans D.H., Claiborne J.B., editors. The Physiology of Fishes, third edition. CRC Press; Boca Raton, FL: 2006. pp. 319–342. [Google Scholar]
- 2.Nakano T. Microorganisms. In: Nakagawa H., Sato M., Gatlin, III D.M., editors. Dietary Supplements for the Health and Quality of Cultured Fish. CAB International; Oxfordshire, UK: 2007. pp. 86–108. [Google Scholar]
- 3.Nakano T. Stress in fish. Yoshoku (Aquaculture Magazine) 2011;48:64–67. [Google Scholar]
- 4.Nakano T., Takeuchi M. Relationship between fish and reactive oxygen species. Yoshoku (Aquaculture Magazine) 1997;34:69–73. [Google Scholar]
- 5.Pickering A.D. Growth and stress in fish production. Aquaculture. 1993;111:51–63. [Google Scholar]
- 6.Prunet P., Øverli Ø., Douxfils J., Bernardini G., Kestemont P., Baron D. Fish welfare and genomics. Fish Physiology and Biochemistry. 2012;38:43–60. doi: 10.1007/s10695-011-9522-z. [DOI] [PubMed] [Google Scholar]
- 7.Barton B.A., Iwama G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annual Review of Fish Diseases. 1991;1:3–26. [Google Scholar]
- 8.Basu N., Todgham A.E., Ackerman P.A., Bibeau M.R., Nakano K., Schulte P.M., Iwama G.K. Heat shock protein genes and their functional significance in fish. Gene. 2002;295:173–183. doi: 10.1016/s0378-1119(02)00687-x. 12354651 [DOI] [PubMed] [Google Scholar]
- 9.Feder M.E., Hofmann G.E. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 10.Iwama G.K., Thomas P.T., Forsyth R.B., Vijayan M.M. Heat shock protein expression in fish. Reviews in Fish Biology and Fisheries. 1998;8:35–56. [Google Scholar]
- 11.Nakano T., Afonso L.O.B., Beckman B.R., Iwama G.K., Devlin R.H. Acute physiological stress down-regulates mRNA expressions of growth-related genes in coho salmon. PLoS One. 2013;8:e71421. doi: 10.1371/journal.pone.0071421. 23990952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deane E.E., Woo N.Y.S. Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: a review. Reviews in Fish Biology and Fisheries. 2009;19:97–120. [Google Scholar]
- 13.M. Kameda, T. Nakano, T. Yamaguchi, M. Sato, L.O.B. Afonso, G.K. Iwama, R.H. Devlin, Effects of heat shock on growth hormone receptor expression in coho salmon, in: Proceedings of the 5th World Fisheries Congress, Yokohama, October 20–25, 2008 .3f-16.
- 14.Moriyama S., Ayson F.G., Kawauchi H. Growth regulation by insulin-like growth factor-I in fish. Bioscience, Biotechnology, and Biochemistry. 2000;64:1553–1562. doi: 10.1271/bbb.64.1553. 10993139 [DOI] [PubMed] [Google Scholar]
- 15.Peter R.E. The brain and feeding behavior. In: Hoar W.S., Randall D.J., Brett J.R., editors. Bioenergetics and Growth. Academic Press; New York, NY: 1979. pp. 121–159. [Google Scholar]
- 16.Reinecke M. Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. Journal of Fish Biology. 2010;76:1233–1254. doi: 10.1111/j.1095-8649.2010.02605.x. 20537012 [DOI] [PubMed] [Google Scholar]
- 17.Donaldson E.M., Fagerlund U.H.M., Higgs D.A., McBride J.R. Hormonal enhancement of growth. In: Hoar W.S., Randall D.J., Brett J.R., editors. Bioenergetics and Growth. Academic Press; New York, NY: 1979. pp. 455–597.884630 [Google Scholar]
- 18.Ohya S., Shimizu T., Horikawa Y., Yamamoto S. Relationship between temperature of rearing water and numbers of dead coho salmon (Oncorhynchus kisutch) Bulletin of the Fisheries Laboratory of Kinki University. 1989;3:73–77. [Google Scholar]
- 19.Basu N., Nakano T., Grau E.G., Iwama G.K. The effects of cortisol on heat shock protein 70 levels in two fish species. General and Comparative Endocrinology. 2001;124:97–105. doi: 10.1006/gcen.2001.7688. 11703075 [DOI] [PubMed] [Google Scholar]
- 20.Wong S.H.Y., Knight J.A., Hopfer S.M., Zaharia O., Leach C.N., Jr., Sunderman F.W., Jr. Lipoperoxides in plasma as measured by liquid–chromatographic separation of malondialdehyde–thiobarbituric acid adduct. Clinical Chemistry. 1987;33:214–220. [PubMed] [Google Scholar]
