Abstract
Regular consumption of moderate doses of wine is an integral part of the Mediterranean diet, which has long been considered to provide remarkable health benefits. Wine׳s beneficial effect has been attributed principally to its non-alcoholic portion, which has antioxidant properties, and contains a wide variety of phenolics, generally called polyphenols. Wine phenolics may prevent or delay the progression of intestinal diseases characterized by oxidative stress and inflammation, especially because they reach higher concentrations in the gut than in other tissues. They act as both free radical scavengers and modulators of specific inflammation-related genes involved in cellular redox signaling. In addition, the importance of wine polyphenols has recently been stressed for their ability to act as prebiotics and antimicrobial agents.
Wine components have been proposed as an alternative natural approach to prevent or treat inflammatory bowel diseases. The difficulty remains to distinguish whether these positive properties are due only to polyphenols in wine or also to the alcohol intake, since many studies have reported ethanol to possess various beneficial effects. Our knowledge of the use of wine components in managing human intestinal inflammatory diseases is still quite limited, and further clinical studies may afford more solid evidence of their beneficial effects.
Abbreviations: AKT, serine/threonine protein kinase (v-akt murine thimoma viral oncogene homolog1); apoB48, apolipoprotein B48; CD, Crohns disease; COX-2, cyclooxygenase-2; Cys, cysteine; DSS, dextran sodium sulfate; ERK, extracellular signal-regulated kinase; GRP, grape reaction product; GSH, reduced glutathione; IBD, inflammatory bowel disease; IKB, inhibitor of NF-κB; IL, interleukin; iNOS, inducible nitric oxide synthase; IFN, interferon; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NADPH, nicotinamide adenine dinucleotide phosphate reduced; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid-2-related factor 2; PGE-2, prostaglandin E-2; ROS, reactive oxygen species; SIRT-1, silent mating type information regulation-1; TNF-α, tumor necrosis factor alpha; UC, Ulcerative Colitis
Keywords: Polyphenols, Wine, Antioxidants, Gut, Inflammation, Oxidative stress
Graphical abstract
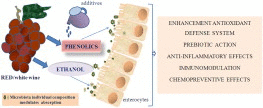
Influence of wine phenolic components on intestinal cell function. The scheme summarized the main activities of phenolics, which are the predominant non-alcoholic components of wine. They can interfere with the development of inflammatory intestinal diseases and colorectal cancer. Phenolics multiple properties are due to both direct antioxidant effects and indirect activation of redox-sensitive cell pathways involved in negative regulation of inflammation and immune modulation.
Furthermore, microbiota plays a role in phenolics activity: it is essential for their metabolism, enabling them to reach highest concentrations in the gut. Diversity of phenolic composition is a reflection of interindividual variation in colonic microflora; on the other hand, phenolics act as prebiotics, increasing microflora growth.
Ethanol matrix has an undoubted role in the impact of wine on intestinal functions. Despite the well-known deleterious consequences of high ethanol consumption, it has been proved that small concentrations of ethanol are able to act as cell signals, and influence microbiota growth together with phenolics.
Highlights
-
•
Wine compounds regulate inflammation and redox signaling in intestinal cells.
-
•
Experimental and clinical data on wine impact on intestinal function are reviewed.
-
•
Among different antioxidants in wine, phenolic compounds are the most representative.
-
•
Microbiota has a principal role in the high availability of wine polyphenols in gut.
-
•
The influence of wine ethanol fraction on intestinal disease is also discussed.
Introduction
A large number of studies, both in experimental models and in humans, have investigated the antioxidant properties of wine. The majority of studies on wine׳s effects have been performed on the cardiovascular system; its moderate consumption is generally considered to protect against coronary heart disease, and to be associated with a lower incidence of oxidative stress-related degenerative diseases [1]. However, during absorption wine components accumulate in the intestinal mucosa, where they reach higher concentrations than in other tissues, and can exert their activities.
The gastrointestinal tract is the first barrier against environmental agents, and is responsible for immune tolerance of microbiota [2]; it is prone to oxidative damage due to its exposure to the luminal oxidants present in foods. For instance, discrete quantities of reactive oxygen species (ROS) are essential for the recognition of nutrients, commensal and pathogenic bacteria, as well as for killing and processing pathogens during inflammatory immune response. However, increased free radical production and impaired antioxidant defenses have been related to the progression of intestinal chronic inflammation, which characterize a group of human diseases known as Inflammatory Bowel Diseases (IBD) [3]. Maintenance of the correct gastro-intestinal redox balance, which depends on dietary compounds, is thus an important aspect for human health.
The beneficial effect of wine has been mainly attributed to its non-alcoholic portion having antioxidant properties, which contains a large variety of phenolic compounds; these are mostly present in red wine [4]. Likewise implicated are antioxidants, such as ascorbic acid and sulfur dioxide, normally added during white-wine processing [5].
This review will discuss experimental and clinical data suggesting a link between wine consumption and intestinal function. In particular, it will focus on the relevant potential protective mechanisms that wine components may exercise on the cell signal network, specifically induced during tissue damage characterized by oxidative and inflammatory reactions.
Maintenance of redox intestinal homeostasis
The intestinal tract is continually attacked by luminal microbes and by oxidized compounds from the diet, exposing it to recurrent oxidative changes. Intestinal epithelial cells act as a selective permeable barrier, which allows the absorption of nutrients, electrolytes and water by transcellular and paracellular pathways, also affording their intracellular compartmentalization and trafficking towards the body. These cells are able to regulate the traffic of antigens towards gut-associated lymphoid tissues, in order to discriminate between innocuous and pathogenic antigens, acting as a crossroad between tolerance and the immune response.
Evidence for the need of a proper dietary intake of antioxidants to maintain low intracellular levels of oxidative species is manifold. ROS and their oxidized products may be considered as part of a network signaling system controlled by antioxidant defenses. For instance, moderate quantities of ROS can act as biological signal molecules, which are involved in different phases of the inflammatory immune response and of autophagic processes activated by luminal agents in intestinal cells. These events imply the production of hydrogen peroxide (H2O2) and superoxide anion (O2−•) or nitric oxide (NO) at specific intracellular sites, i.e. mitochondria, membrane nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase, endothelial inducible NO synthase (iNOS) and myeloperoxidase in inflammatory cells [6]. H2O2 regulate redox sensitive transduction pathways, such as phosphatidylinositol 3-kinase/AKT, mitogen-activated protein kinase/extracellular signal regulated kinase kinase / extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase, and also regulate activation of the oxidative stress-responsive nuclear transcription factor-κB (NF-κB), which is involved in inflammatory reactions [7,8]. However, although cell inflammation and oxidative reactions are considered to be a primary host defense, excessive inflammatory reactions, with overproduction of O2−•, H2O2, NO and HOCl by activated leukocytes, can overwhelm the tissue׳s antioxidant defenses and may contribute to functional impairment of the enteric mucosa, leading to an aberrant response to luminal agents. These events have been extensively considered in the pathogenesis of IBD and have been associated to chronic abnormal inflammatory and immune responses [3].
