Abstract
It has been recently shown that mammalian spermatozoa were hyperactivated by steroids, amines and amino acids. In the present study, we investigated whether hyperactivation of hamster sperm is regulated by progesterone (P) and γ-aminobutyric acid (GABA). Although sperm hyperactivation was enhanced by P, GABA significantly suppressed P-enhanced hyperactivation in a dose-dependent manner. Suppression of P-enhanced hyperactivation by GABA was significantly inhibited by an antagonist of the GABAA receptor (bicuculline). Moreover, P bound to the sperm head, and this binding was decreased by GABA. Because the concentrations of GABA and P change in association with the estrous cycle, these results suggest that GABA and P competitively regulate the enhancement of hyperactivation through the GABAA receptor.
Keywords: γ-aminobutyric acid (GABA), Hyperactivation, Progesterone, Spermatozoa
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter in the mammalian central nervous system, and is formed in the brain by α-decarboxylation of L-glutamate. It exerts its effects through three types of membrane receptor: GABAA, GABAB and GABAC. The GABAA and GABAC receptors are ligand-gated ion channels. The former is a multi-subunit receptor complex linked to the Cl- channel, while the latter has not been studied as extensively [1]. The GABAB receptor is a G-protein-coupled receptor linked to the K+ and Ca2+ channels [2].
Mammalian spermatozoa must undergo a period of physiological preparation in the female reproductive tract in order to acquire the ability to fertilize an egg [3, 4]. These changes are referred as “capacitation”. During capacitation, spermatozoa show several modifications; for example, a change in the fluidity of the spermatic plasma membrane by the removal of cholesterol, tyrosine phosphorylation of spermatic proteins, hyperactivation of the flagellar movement, and the acrosome reaction (AR) in the head of the sperm [5, 6]. The removal of cholesterol is induced by albumin [7], and the proteins are phosphorylated at a tyrosine residue in a time-dependent manner [6, 8,9,10]. Hyperactivation is the specialized movement of the sperm’s flagellum that creates the propulsive force necessary for penetration of the zona pellucida (ZP). The flagella of hyperactivated spermatozoa exhibit an asymmetrical beating pattern with a large amplitude [5, 11, 12]. The AR is a modified exocytosis of the acrosome and is also required for penetration of the ZP of the egg and subsequent sperm-egg plasma membrane fusion [5].
It was recently reported that hyperactivation is enhanced by several ligands [6]. In human spermatozoa, progesterone (P) and melatonin regulate and/or increase hyperactivation [13, 14]. In hamster spermatozoa, P enhances hyperactivation in a dose-dependent manner, as do serotonin and melatonin [15,16,17]. Moreover, P-enhanced hyperactivation is competitively suppressed by 17β-estradiol (E) [18]. In rat spermatozoa, P and GABA increase hyperactivation through the GABAA receptor/Cl- channel [19].
In humans, rams and rats, GABA appears to increase sperm hyperactivation via the GABAA receptor/Cl- channel [19,20,21,22]. Because the GABAA receptor is the Cl- channel, it is thought that hyperactivation is increased through activation of the Cl- channel and hyperpolarization by Cl- influx [19, 20]. Interestingly, it has been suggested that GABA has the same effect as P on hyperactivation in humans and rats [19, 20]. Moreover, the increased hyperactivation of rat sperm by P is inhibited by a GABAA receptor antagonist [19]. Although the GABAB receptor also exists in rat spermatozoa and is localized in the sperm head [23,24,25], it is unclear whether the GABAB receptor is involved in regulation of hyperactivation.
In the present study, we examined whether GABA enhances and/or increases sperm hyperactivation in hamsters because hyperactivation is enhanced by P in this species [16, 18].
Materials and Methods
Chemicals
Fluorescein isothiocyanate (FITC) and bovine serum albumin (BSA) conjugated progesterone (FITC/BSA-P), GABA, bicuculline, P and RU486 (11β-(4-dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one, mifepristone) were purchased from Sigma-Aldrich (St Louis, MO, USA), BSA fraction V was purchased from Merck KGaA (Darmstadt, Germany), and all other reagent-grade chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of hyperactivated spermatozoa
Spermatozoa were obtained from the caudal epididymis of sexually mature male golden hamsters (Mesocricetus auratus). The experimental protocols were approved by the Animal Care and Use Committee of the Dokkyo Medical University, and the experiment was carried out under the control of the Guidelines for Animal Experimentation in the Dokkyo Medical University.