- 21.Lesser M.P. Oxidative stress in marine environments: biochemistry and physiological ecology. Annual Review of Physiology. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 22.Lushchak V.I. Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. 21074869 [DOI] [PubMed] [Google Scholar]
- 23.Verlecar X.N., Jena K.B., Chainy G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chemico-Biological Interactions. 2007;167:219–226. doi: 10.1016/j.cbi.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Nakano T., Tosa M., Takeuchi M. Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. Journal of Agricultural and Food Chemistry. 1995;43:1570–1573. [Google Scholar]
- 25.Heise K., Puntarulo S., Nikinmaa M., Abele D., Pörtner H.-O. Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. Journal of Experimental Biology. 2006;209:353–363. doi: 10.1242/jeb.01977. 16391357 [DOI] [PubMed] [Google Scholar]
- 26.Ho E., Galougahi K.K., Liu C.-C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biology. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano T., Kanmuri T., Sato M., Takeuchi M. Effect of astaxanthin rich red yeast (Phaffia rhodozyma) on oxidative stress in rainbow trout. Biochimica et Biophysica Acta. 1999;1426:119–125. doi: 10.1016/s0304-4165(98)00145-7. 9878705 [DOI] [PubMed] [Google Scholar]
- 28.Olsen R.E., Sundell K., Mayhew T.M., Myklebust R., Ringø E. Acute stress alters intestinal function of rainbow trout, Oncorhynchus mykiss (Walbaum) Aquaculture. 2005;250:480–495. [Google Scholar]
- 29.Rau M.A., Whitaker J., Freedman J.H., Di Giulio R.T. Differential susceptibility of fish and rat liver cells to oxidative stress and cytotoxicity upon exposure to prooxidants. Comparative Biochemistry and Physiology. C: Toxicology & Pharmacology. 2004;137C:335–342. doi: 10.1016/j.cca.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Arrigo A.-P. Gene expression and the thiol redox state. Free Radical Biology and Medicine. 1999;27:936–944. doi: 10.1016/s0891-5849(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 31.Niki E. Ascorbic acid, glutathione. In: Nakano M., Asada K., Oyanagui Y., editors. Reactive Oxygen. Kyoritsu Shuppan; Tokyo: 1988. pp. 321–326. [Google Scholar]
- 32.Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 33.Nakano T., Sato M., Takeuchi M. Glutathione peroxidase of fish. Journal of Food Science. 1992;57:1116–1119. [Google Scholar]
- 34.Nakano T., Sato M., Takeuchi M. Partial purification and properties of glutathione peroxidase from carp hepatopancreas. Comparative Biochemistry and Physiology. B: Comparative Biochemistry. 1992;102B:31–35. doi: 10.1016/0305-0491(92)90268-v. [DOI] [PubMed] [Google Scholar]
- 35.Ploch S.A., Lee Y.-P., MacLean E., Di Giulio R.T. Oxidative stress in liver of brown bullhead and channel catfish following exposure to tert-butyl hydroperoxide. Aquatic Toxicology. 1999;46:231–240. [Google Scholar]
- 36.Valavanidis A., Vlahogianni T., Dassenakis M., Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. 16406578 [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa T., Matsuda Y. Transport of glutathione. Tanpakushitsu, Kakusan, Koso (Proteins, Nucleic Acids, and Enzymes) 1988;33:1450–1458. [PubMed] [Google Scholar]
- 38.Poly W.J. Nongenetic variation, genetic-environmental interactions and altered gene expression. II Disease, parasite and pollution effects. Comparative Biochemistry and Physiology. B: Comparative Biochemistry. 1997;117B:61–74. doi: 10.1016/s0305-0491(96)00329-x. [DOI] [PubMed] [Google Scholar]
- 39.Craig P.M., Wood C.M., McClelland G.B. Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio) American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;293:R1882–R1892. doi: 10.1152/ajpregu.00383.2007. [DOI] [PubMed] [Google Scholar]