An antioxidant intestinal environment reflects the intestinal mucosa׳s response aimed at preventing oxidative damage, and is maintained by a complex dynamic recycling system in which different molecules undergo well-established oxido-reductions. The chief molecules involved are antioxidant enzymes, i.e. superoxide dismutase, catalase and glutathione peroxidases, as well as non-enzymatic molecules some of which originate from the diet, such as ascorbic acid, tocopherols and amino-thiols compounds [9]. To a great extent, ascorbate, reduced glutathione (GSH), cysteine (Cys) and sulfur dioxide are produced under conditions used in winemaking (see section below).
Principal non-alcoholic components of wine
There is a wide variation in wine compounds, the composition being affected by several factors including the grape varieties used, winemaking technology, and conditions under which the wine is aged and stored. The most abundant non-alcoholic components are phenolic compounds, which are generally called polyphenols, and that may roughly be divided as flavonoid and non-flavonoid compounds. Wine flavonoids can in turn be subdivided into flavan-3-ols, anthocyanins and flavonols. Catechin and epicatechin are the most representative flavan-3-ols in monomeric form; proanthocyanidins are oligomers, while condensed tannins are polymers. Non-flavonoid wine components are benzoic and hydroxycinnamic acids (caffeic, ferulic and p-coumaric acids) with their tartaric esters (caftaric, fertaric and coutaric acids), and stilbenes [10,11].
Although Vitis vinifera is the dominant grape used in wine production, there are significant varietal differences within this species [12]; the phenolic composition of wine also depends on processing in the winery [13]. Red wine is a whole-berry extract made by fermenting the juice in the presence of grape skin and seeds (which contain most of the phenols), while white wine is a juice product. Thus the total amount of phenols found in a typical glass of red wine is in the order of 100–200 mg, versus 25–50 mg in a glass of white wine [10].
In red wine, tannins contribute to the mouth feel of wines, but they also form pigmented polymers in association with anthocyanins, to provide the stable pigments that give red wine its long-term color stability. In white wine, the most important phenolic compounds are hydroxycinnamic acids and, in minor quantities, flavan-3-ol monomers [10].
Phenolic concentration and composition change significantly during vinification when considerable amounts of phenolics are degraded or oxidized [14]. Aging or fermenting in oak barrels enables oxygenation to occur, and various different compounds with organoleptic properties are released from the wood. Oak-treated wines contain volatile phenols including vanillin, furanic derivatives, lactones, terpenes and hydrolysable tannins (mainly gallotannins and ellagitannins), which affect the color, flavor and texture of the wine [15,16].
An important common practice in winemaking, especially when bottling white wines, is to add compounds that eliminate oxidative spoilage, by efficient free-radical scavenging. Sulfated compounds are the main antimicrobial and antioxidant additives of white wine; they are present in the wine in different forms depending on their solubility and pH, but mainly as sulfur dioxide, sulfates and sulfites. These compounds are often used in combination with ascorbic acid, which appears to be important for its ability to protect sulfur dioxide and wine phenolic components against oxidation. However, ascorbic acid should only be employed as a complement to sulfur dioxide, because it can also exhibit pro-oxidant activity depending on its concentration, and may be responsible for the browning of white wine due to its reaction with (+)catechin, to form yellow xanthylium cations pigments [17].
Quantitative and qualitative analyses of the amino-thiols present in grapes and wine have been reported; compounds found include GSH, N-acetylcysteine, Cys and omoCys, especially in white wines [18]. These compounds play an important role in slowing down the browning process due to natural oxidation. An important role in wine quality has been attributed to GSH for its high antioxidant activity and its influence on wine aroma [19]. Once grapes are crushed, hydroxycinnamic acids are rapidly oxidized by polyphenol oxidase enzymes, forming quinones, which in turn polymerize and lead to the formation of brown polymerized pigments in the must. GSH can avoid the formation of polymers, quickly reacting with quinones, and producing glutathionil-adducts (called grape reaction products—GRP). These compounds cannot be oxidized by polyphenol oxidase: thus the browning potential of musts may depend on their containing large amounts of GSH, and consequently having a high GRP concentration [20].
GSH has been also detected in red wines, but in lower concentrations than in white wines; the content varies widely depending on the winemaking process and aging conditions [21]. It has recently been proposed that glutathione could be used as an antioxidant additive, to maintain the antioxidant efficacy of sulfur dioxide and ascorbic acid high, thus avoiding the formation of xanthylium cations [22]. Interestingly, changes in GSH levels during fermentation depend on the yeast strain; this points to a direct relationship between GSH metabolism and yeast metabolism [23]. Finally, the addition of GSH to grape juice before fermentation has been found to increase the hydrogen sulfide content [24].
However, rather than directly contributing to increasing exogenous intestinal antioxidant defenses, amino-thiols and ascorbic acid in wine act to avoid oxidation of phenolic compounds that, in turn, improve the activity and expression of endogenous GSH-related enzymes [25,26]. The real players in terms of the antioxidant and anti-inflammatory activities attributed to wine are the polyphenols, which can reach higher concentrations in the gut than other known antioxidants contained in the diet.
Bioavailability of wine phenolics in the intestinal tract: the importance of microbiota
Some classes of wine phenolics are quite resistant to gastric digestion and reach the intestinal lumen as native compounds [27]. Generally, polyphenols are ingested in the conjugated form; once in the lumen phenols may directly cross the intestinal barrier and be metabolized by the enterocytes, or may function as a substrate for bacterial metabolism [28].
The interaction between gut microflora and wine phenolics is important for the bioavailability of most of the latter, and appears to be closely dependent on the bacterial population and intestinal transit time. Feeding studies with specific phenolics consumed by either healthy volunteers or ileostomy subjects underlined the contribution of microbiota to the interindividual variability observed in the metabolism of these compounds [29,30].
Proanthocyanidins and condensed tannins must be biotransformed by colonic bacteria before absorption, in order to yield a great variety of phenolic acids metabolites [31]. Hydrolysable tannins undergo similar bacterial degradation, yielding urolithin A and B metabolites that are absorbed in large amounts into the bloodstream, and may be detected in human urine and feces [32–34].
The anthocyanin fraction, which is stable to gastric conditions [35], may be partly absorbed from the stomach [36,37], but most of it undergoes gut microflora metabolization, via glycosylation and C-ring fission, leading to phenolic acids and aldehydes [38,39].
A good proportion of flavonols is absorbed in the intestinal tract. Quercetin is predominantly present in wine as quercetin-β-glycoside; it is absorbed in larger quantities in the rat intestine when ethanol is present [40]. In addition, low levels of quercetin-3-O-rhamnoglucoside (rutin) and quercetin-3-O-rhamnoside (quercitrin) are also found in wine [41], and can be hydrolyzed to quercetin only by α-rhamnosidases from colonic microflora in the large intestine [42].
Bioavailability of flavan-3-ols is greatly influenced by the degree of polymerization: catechin and epicatechin are readily absorbed in the small intestine by both passive and carrier-mediated diffusion [43]. However, their most abundant metabolites also arise from intestinal microbial catabolism [44]. Among the most common non-flavonoid compounds present in wine, hydroxycinnamic acids are esterified to quinic acids to form chlorogenic acids mainly by colonic commensal bacteria [42].
Some of the antioxidant, anti-inflammatory and anti-carcinogenic effects induced by dietary wine phenolics and their metabolites might also be attributed to their ability to modulate intestinal microbiota populations, as prebiotics. Overall, the prebiotic activity of wine phenolics may derive from their ability to interact with microbes׳ growth, both by inhibiting detrimental bacteria and by stimulating beneficial bacteria in the gut, in particular Lactobacteria and Bifidobacteria [45,46]; this influence depends on the bacterial concentration/type, the strains, the specific intestinal tract and interindividual variation [29]. Combined analytical methods provided comprehensive information on different microbial metabolites obtained from in vitro fermentation of fecal batches from 10 healthy volunteers with a mixture of red wine and grape juice [47].