Hyperactivated spermatozoa were prepared according to the method described previously [10] using a modified Tyrode’s albumin lactate pyruvate (mTALP) medium containing 101.02 mM NaCl, 2.68 mM KCl, 2 mM CaCl2, 1.5 mM MgCl2-6H2O, 0.36 mM NaH2PO4-2H2O, 35.70 mM NaHCO3, 4.5 mM D-glucose, 0.09 mM sodium pyruvate, 9 mM sodium lactate, 0.5 mM hypotaurine, 0.05 mM (-)epinephrine, 0.2 mM sodium taurocholate, 5.26 μM sodium metabisulfite, 0.05% (w/v) streptomycin sulfate, 0.05% (w/v) potassium penicillin G and 15 mg/ml BSA (pH 7.4 at 37 C under 5% (v/v) CO2 in air). An aliquot (~ 5 μl) of caudal epididymal spermatozoa was placed in a 35-mm dish (culture plate), and 3 ml of mTALP medium were carefully added before incubation for 5 min to allow the spermatozoa to swim up. The supernatant containing motile spermatozoa was collected, placed in a culture plate and incubated for 4 h at 37 C under 5% CO2 in air to accomplish hyperactivation. P dissolved in ethanol, GABA or antagonists were added to the medium after placing motile spermatozoa on the culture plate. In all experiments, the maximal concentration of vehicle was 0.1% by volume.
Measurement of the motility and hyperactivation of spermatozoa
Motility and hyperactivation measurements were performed according to the method of Fujinoki et al. (2006) [10] with some modifications. Motile spermatozoa suspended in the mTALP medium were recorded on VHS via a CCD camera (Progressive 3CCD, Sony, Tokyo, Japan) attached to a microscope (IX70, Olympus, Tokyo, Japan) with phase-contrast illumination and a small CO2 incubator (MI-IBC, Olympus). Each observation was performed at 37 C, recorded for 2 min, and analyzed by manually counting the numbers of total spermatozoa, motile spermatozoa and hyperactivated spermatozoa in 10 different fields. Motile spermatozoa that exhibited asymmetric and whiplash flagellar movements [11] and a circular and/or octagonal swimming locus were defined as hyperactivated. Motile spermatozoa (%) and hyperactivated spermatozoa (%) were defined as the number of motile spermatozoa/number of total spermatozoa × 100 and as the number of hyperactivated spermatozoa/number of total spermatozoa × 100, respectively. Experiments were performed four times using four hamsters.
Statistical analysis was carried out using Tukey’s post hoc analysis of variance test. P < 0.05 was considered significant.
Ligand assays of GABA and P
Ligand assays of GABA and P were performed according to the method described previously [16, 18]. An aliquot (~ 5 μl) of caudal epididymal spermatozoa was placed in the bottom of 35-mm dish (culture plate), and 3 ml of mTALP medium with 12.6 nM FITC/BSA-P, which is converted into approximately 20 ng/ml P, were carefully added after spermatozoa were exposed to 0.1% ethanol (as vehicle) or other chemicals for 5 min. The supernatant containing motile spermatozoa was collected, placed on a culture dish and incubated for 5 min at 37 C under 5% (v/v) CO2 in air. After incubation, several microliters of the supernatant were placed on a glass slide without fluorescence and observed using a CCD camera (LucaEM-R 604, AndorTM Technology, Belfast, UK) attached to a light microscope (IX70, Olympus) with phase-contrast illumination and a fluorescence unit.