- 40.Asada K. Production, scavenging and action of active oxygen. Tanpakushitsu, Kakusan, Koso (Proteins, Nucleic Acids, and Enzymes) 1988;33:7–12. [PubMed] [Google Scholar]
- 41.Nakano T., Sato M., Takeuchi M. Superoxide dismutase activity in the skin of fish. Journal of Fish Biology. 1993;43:492–496. [Google Scholar]
- 42.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 43.Dröge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. 11773609 [DOI] [PubMed] [Google Scholar]
- 44.Heise K., Puntarulo S., Pörtner H.O., Abele D. Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica (King and Broderip) under heat stress. Comparative Biochemistry and Physiology. C: Toxicology & Pharmacology. 2003;134C:79–90. doi: 10.1016/s1532-0456(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 45.Keller M., Sommer A.M., Pörtner H.O., Abele D. Seasonality of energetic functioning and production of reactive oxygen species by lugworm (Arenicola marina) mitochondria exposed to acute temperature changes. Journal of Experimental Biology. 2004;207:2529–2538. doi: 10.1242/jeb.01050. 15184524 [DOI] [PubMed] [Google Scholar]
- 46.Ji L.L. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radical Biology and Medicine. 1995;18:1079–1086. doi: 10.1016/0891-5849(94)00212-3. [DOI] [PubMed] [Google Scholar]
- 47.Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poultry Science. 2005;84:307–314. doi: 10.1093/ps/84.2.307. 15742968 [DOI] [PubMed] [Google Scholar]
- 48.Shin M.H., Moon Y.J., Seo J.-E., Lee Y., Kim K.H., Chung J.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radical Biology and Medicine. 2008;44:635–645. doi: 10.1016/j.freeradbiomed.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Peng J., Jones G.L., Watson K. Stress proteins as biomarkers of oxidative stress: effects of antioxidant supplements. Free Radical Biology and Medicine. 2000;28:1598–1606. doi: 10.1016/s0891-5849(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 50.Klein S.E., Sheridan M.A. Somatostatin signaling and the regulation of growth and metabolism in fish. Molecular and Cellular Endocrinology. 2008;286:148–154. doi: 10.1016/j.mce.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Kopchick J.J., Andry J.M. Growth hormone (GH), GH receptor, and signal transduction. Molecular Genetics and Metabolism. 2000;71:293–314. doi: 10.1006/mgme.2000.3068. [DOI] [PubMed] [Google Scholar]
- 52.Allen R.G., Tresini M. Oxidative stress and gene regulation. Free Radical Biology and Medicine. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 53.Franco A.A., Odom R.S., Rando T.A. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radical Biology and Medicine. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths H.R., Dias I.H.K., Willetts R.S., Devitt A. Redox regulation of protein damage in plasma. Redox Biology. 2014;2:430–435. doi: 10.1016/j.redox.2014.01.010. 24624332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakano T., Miura Y., Wazawa M., Sato M., Takeuchi M. Red yeast Phaffia rhodozyma reduces susceptibility of liver homogenate to lipid peroxidation in rainbow trout. Fisheries Science. 1999;65:961–962. [Google Scholar]
- 56.Nakano T., Wazawa M., Yamaguchi T., Sato M., Iwama G.K. Positive biological actions of astaxanthin in rainbow trout. Marine Biotechnology. 2004;6:S100–S105. [Google Scholar]
- 57.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nature Reviews Drug Discovery. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 58.Milisav I., Poljsak B., Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. International Journal of Molecular Sciences. 2012;13:10771–10806. doi: 10.3390/ijms130910771. 23109822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. 23102266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan L.-J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biology. 2014;2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