The effect on bacterial growth of different grape-seed extracts, characterized by their content of monomeric flavanols, procyanidins and gallates, was tested directly on various Lactobacteria and Bifidobacteria species: growth inhibitory effects within species were reported, inhibition being specifically dependent on increased procyanidin content [45]. in vivo studies on male broiler Cobb chicks fed with grape-seed extract showed greater biodiversity in their gut microbiota populations of Lactobacillus, increased Enterococcus than those fed an ordinary diet, depending on the portion of intestinal tract [48]. There is some evidence of a possible antimicrobial effect of pure phenolics and of wine extracts, either from Argentinian wine varieties against Escherichia coli [49] or from Serbian red wines against different Gram-positive and Gram-negative strains [50].
Regarding the effect of ethanolic fraction of wine on intestinal bacteria growth, acute alcohol abuse increases mucosal susceptibility to microbial pathogens, probably by altering molecules involved in pathogen recognition, for example Toll like receptor 4, and consequently triggering inflammatory cell signals [51]. Conversely, low doses of alcohol have long been known to exercise bactericidal activity, which is improved by the acid pH of wine; the activity against the gastrointestinal pathogens Salmonella enterica and E. coli was markedly increased in whole wine compared to either dealcoholized wine or ethanol alone, suggesting that suggesting that other substances in non-alcoholic fraction of wine might synergize the anti-bacterial effect of ethanol [52].
The antiviral properties of red wine have been studied in depth. Among more than 100 red wine extracts, from the USA, Europe and South America, Cabernet Sauvignon has been found to strongly inhibit calcium transport through chloride channels on the apical plasma membrane of intestinal epithelial cells, and consequently to reduce diarrhea caused by rotavirus in neonatal mice [53].
Effects of wine on intestinal damage
Epidemiological studies have demonstrated that five to seven servings of fresh fruit and vegetables and two glasses of wine per day can lead to a prolonged healthy life [54]. A relationship between life expectancy and wine consumption was found in a cohort study in middle aged men drinkers from seven Italian villages [55].
Regular consumption of moderate doses of red or white wine is an integral part of the Mediterranean diet, in which the maintenance of a correct intake of oxidizable foodstuffs, such as ω-6 polyunsaturated fatty acids or cholesterol, and antioxidants is considered the key mechanism of protection against the occurrence of coronary events [56]. Increasing interest has been shown in the effect of wine compounds, especially polyphenols, to protect against human inflammatory and degenerative diseases other than cardiovascular. In fact, even if polyphenols are present in tissues in quite low concentrations, they can target signaling cascades involved in different cellular functions, thus exerting many health benefits [29]. As a consequence, assuming that these compounds reach very high levels in the intestine, their maximal effects in this organ might be hypothesized. However, of the many studies concerning wine׳s beneficial effects on human intestinal diseases, most are either in vitro studies in which phenolic components were added singly to cells, or experimental animal models of colitis (Fig. 1).
Fig. 1.
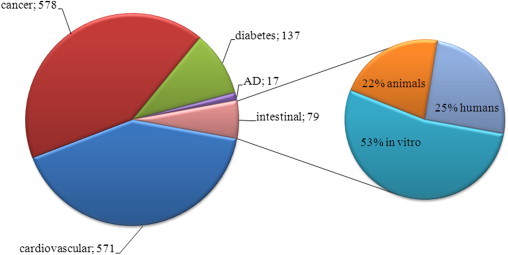
Distribution of articles on wine effects on different diseases according to PubMed citations during the last 10 years. Left panel: number of articles published from 2004 to 2013 with the exclusion of reviews; word “wine”, together with the words “cardiovascular”, or “cancer”, or “diabetes”, or “Alzheimer disease” (AD) or “intestinal”, have been considered as the keywords. Right panel: percentage of articles focused on words “wine” and “intestinal”, which included studies on animals, cell lines, or human trials.
in vitro evidence
ROS and their oxidized products may be considered as part of a network signaling system controlled by antioxidant defenses in the gut. The protective effect of wine polyphenols is chiefly due to their antioxidant properties, acting directly as free-radical scavengers, or indirectly, by interfering with specific inflammatory molecules involved in cellular redox signaling pathways. These molecules include COX-2, iNOS, ILs, transcription factors, i.e. nuclear factor erythroid-2-related factor 2 (Nrf2), NF-κB and activator protein 1 [57,58].
Among different polyphenols, resveratrol is widely recognized as the best wine component for its ability to inhibit cancer progression by modulating in vitro cell signals activated by oxidative changes [59]. Its antiproliferative activity has been tested in different intestinal cancer cells [60]. In CaCo-2 and SW480 human colon cancer cells resveratrol has been shown to inhibit lipopolysaccharide (LPS)-induced NF-κB activation [61], to induce cell–cycle arrest and apoptosis, and to decrease collagen synthesis in primary rat intestinal smooth muscle cells isolated from colon muscularis externa [62].
Tannin procyanidin B2 has also been shown to protect CaCo-2 cells against oxidative stress, by regulating cascade signals involving ERK and p38 MAPK activation, translocation of Nrf2 and consequent expression of glutathione S-transferase P1 [63].
With regard to experimental data on the modulation of intestinal cell signaling by whole-wine components, chronic treatment of CaCo-2 cells with a grape seed extract, at concentration similar to that due to drinking 250 mL of red wine per meal in a human subject (containing approximately 67.4% procyanidins and 13.5% catechins and procyanidin dimers) induced morphological and functional cell changes leading to a differentiated phenotype [64].
A Portuguese red-wine extract enriched in anthocyanins has been found to possess anti-inflammatory capacity in HT-29 colon epithelial cells stimulated with a mix of TNF-α, IFN-γ and IL-1. Wine extract decreased the degradation of Inhibitor of NF-κB, the synthesis of inducible nitric oxide synthase, COX-2 and IL-8 [65]. Similarly, human colon-derived CCD-18Co myofibroblast cells pre-treated with Port Barrel Reserve red wine polyphenolic extract (LeNoir grapes—Texas) and then treated with LPS showed decreased mRNA of both NF-kB and adhesion molecules, depending on the wine-extract concentration. Interestingly, this wine extract induced miR-126, a microRNA that is involved in negative regulation of vascular cell adhesion molecule-1 expression [66]. CaCo-2 cells treated with TNF-α confirmed the anti-inflammatory effect of grape seeds and of grape marc meal-based feed additives used for livestock nutrition, in that they inhibited NF-κB transactivation and IL-1β, IL-8, monocyte chemotactic protein-1, chemokine CXC ligand 1 expression [67].
Extracts of Sardinian wines (Cannonau and Vermentino) were shown to possess strong antioxidant properties against pro-oxidant tert-butyl hydroperoxide [41] and anti-inflammatory activity in differentiated enterocyte-like CaCo-2 cells in presence of a mixture of dietary oxysterols (cholesterol oxidation products), which are able to induce inflammation by up-regulating intestinal NADPH oxidase 1 and enhancing ROS production [68]. Notably, Cannonau extract exerted a more efficient protective effect on the oxysterol-dependent production of IL-6 and IL-8 than Vermentino white wine extract [68]. This difference was ascribed to the phenolic fraction, which is more abundant in red wine than in white wine. In this connection, ex vitro studies showed that red wine possesses great antioxidant capability on cholesterol oxidation [69].