Results
Effects of GABA and GABAA receptor on sperm hyperactivation
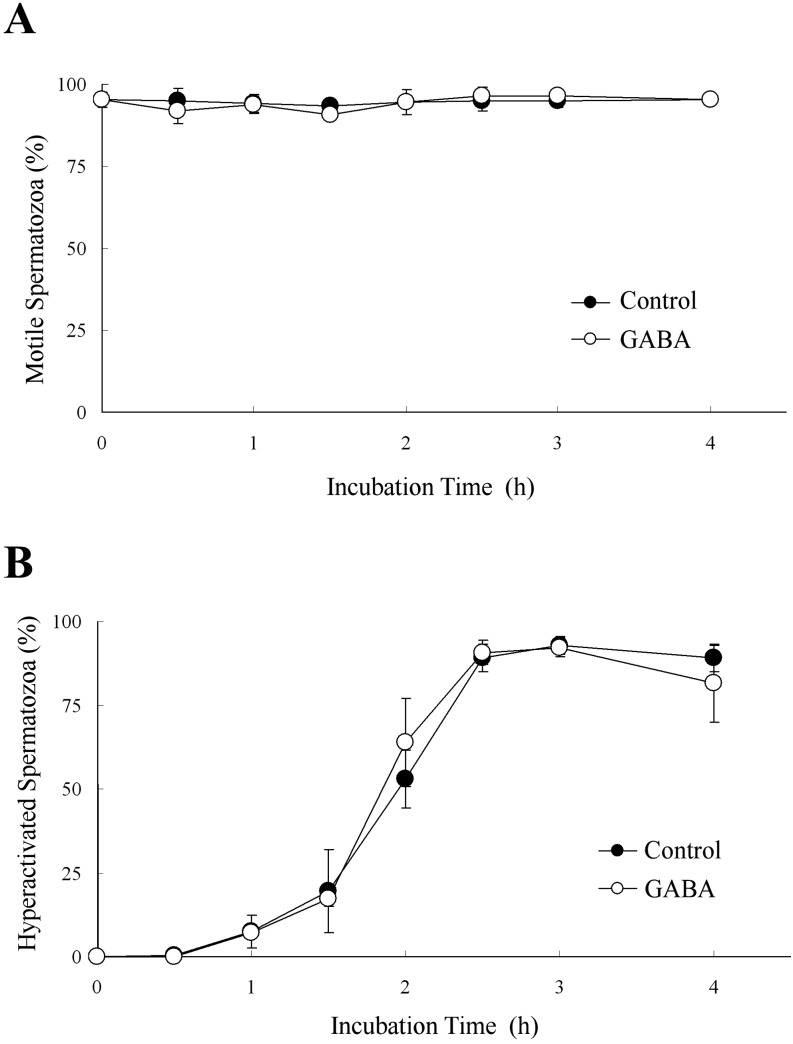
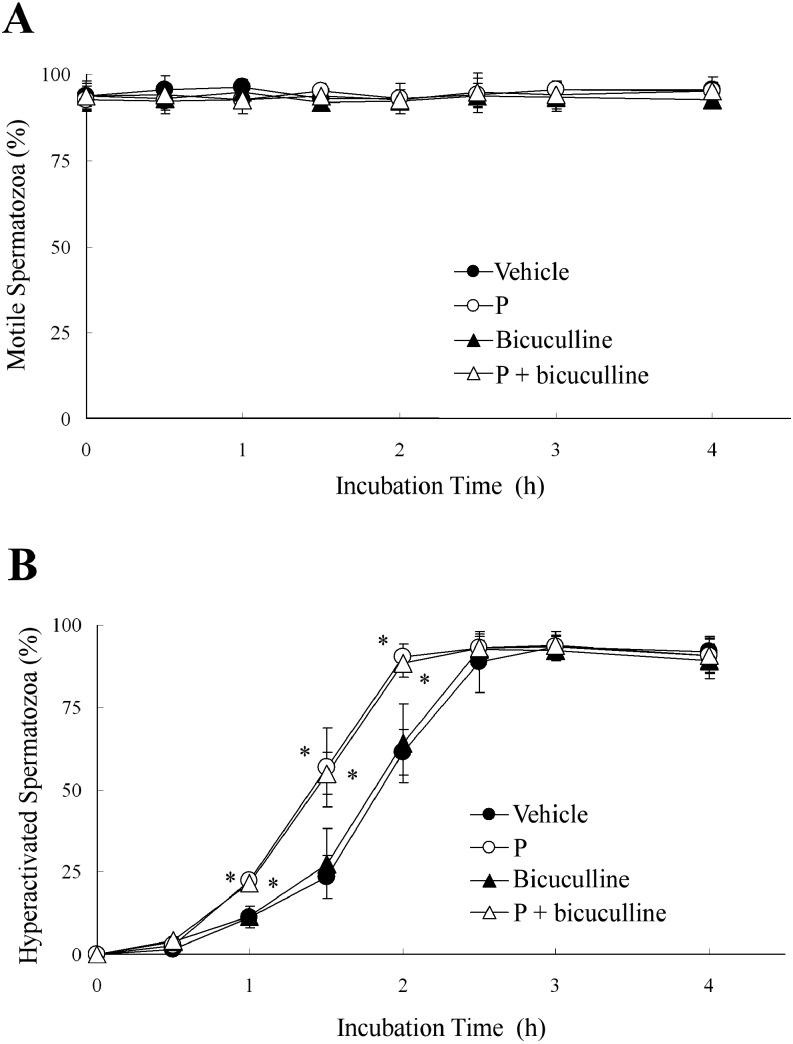
When hamster spermatozoa were exposed to 5 μM GABA, which increased rat sperm hyperactivation [19], GABA did not affect the percentages of motile or hyperactivated spermatozoa (Fig. 1). Moreover, 1 μM bicuculline, which is a GABAA receptor antagonist (IC50 value is 1.2 μM [26]), also did not affect these percentages when hamster spermatozoa were exposed to bicuculline before exposure to 20 ng/ml P (Fig. 2). Therefore, none of the effects reported in previous studies [19, 20] was observed for hamster spermatozoa.
Fig. 1.
Effects of γ-aminobutyric acid (GABA) on sperm hyperactivation. The percentages of motile spermatozoa (A) and hyperactivated spermatozoa (B) are shown when 5 μM GABA were added to the mTALP medium. Data are expressed as means ± SD. Control, mTALP; GABA, Control + 5 μM GABA.
Fig. 2.
Effects of an antagonist of the γ-aminobutyric acid (GABA) A receptor (GABAA receptor) on progesterone (P)-enhanced hyperactivation. The percentages of motile spermatozoa (A) and hyperactivated spermatozoa (B) are shown when spermatozoa were exposed to 20 ng/ml P after exposure to 1 μM bicuculline (an antagonist of the GABAA receptor) for 5 min. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; Bicuculline,Vehicle + 1 μM bicuculline; P + bicuculline, Vehicle + 20 ng/ml P + 1 μM bicuculline. *Significant difference compared with Vehicle (P < 0.05).
Effects of GABA on P-enhanced hyperactivation
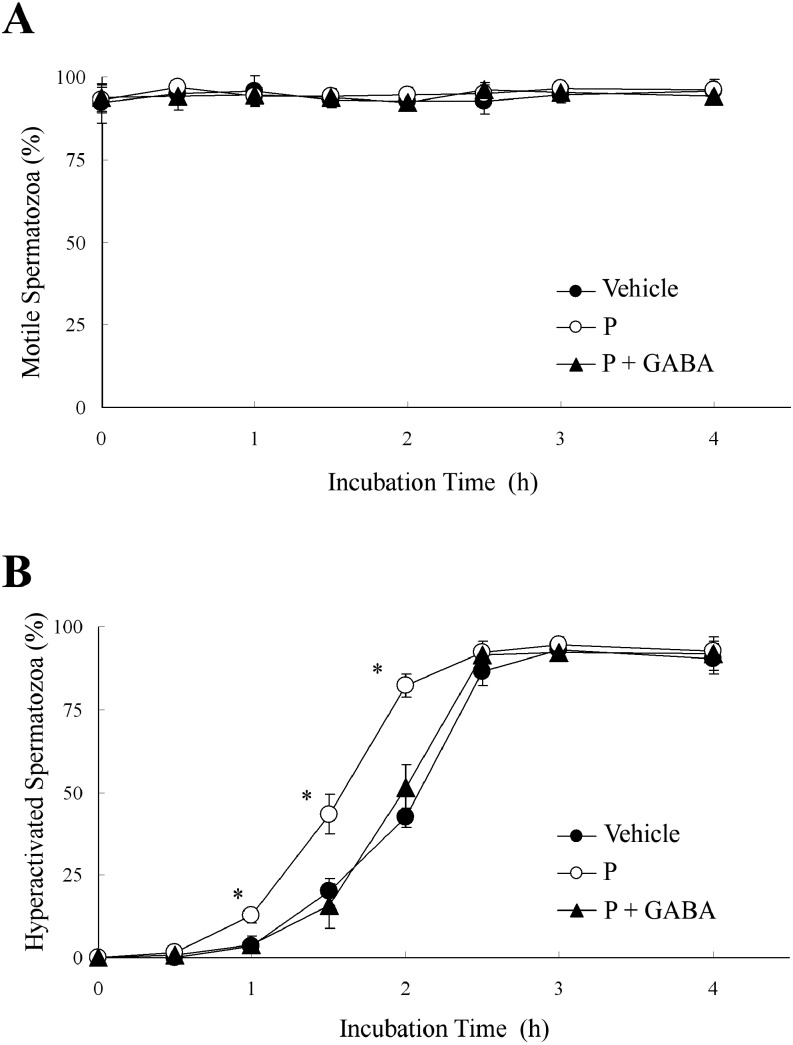
Next, we examined whether GABA suppressed P-enhanced hyperactivation similarly to E [18] because GABA is generally an inhibitory neurotransmitter. As shown in Fig. 3, 5 μM GABA significantly suppressed P-enhanced hyperactivation when hamster spermatozoa were exposed to 20 ng/ml P after exposure to GABA, although GABA did not affect the percentage of motile spermatozoa.