Interestingly, 9 years ago Cabernet Shiraz red-wine extract (South Australia) was found to reduce the secretion by differentiated CaCo-2 cells of apolipoprotein B48, the marker of intestinal chylomicrons. This inhibition might limit the availability of total and free cholesterol content, thus reducing pro-atherogenic events [70]. However, a recent study showed that red wine added to apical compartment of these cytotypes in concentrations considered similar to dietary intake, inhibited LPS-dependent IL-6 induction, without altering high density lipoprotein-cholesterol functionality [71].
Particular attention should be paid on intestinal barrier affected by ethanolic matrix of wine. Acute low concentrations of ethanol have been shown to disrupt confluent epithelial Caco-2 cells by inducing apoptosis [72]. Conversely, chronic treatment of Caco-2 cells with 1% ethanol stimulated cell differentiation and morphological changes, i.e. microvillus density and elongation [64]. Studies on the effect of wine on thiamine and folate absorption, the deficiency of which has long been known to be involved in chronic alcoholism, showed that both red and white wines inhibited apical uptake of folate by Caco-2 cells; the same was true of alcohol-free wines. Ethanol alone had a weak effect on reducing vitamin absorption; at least some of this effect was concentration-dependent [73]. Further, studies by Pal and coworkers showed that both dealcoholized and alcoholized red wines had similar effects on apoB48 secretion in intestinal CaCo-2 cells [70], thus supporting the importance of wine components other than ethanol in modulating intestinal function.
Evidence in animals
As in the case of cellular studies, resveratrol in wine has been proposed to induce suppression of colon-cancer incidence associated with colitis in animals. Fischer F344 rats, in which experimental colitis was induced with dextran sodium sulfate (DSS), fed low doses of resveratrol similar to those hypothetically contained in the daily diet of a person weighing 70 kg, have shown reduced mucosal levels of inflammatory markers, such as prostaglandin E (PGE)-2, COX-2 and PGE synthase-1, and improved colonic mucosa architecture, with the specific induction of Bifidobacterium and Lactobacillus, and reduction of E. coli growth [74]. Colitic C57BL/6 mice treated with resveratrol also showed a decrease in the neutrophil percentage and T lymphocytes number expressing TNF-α and IFN-γ in the mesenteric lymph nodes and colonic lamina propria, as well as silent mating type information regulation-1 (SIRT-1) protein increase [75].
Interestingly, powdered grape-seed extract, produced from wine and grape-juice industrial by-products, having high concentration of procyanidins, was also able to reduce histological severity score in DSS-induced colitis in rats [76].
Studies on weaning piglets fed red wine pomace added to a standard diet have shown it to inhibit gut-associated lymphoid tissue activity, suggesting an immune preventive effect of wine phenolic compounds in these animals’ intestinal tract. These findings are important in view of attempts to counteract intestinal inflammation and diarrhea, as well as growth deficiency, which often occur in piglets during the weaning phase [77].
Beneficial effects of red wine extract (Rubesco, Rosso di Torgiano, Italy) were observed in male Sprague-Dawley rats fed a zinc-deficient diet, which induces severe intestinal morphological changes, oxidative damage and inflammation. The red-wine extract improved these morphological alterations and increased glutathione peroxidase, catalase and myeloperoxidase activities in the different parts of the small intestine. Further, increased mRNA levels of the anti-inflammatory IL-10 were detected in jejunum, as well as decreased expressions of cytokine-induced neutrophil chemoattractant and of TNFα [78]. Wine-complex polyphenols and tannins have been found to induce a significant decrease in oxidative DNA damage in colonocytes isolated from male Fisher 344 rats fed a high-fat diet, which mimics the typical diet of humans at high risk of colon cancer [79].
Human studies: influence of alcoholic and non-alcoholic fractions
Based on experimental data on the reduction of symptoms in animals with acute colitis, wine compounds have attracted major scientific interest for their potential applications as food supplements, in a physiological approach to treating IBD, with the possibility of altering the natural history of these chronic relapsing diseases. In particular, phenolic compounds may be of interest in preventing IBD and colitis-associated colorectal cancer development [58,80]. However, relatively few studies have concerned the effects of wine-related phenolics on human intestinal diseases, and those reported have yielded conflicting results. An important point on wine consumption is associated to alcohol intake. The most important and abundant alcohol in wine is ethanol, which is produced during grape fermentation, and concurs to characterizing the properties of wine.
Scientific communities in different countries agreed that “moderate” consumption of alcohol comprises about 15 g/day for women and 30 g/day for men; this corresponds to 1–3 glasses per day of wine containing 12–14% alcoholic fraction. The alcohol content depends on the sugar concentration in the grapes and on the yeast used; thus, the effects of different doses of ethanol on intestinal functions must be investigated in adequate depth.
Numerous studies have aimed to establish the effect of a moderate alcohol intake on human diseases. Unlike the effects induced by high consumption of ethanol in beverages, low concentrations of alcohol appear to protect diabetic and atherosclerotic patients against cardiovascular diseases, especially lowering the risk of ischemic coronary events. However, controversial results from epidemiological studies suggest caution in making any recommendations related to alcohol consumption [81].
Regarding the possible impact of ethanol on the gastrointestinal tract, the majority of studies have enrolled patients with sustained alcohol consumption, which affects the motility of the esophagus, stomach and small bowel, thus contributing to intestinal absorptive dysfunction. Alcohol׳s mechanism of action in intestinal dysmotility appears mainly to be due to its influence on brain–gut axis neurotransmission and on the muscular layers. High concentrations of ethanol induce sustained oxidative and inflammatory reactions. These events may contribute to motility disorders, as well as causing serious mucosal injury, which is considered high risk for colorectal cancer development [82,83].
However, short-term moderate social drinking in patients with inactive IBD was evaluated in a prospective cohort study, including Crohn׳s disease (CD) or Ulcerative Colitis (UC) subjects in clinical remission, who drank red wine daily for 7 days (1–3 glasses of red wine/day). This study showed that patients had delayed long-term risk for disease relapse; the study authors suggested that moderate red wine intake might be safe in inactive IBD. However, contradictory effects on stool calprotectin and intestinal permeability were found: a reduction in stool calprotectin was found in subjects with UC, but there was a significant disruption of the epithelial barrier in the small bowel of CD patients. The study authors hypothesized that ethanol exerted different actions depending on the type and intestinal location of the disease. Reduction in stool calprotectin might be due to the effects of alcohol, inhibiting the systemic immune system and neutrophils migration in UC; conversely, alcohol might be deleterious in those intestinal areas that are already weak, and thus more susceptible to the negative effects of alcohol. It remains difficult to distinguish whether certain positive properties are actually due to alcohol itself, or to other components in the wine [84].