Fig. 3.
Effects of γ-aminobutyric acid (GABA) on progesterone (P)-enhanced hyperactivation. The percentages of motile spermatozoa (A) and hyperactivated spermatozoa (B) are shown when spermatozoa were exposed to 20 ng/ml P after exposure to 5 μM GABA for 5 min. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; P + GABA, Vehicle + 20 ng/ml P + 5 μM GABA. *Significant difference compared with Vehicle (P < 0.05).
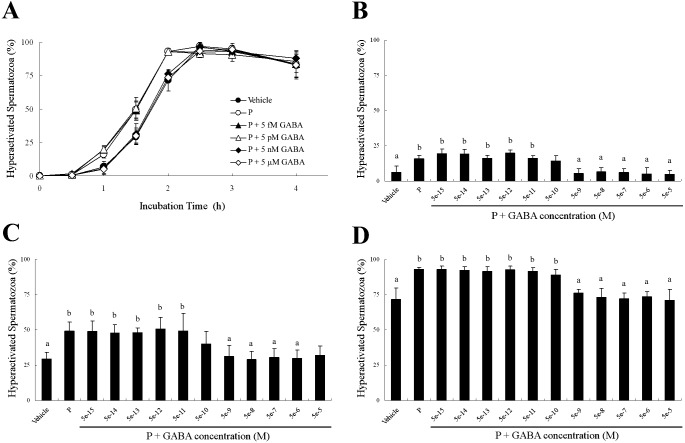
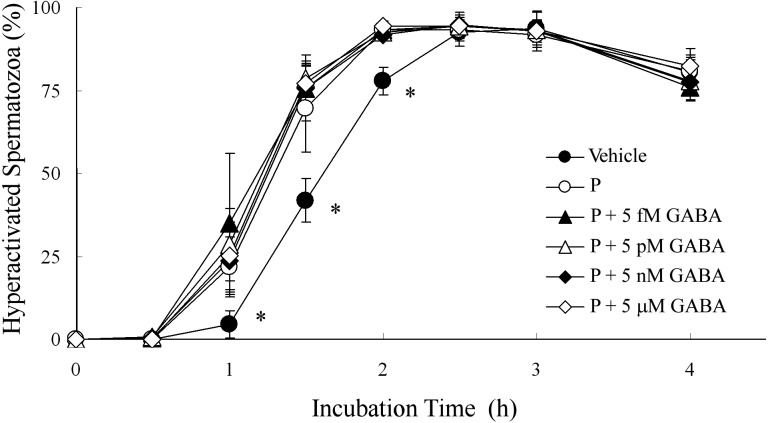
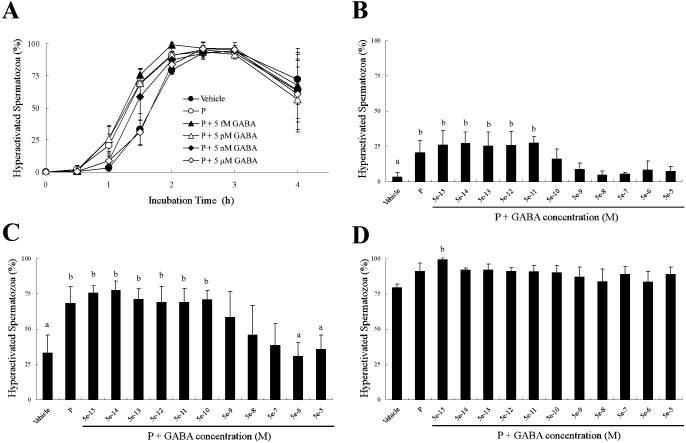
Because 5 μM GABA significantly suppressed P-enhanced hyperactivation (Fig. 3), we examined the dose-dependent effects of GABA for P-enhanced hyperactivation (Figs. 4–6). When spermatozoa were exposed to 20 ng/ml P after exposure to GABA, as shown in Fig. 4, P-enhanced hyperactivation was significantly suppressed by GABA in a dose-dependent manner. After incubation for 1 h, 5 nM to 50 μM GABA significantly suppressed P-enhanced hyperactivation, although the effect of 500 pM GABA was not significantly different from that of the vehicle or P (Fig. 4B). After incubation for 1.5 h, 5 nM to 5 μM GABA significantly suppressed P-enhanced hyperactivation, although the effects of 500 pM or 50 μM GABA were not significantly different compared with the vehicle and P (Fig. 4C). After incubation for 2 h, 5 nM to 50 μM GABA significantly suppressed P-enhanced hyperactivation (Fig. 4D).