Further, the role of exogenous sulfur dioxide present in wine in the form of hydrogen sulfide as a potential player in the etiology of intestinal disorders such as IBD and colorectal cancer has been stressed: in this study, 81 patients with UC were recruited in order to elucidate any association between individual dietary items and disease activity. In this case, wine consumption was associated to the presence of high doses of sulfites, which might be harmful for UC [85]. Hydrogen sulfide has recently been hypothesized to induce pro-inflammatory and genotoxic effects, for its interfering activity on microbial growth and colonocyte metabolism in butyrate oxidation [86]. A double-blind, placebo-controlled, randomized cross-over study was performed in healthy volunteers underwent over a period of 4 weeks a diet rich in polyphenols with grape juice or grape juice combined with wine polyphenol extract. Metabonomic analyses showed quantitative changes of microbiota, reflected as metabolic outcome in feces only in subjects who consumed the mixture of grape juice and wine extract with a reduction in isobutyrate, thus suggesting the role of polyphenols in the modulation of the microbial ecology of the gut [87].
In a crossover study with 20 patients with CD in remission, who consumed randomly different alcoholic drinks with moderate ethanol content, included red and white wine, and pure ethanol, any differences in alcohol absorption compared to healthy subjects were found. However, abdominal pain manifestations were detected in CD patients after consumption of Smirnoff Ice and Elephant beer with high glucose concentration. This suggests that fermentation could be responsible of intestinal bacteria overgrowth, thus playing a role in abdominal pain and intestinal damage in IBD [88].
The great discrepancy among data concerning the action of the alcohol contained in wine strengthens the notion that attention should be paid in discriminating between the effects of alcohol and those of other wine components.
Conclusions
In Western countries, consumers׳ demand for foods or food supplements that help to prevent or manage diseases is continuously increasing. Evidence strongly supports the hypothesis that moderate wine consumption can prolong healthy life, and might also possess preventive and/or therapeutic value in different human inflammatory diseases. The beneficial activity of wine, especially that of red wine, mainly arises from its high content of phenolic compounds (graphical abstract); these can act directly, as free radical scavengers, or indirectly, by interfering with specific inflammation-related genes involved in cellular redox signaling pathways. With regard to maintaining intestinal functions, wine phenolics reach much higher concentrations in the intestinal mucosa than in other tissues; they are thus strongly implicated in safeguarding the oxidative/antioxidant balance of the intestinal epithelial layer, concurring to increase intracellular levels of antioxidants. In addition, most wine phenolics primarily target the activity of the redox sensitive transcription factor NF-κB, which controls the inflammatory cell signaling cascades that have been implicated in the pathogenesis of IBD and colorectal cancer development.
The importance of the changes in the colonic bacterial population induced by wine phenolic compounds acting as prebiotics has recently been stressed. The use of natural wine phenolics, by restoring the microbial balance between detrimental and protective luminal bacteria, might thus prevent inflammatory and oxidative reactions, reducing mucosal injury in IBD. The influence on intestinal diseases of the ethanolic wine fraction has also been investigated: alcohol has long been known for its bactericidal effects, and has been recently thought to positively modulate inflammatory and immune responses, at least in low concentrations. However, most of wine׳s antioxidant and anti-inflammatory capacity does not arise from its alcohol content, but rather from its polyphenol content.
There is increasing pharmacological interest in identifying and characterizing the natural compounds present in wine, which can protect intestinal redox homeostasis and guard against inflammation. Several studies on intestinal cell lines or on experimental animal models have shown that wine compounds are able to reduce colonic injury.
However, knowledge on the beneficial effect of wine in human intestinal diseases is not sufficient and is still limited because of the small number of clinical trials. Furthermore, any such benefit must be weighed against the unfavorable effects of the corresponding consumption of ethanol.
Future perspectives thus require additional studies in humans in order to reinforce the experimental evidence concerning the beneficial effect of wine consumption on intestinal function, and to correlate the right quantity of its specific compounds intake with positive health outcomes.
Acknowledgments
The authors thank the Italian Ministry for the University, PRIN 2009, 2009M8FKBB_005, the CRT Foundation, Turin, 2013.1044, Banco di Sardegna Foundation, 2153/2011.1088, and the University of Turin, Italy, D85E12001730005, for supporting this work. The authors state that there is not conflict of interest in the present paper.
References
- 1.Rodrigo R., Miranda A., Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clinica Chimica Acta. 2011;412:410–424. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart D.C., Dignass A.U. Intestinal barrier function. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:685–694. doi: 10.1097/00075197-200211000-00012. 12394645 [DOI] [PubMed] [Google Scholar]
- 3.Biasi F., Leonarduzzi G., Oteiza P.I., Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxidants & Redox Signaling. 2013;19:1711–1747. doi: 10.1089/ars.2012.4530. 23305298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covas M.I., Gambert P., Fitó M., de la Torre R. Wine and oxidative stress: up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis. 2010;208:297–304. doi: 10.1016/j.atherosclerosis.2009.06.031. 19660752 [DOI] [PubMed] [Google Scholar]
- 5.Barril C., Clark A.C., Scollary G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Analytica Chimica Acta. 2012;732:186–193. doi: 10.1016/j.aca.2011.11.011. 22688051 [DOI] [PubMed] [Google Scholar]
- 6.Patel K.K., Stappenbeck T.S. Autophagy and intestinal homeostasis. Annual Review of Physiology. 2013;75:241–262. doi: 10.1146/annurev-physiol-030212-183658. 23216414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonarduzzi G., Sottero B., Testa G., Biasi F., Poli G. New insights into redox-modulated cell signaling. Current Pharmaceutical Design. 2011;17:3994–4006. doi: 10.2174/138161211798764906. 22188450 [DOI] [PubMed] [Google Scholar]
- 8.Pasparakis M. Role of NF-κB in epithelial biology. Immunological Reviews. 2012;246:346–358. doi: 10.1111/j.1600-065X.2012.01109.x. 22435565 [DOI] [PubMed] [Google Scholar]
- 9.Circu M.L., Aw T.Y. Intestinal redox biology and oxidative stress. Seminars in Cell & Developmental Biology. 2012;23:729–737. doi: 10.1016/j.semcdb.2012.03.014. 22484611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterhouse A.L. Wine phenolics. Annals of the New York Academy of Sciences. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. 12074959 [DOI] [PubMed] [Google Scholar]
- 11.Stockley C., Teissedre P.L., Boban M., Di Lorenzo C., Restani P. Bioavailability of wine-derived phenolic compounds in humans: a review. Food & Function. 2012;3:995–1007. doi: 10.1039/c2fo10208k. 22728778 [DOI] [PubMed] [Google Scholar]
- 12.Pelsy F. Molecular and cellular mechanisms of diversity within grapevine varieties. Heredity. 2010;104:331–340. doi: 10.1038/hdy.2009.161. 19935824 [DOI] [PubMed] [Google Scholar]
- 13.Chira K., Lorrain B., Ky I., Teissedre P.L. Tannin composition of cabernet-sauvignon and merlot grapes from the Bordeaux area for different vintages (2006–2009) and comparison to tannin profile of five 2009 vintage Mediterranean grapes varieties. Molecules (Basel, Switzerland) 2011;16:1519–1532. doi: 10.3390/molecules16021519. 21317842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B., Neves A.C., Fernandes T.A., Fernandes A.L., Mateus N., De Freitas V., Leandro C., Spranger M.I. Evolution of phenolic composition of red wine during vinification and storage and its contribution to wine sensory properties and antioxidant activity. Journal of Agricultural and Food Chemistry. 2011;59:6550–6557. doi: 10.1021/jf201383e. 21561162 [DOI] [PubMed] [Google Scholar]
- 15.De Simón B.F., Hernández T., Cadahía E., Dueñas M., Estrella I. Phenolic compounds in a Spanish red wine aged in barrels made of Spanish, French and American oak wood. European Food Research and Technology. 2003;216:150–156. [Google Scholar]
- 16.Michel J., Jourdes M., Silva M.A., Giordanengo T., Mourey N., Teissedre P.L. Impact of concentration of ellagitannins in oak wood on their levels and organoleptic influence in red wine. Journal of Agricultural and Food Chemistry. 2011;59:5677–5683. doi: 10.1021/jf200275w. 21480590 [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw M.P., Barril C., Clark A.C., Prenzler P.D., Scollary G.R. Ascorbic acid: a review of its chemistry and reactivity in relation to a wine environment. Critical Reviews in Food Science and Nutrition. 2011;51:479–498. doi: 10.1080/10408391003690559. 21929328 [DOI] [PubMed] [Google Scholar]
- 18.Sarakbi A., Aydogmus Z., Dago A., Mertens D., Dewert J.Y., Kauffmann J.M. Determination of aminothiols by liquid chromatography with amperometric detection at a silver electrode: application to white wines. Analytica Chimica Acta. 2013;786:22–28. doi: 10.1016/j.aca.2013.04.070. 23790287 [DOI] [PubMed] [Google Scholar]
- 19.Kritzinger E.C., Bauer F.F., du Toit W.J. Role of glutathione in winemaking: a review. Journal of Agricultural and Food Chemistry. 2013;61:269–277. doi: 10.1021/jf303665z. 23240621 [DOI] [PubMed] [Google Scholar]
- 20.Cejudo-Bastante M.J., Pérez-Coello M.S., Hermosín-Gutiérrez I. Identification of new derivatives of 2-S-glutathionylcaftaric acid in aged white wines by HPLC-DAD-ESI-MS(n.) Journal of Agricultural and Food Chemistry. 2010;58:11483–11492. doi: 10.1021/jf102920q. 20942401 [DOI] [PubMed] [Google Scholar]
- 21.Marchand S., de Revel G. A HPLC fluorescence-based method for glutathione derivatives quantification in must and wine. Analytica Chimica Acta. 2010;660:158–163. doi: 10.1016/j.aca.2009.09.042. 20103157 [DOI] [PubMed] [Google Scholar]
- 22.Sonni F., Clark A.C., Prenzler P.D., Riponi C., Scollary G.R. Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. Journal of Agricultural and Food Chemistry. 2011;59:3940–3949. doi: 10.1021/jf104575w. 21384873 [DOI] [PubMed] [Google Scholar]
- 23.Lavigne V., Pons A., Dubourdieu D. Assay of glutathione in must and wines using capillary electrophoresis and laser-induced fluorescence detection. Changes in concentration in dry white wines during alcoholic fermentation and aging. Journal of Chromatography A. 2007;1139:130–135. doi: 10.1016/j.chroma.2006.10.083. 17125780 [DOI] [PubMed] [Google Scholar]
- 24.Winter G., Henschke P.A., Higgins V.J., Ugliano M., Curtin C.D. Effects of rehydration nutrients on H2S metabolism and formation of volatile sulfur compounds by the wine yeast VL3. AMB Express. 2011;2:36. doi: 10.1186/2191-0855-1-36. 22044590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannini C., Scazzocchio B., Matarrese P., Varì R., D’Archivio M., Di Benedetto R., Casciani S., Dessì M.R., Straface E., Malorni W., Masella R. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: Protective effects of phenolic compounds. Journal of Nutritional Biochemistry. 2008;19:118–128. doi: 10.1016/j.jnutbio.2007.01.010. 17588737 [DOI] [PubMed] [Google Scholar]
- 26.Noguer M.A., Cerezo A.B., Donoso Navarro E., Garcia-Parrilla M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacological Research: The Official Journal of the Italian Pharmacological Society. 2012;65:609–614. doi: 10.1016/j.phrs.2012.03.003. 22484523 [DOI] [PubMed] [Google Scholar]
- 27.Noguer M., Cerezo A.B., Rentzsch M., Winterhalter P., Troncoso A.M., García-Parrilla M.C. Simulated digestion and antioxidant activity of red wine fractions separated by high speed countercurrent chromatography. Journal of Agricultural and Food Chemistry. 2008;56:8879–8884. doi: 10.1021/jf8007376. 18778068 [DOI] [PubMed] [Google Scholar]
- 28.Barnes S., Prasain J., D’Alessandro T., Arabshahi A., Botting N., Lila M.A., Jackson G., Janle E.M., Weaver C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food & Function. 2011;2:235–244. doi: 10.1039/c1fo10025d. 21779561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Product Reports. 2009;26:1001–1043. doi: 10.1039/b802662a. 19636448 [DOI] [PubMed] [Google Scholar]
- 30.Stalmach A., Edwards C.A., Wightman J.D., Crozier A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food & Function. 2013;4:52–62. doi: 10.1039/c2fo30151b. [DOI] [PubMed] [Google Scholar]
- 31.Monagas M., Quintanilla-López J.E., Gómez-Cordovés C., Bartolomé B., Lebrón-Aguilar R. MALDI-TOF MS analysis of plant proanthocyanidins. Journal of Pharmaceutical and Biomedical Analysis. 2010;51:358–372. doi: 10.1016/j.jpba.2009.03.035. 19410413 [DOI] [PubMed] [Google Scholar]
- 32.Borges G., Roowi S., Rouanet J.M., Duthie G.G., Lean M.E., Crozier A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Molecular Nutrition & Food Research. 2007;51:714–725. doi: 10.1002/mnfr.200700024. 17533654 [DOI] [PubMed] [Google Scholar]
- 33.Espín J.C., González-Barrio R., Cerdá B., López-Bote C., Rey A.I., Tomás-Barberán F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. Journal of Agricultural and Food Chemistry. 2007;55:10476–10485. doi: 10.1021/jf0723864. 17990850 [DOI] [PubMed] [Google Scholar]
- 34.Cerdá B., Periago P., Espín J.C., Tomás-Barberán F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. Journal of Agricultural and Food Chemistry. 2005;53:5571–5576. doi: 10.1021/jf050384i. 15998116 [DOI] [PubMed] [Google Scholar]