Fig. 4.
Dose-dependent effects of γ-aminobutyric acid (GABA) on progesterone (P)-enhanced hyperactivation when spermatozoa were exposed to P after exposure to GABA for 5 min. The percentages of hyperactivated spermatozoa are shown (A) as an overview of the effects after incubation for (B) 1, (C) 1.5 and (D) 2 h. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; P + 5 fM or 5e-15,: Vehicle + 20 ng/ml P + 5 fM GABA; 5e-14, Vehicle + 20 ng/ml P + 50 fM GABA; 5e-13, Vehicle + 20 ng/ml P + 500 fM GABA; P + 5 pM or 5e-12, Vehicle + 20 ng/ml P + 5 pM GABA; 5e-11, Vehicle + 20 ng/ml P + 50 pM GABA; 5e-10, Vehicle + 20 ng/ml P + 500 pM GABA; P + 5 nM or 5e-9, Vehicle + 20 ng/ml P + 5 nM GABA; 5e-8, Vehicle + 20 ng/ml P + 50 nM GABA; 5e-7, Vehicle + 20 ng/ml P + 500 nM GABA; P + 5 μM or 5e-6, Vehicle + 20 ng/ml P + 5 μM GABA; 5e-5, Vehicle + 20 ng/ml P + 50 μM GABA. a Significant difference compared with P (P < 0.05). b Significant difference compared with Vehicle (P < 0.05).
Fig. 6.
Dose-dependent effects of γ-aminobutyric acid (GABA) on progesterone (P)-enhanced hyperactivation when spermatozoa were exposed to P for 5 min before exposure to GABA. The percentage of hyperactivated spermatozoa is shown. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; P + 5 fM, Vehicle + 20 ng/ml P + 5 fM GABA; P + 5 pM, Vehicle + 20 ng/ml P + 5 pM GABA; P + 5 nM, Vehicle + 20 ng/ml P + 5 nM GABA; P + 5 μM, Vehicle + 20 ng/ml P + 5 μM GABA. *Significant difference compared with P (P < 0.05).
When spermatozoa were simultaneously exposed to 20 ng/ml P and GABA, P-enhanced hyperactivation was significantly suppressed by GABA in a dose-dependent manner (Fig. 5). After incubation for 1 h, 500 pM to 50 μM GABA weakly suppressed P-enhanced hyperactivation, but the effects were not significantly different compared with the vehicle and P (Fig. 5B). After incubation for 1.5 h, 5 μM and 50 μM GABA significantly suppressed P-enhanced hyperactivation, although the effects of 5 nM to 500 nM GABA were not significantly different compared with the vehicle and P (Fig. 5C). After incubation for 2 h, GABA did not suppress P-enhanced hyperactivation (Fig. 5D).
Fig. 5.
Dose-dependent effects of γ-aminobutyric acid (GABA) on progesterone (P)-enhanced hyperactivation when spermatozoa were exposed to P and GABA at the same time. The percentages of hyperactivated spermatozoa are shown (A) as an overview of the effects after incubation for (B) 1, (C) 1.5 and (D) 2 h. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; P + 5 fM or 5e-15, Vehicle + 20 ng/ml P + 5 fM GABA; 5e-14, Vehicle + 20 ng/ml P + 50 fM GABA; 5e-13, Vehicle + 20 