- 35.McDougall G.J., Fyffe S., Dobson P., Stewart D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry. 2005;66:2540–2548. doi: 10.1016/j.phytochem.2005.09.003. 16242736 [DOI] [PubMed] [Google Scholar]
- 36.Matuschek M.C., Hendriks W.H., McGhie T.K., Reynolds G.W. The jejunum is the main site of absorption for anthocyanins in mice. Journal of Nutritional Biochemistry. 2006;17:31–36. doi: 10.1016/j.jnutbio.2005.04.005. 16098729 [DOI] [PubMed] [Google Scholar]
- 37.Talavéra S., Felgines C., Texier O., Besson C., Lamaison J.L., Rémésy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. Journal of Nutrition. 2003;133:4178–4182. doi: 10.1093/jn/133.12.4178. 14652368 [DOI] [PubMed] [Google Scholar]
- 38.Aura A.M., Martin-Lopez P., O’Leary K.A., Williamson G. in vitro metabolism of anthocyanins by human gut microflora. European Journal of Nutrition. 2005;44:133–142. doi: 10.1007/s00394-004-0502-2. 15309431 [DOI] [PubMed] [Google Scholar]
- 39.Forester S.C., Waterhouse A.L. Identification of Cabernet Sauvignon anthocyanin gut microflora metabolites. Journal of Agricultural and Food Chemistry. 2008;56:9299–9304. doi: 10.1021/jf801309n. 18767860 [DOI] [PubMed] [Google Scholar]
- 40.Dragoni S., Gee J., Bennett R., Valoti M., Sgaragli G. Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro. British Journal of Pharmacology. 2006;147:765–771. doi: 10.1038/sj.bjp.0706662. 16444288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deiana M., Loru D., Incani A., Rosa A., Atzeri A., Melis M.P., Cabboi B., Hollecker L., Pinna M.B., Argiolas F., Murru M., Dessì M.A. Wine extracts from Sardinian grape varieties attenuate membrane oxidative damage in Caco-2 cell monolayers. Food Chemistry. 2012;134:2105–2113. doi: 10.1016/j.foodchem.2012.04.014. 23442662 [DOI] [PubMed] [Google Scholar]
- 42.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. 10917926 [DOI] [PubMed] [Google Scholar]
- 43.Starp C., Alteheld B., Stehle P. Characteristics of (+)-catechin and (−)-epicatechin transport across pig intestinal brush border membranes. Annals of Nutrition & Metabolism. 2006;50:59–65. doi: 10.1159/000089640. 16282679 [DOI] [PubMed] [Google Scholar]
- 44.Takagaki A., Nanjo F. Catabolism of (+)-catechin and (−)-epicatechin by rat intestinal microbiota. Journal of Agricultural and Food Chemistry. 2013;61:4927–4935. doi: 10.1021/jf304431v. 23621128 [DOI] [PubMed] [Google Scholar]
- 45.Tabasco R., Sánchez-Patán F., Monagas M., Bartolomé B., Victoria Moreno-Arribas M., Peláez C., Requena T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiology. 2011;28:1345–1352. doi: 10.1016/j.fm.2011.06.005. 21839384 [DOI] [PubMed] [Google Scholar]
- 46.Dolara P., Luceri C., De Filippo C., Femia A.P., Giovannelli L., Caderni G., Cecchini C., Silvi S., Orpianesi C., Cresci A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutation Research. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. 16293270 [DOI] [PubMed] [Google Scholar]
- 47.Gross G., Jacobs D.M., Peters S., Possemiers S., van Duynhoven J., Vaughan E.E., van de Wiele T. in vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. Journal of Agricultural and Food Chemistry. 2010;58:10236–10246. doi: 10.1021/jf101475m. 20726519 [DOI] [PubMed] [Google Scholar]
- 48.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poultry Science. 2011;90:566–578. doi: 10.3382/ps.2010-00889. 21325227 [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez Vaquero M.J., Manca de Nadra M.C. Growth parameter and viability modifications of Escherichia coli by phenolic compounds and Argentine wine extracts. Applied Biochemistry and Biotechnology. 2008;151:342–352. doi: 10.1007/s12010-008-8197-0. 18594776 [DOI] [PubMed] [Google Scholar]
- 50.Radovanović A.N., Jovančićević B.S., Radovanović B.C., Mihajilov-Krstev T., Zvezdanović J.B. Antioxidant and antimicrobial potentials of Serbian red wines produced from international Vitis vinifera grape varieties. Journal of the Science of Food and Agriculture. 2012;15:2154–2161. doi: 10.1002/jsfa.5601. [DOI] [PubMed] [Google Scholar]
- 51.Zhou C., Zhao J., Li J., Wang H., Tang C. Acute ethanol administration inhibits Toll-like receptor 4 signaling pathway in rat intestinal epithelia. Alcohol (Fayetteville, N.Y.) 2013;47:231–239. doi: 10.1016/j.alcohol.2013.01.003. 23428594 [DOI] [PubMed] [Google Scholar]
- 52.Boban N., Tonkic M., Budimir D., Modun D., Sutlovic D., Punda-Polic V., Boban M. Antimicrobial effects of wine: separating the role of polyphenols, pH, ethanol, and other wine components. Journal of Food Science. 2010;75:M322–M326. doi: 10.1111/j.1750-3841.2010.01622.x. 20629891 [DOI] [PubMed] [Google Scholar]
- 53.Ko E.A., Jin B.J., Namkung W., Ma T., Thiagarajah J.R., Verkman A.S. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut. 2014;63:1120–1129. doi: 10.1136/gutjnl-2013-305663. 24052273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levantesi G., Marfisi R., Mozaffarian D., Franzosi M.G., Maggioni A., Nicolosi G.L., Schweiger C., Silletta M., Tavazzi L., Tognoni G., Marchioli R. Wine consumption and risk of cardiovascular events after myocardial infarction: results from the GISSI-Prevenzione trial. International Journal of Cardiology. 2013;163:282–287. doi: 10.1016/j.ijcard.2011.06.053. 21737162 [DOI] [PubMed] [Google Scholar]
- 55.Farchi G., Fidanza F., Giampaoli S., Mariotti S., Menotti A. Alcohol and survival in the Italian rural cohorts of the Seven Countries Study. International Journal of Epidemiology. 2000;29:667–671. doi: 10.1093/ije/29.4.667. 10922343 [DOI] [PubMed] [Google Scholar]
- 56.Nadtochiy S.M., Redman E.K. Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition (Burbank, los Angeles County, Calif.) 2011;27:733–744. doi: 10.1016/j.nut.2010.12.006. 21454053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical Pharmacology. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. 16920072 [DOI] [PubMed] [Google Scholar]
- 58.Biasi F., Astegiano M., Maina M., Leonarduzzi G., Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Current Medicinal Chemistry. 2011;18:4851–4865. doi: 10.2174/092986711797535263. 21919842 [DOI] [PubMed] [Google Scholar]
- 59.Shukla Y., Singh R. Resveratrol and cellular mechanisms of cancer prevention. Annals of the New York Academy of Sciences. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. 21261635 [DOI] [PubMed] [Google Scholar]
- 60.González-Sarrías A., Gromek S., Niesen D., Seeram N.P., Henry G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. Journal of Agricultural and Food Chemistry. 2011;59:8632–8638. doi: 10.1021/jf201561e. 21761862 [DOI] [PubMed] [Google Scholar]
- 61.Panaro M.A., Carofiglio V., Acquafredda A., Cavallo P., Cianciulli A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. British Journal of Nutrition. 2012;108:1623–1632. doi: 10.1017/S0007114511007227. 22251620 [DOI] [PubMed] [Google Scholar]
- 62.Garcia P., Schmiedlin-Ren P., Mathias J.S., Tang H., Christman G.M., Zimmermann E.M. Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302:G326–G335. doi: 10.1152/ajpgi.00083.2011. 22052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. European Journal of Nutrition. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. 22042007 [DOI] [PubMed] [Google Scholar]
- 64.Laurent C., Besançon P., Auger C., Rouanet J.M., Caporiccio B. Grape seed extract affects proliferation and differentiation of human intestinal Caco-2 cells. Journal of Agricultural and Food Chemistry. 2004;52:3301–3308. doi: 10.1021/jf035231e. 15161187 [DOI] [PubMed] [Google Scholar]
- 65.Nunes C., Ferreira E., Freitas V., Almeida L., Barbosa R.M., Laranjinha J. Intestinal anti-inflammatory activity of red wine extract: unveiling the mechanisms in colonic epithelial cells. Food & Function. 2013;4:373–383. doi: 10.1039/c2fo30233k. 23233037 [DOI] [PubMed] [Google Scholar]
- 66.Angel-Morales G., Noratto G., Mertens-Talcott S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: potential role of microRNA-126. Food & Function. 2012;3:745–752. doi: 10.1039/c2fo10271d. 22572890 [DOI] [PubMed] [Google Scholar]
- 67.Gessner D.K., Ringseis R., Siebers M., Keller J., Kloster J., Wen G., Eder K. Inhibition of the pro-inflammatory NF-κB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. Journal of Animal Physiology and Animal Nutrition. 2012;96:1074–1083. doi: 10.1111/j.1439-0396.2011.01222.x. 21895782 [DOI] [PubMed] [Google Scholar]
- 68.Biasi F., Guina T., Maina M., Cabboi B., Deiana M., Tuberoso C.I., Calfapietra S., Chiarpotto E., Sottero B., Gamba P., Gargiulo S., Brunetto V., Testa G., Dessì M.A., Poli G., Leonarduzzi G. Phenolic compounds present in Sardinian wine extracts protect against the production of inflammatory cytokines induced by oxysterols in CaCo-2 human enterocyte-like cells. Biochemical Pharmacology. 2013;86:138–145. doi: 10.1016/j.bcp.2013.03.024. 23583258 [DOI] [PubMed] [Google Scholar]
- 69.Tian L., Wang H., Abdallah A.M., Prinyawiwatkul W., Xu Z. Red and white wines inhibit cholesterol oxidation induced by free radicals. Journal of Agricultural and Food Chemistry. 2011;59:6453–6458. doi: 10.1021/jf200544r. 21563753 [DOI] [PubMed] [Google Scholar]
- 70.Pal S., Ho S.S., Takechi R. Red wine Polyphenolics suppress the secretion of ApoB48 from human intestinal CaCo-2 cells. Journal of Agricultural and Food Chemistry. 2005;53:2767–2772. doi: 10.1021/jf048309f. 15796623 [DOI] [PubMed] [Google Scholar]
- 71.Nicod N., Chiva-Blanch G., Giordano E., Dávalos A., Parker R.S., Visioli F. Green tea, cocoa, and red wine polyphenols moderately modulate intestinal inflammation and do not increase high-density lipoprotein (HDL) production. Journal of Agricultural and Food Chemistry. 2014;62:2228–2232. doi: 10.1021/jf500348u. 24559192 [DOI] [PubMed] [Google Scholar]
- 72.Asai K., Buurman W.A., Reutelingsperger C.P., Schutte B., Kaminishi M. Low concentrations of ethanol induce apoptosis in human intestinal cells. Scandinavian Journal of Gastroenterology. 2003;38:1154–1161. doi: 10.1080/00365520310006252. 14686719 [DOI] [PubMed] [Google Scholar]
- 73.Lemos C., Peters G.J., Jansen G., Martel F., Calhau C. Modulation of folate uptake in cultured human colon adenocarcinoma Caco-2 cells by dietary compounds. European Journal of Nutrition. 2007;46:329–336. doi: 10.1007/s00394-007-0670-y. 17712586 [DOI] [PubMed] [Google Scholar]
- 74.Larrosa M., Yañéz-Gascón M.J., Selma M.V., González-Sarrías A., Toti S., Cerón J.J., Tomás-Barberán F., Dolara P., Espín J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. Journal of Agricultural and Food Chemistry. 2009;57:2211–2220. doi: 10.1021/jf803638d. 19228061 [DOI] [PubMed] [Google Scholar]
- 75.Singh U.P., Singh N.P., Singh B., Hofseth L.J., Price R.L., Nagarkatti M., Nagarkatti P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. Journal of Pharmacology and Experimental Therapeutics. 2010;332:829–839. doi: 10.1124/jpet.109.160838. 19940103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheah K.Y., Bastian S.E., Acott T.M., Abimosleh S.M., Lymn K.A., Howarth G.S. Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Digestive Diseases and Sciences. 2013;58:970–977. doi: 10.1007/s10620-012-2464-1. 23143736 [DOI] [PubMed] [Google Scholar]
- 77.Sehm J., Lindermayer H., Dummer C., Treutter D., Pfaffl M.W. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. Journal of Animal Physiology and Animal Nutrition. 2007;91:289–296. doi: 10.1111/j.1439-0396.2006.00650.x. 17614999 [DOI] [PubMed] [Google Scholar]
- 78.Canali R., Vignolini F., Nobili F., Mengheri E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radical Biology & Medicine. 2000;28:1661–1670. doi: 10.1016/s0891-5849(00)00285-9. 10938463 [DOI] [PubMed] [Google Scholar]
- 79.Giovannelli L., Testa G., De Filippo C., Cheynier V., Clifford M.N., Dolara P. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. European Journal of Nutrition. 2000;39:207–212. doi: 10.1007/s003940070013. 11131367 [DOI] [PubMed] [Google Scholar]
- 80.O’Connor P.M., Lapointe T.K., Beck P.L., Buret A.G. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflammatory Bowel Diseases. 2010;16:1411–1420. doi: 10.1002/ibd.21217. 20155848 [DOI] [PubMed] [Google Scholar]
- 81.Chiva-Blanch G., Arranz S., Lamuela-Raventos R.M., Estruch R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: evidences from human studies. Alcohol and Alcoholism (Oxford, Oxfordshire) 2013;48:270–277. doi: 10.1093/alcalc/agt007. 23408240 [DOI] [PubMed] [Google Scholar]
- 82.Stermer E. Alcohol consumption and the gastrointestinal tract. Israel Medical Association Journal. 2002;4:200–202. 11908263 [PubMed] [Google Scholar]
- 83.Siegmund S., Spanagel R., Singer M.V. Role of the brain–gut axis in alcohol-related gastrointestinal diseases—what can we learn from new animal models? Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society. 2003;54:191–207. 15075460 [PubMed] [Google Scholar]
- 84.Swanson G.R., Tieu V., Shaikh M., Forsyth C., Keshavarzian A. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion. 2011;84:238–244. doi: 10.1159/000329403. 21876358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magee E.A., Edmond L.M., Tasker S.M., Kong S.C., Curno R., Cummings J.H. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutrition Journal. 2005;4–7:7. doi: 10.1186/1475-2891-4-7. 15705205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Frontiers in Physiology. 2012;3:448. doi: 10.3389/fphys.2012.00448. 23226130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs D.M., Deltimple N., van Velzen E., van Dorsten F.A., Bingham M., Vaughan E.E., van Duynhoven J. (1)H NMR metabolite profiling of feces as a tool to assess the impact of nutrition on the human microbiome. NMR in Biomedicine. 2008;21:615–626. doi: 10.1002/nbm.1233. 18085514 [DOI] [PubMed] [Google Scholar]
- 88.Hey H., Schmedes A., Nielsen A.A., Winding P., Grønbaek H. Effects of five different alcoholic drinks on patients with Crohn׳s disease. Scandinavian Journal of Gastroenterology. 2007;42:968–972. doi: 10.1080/00365520701452241. 17613927 [DOI] [PubMed] [Google Scholar]


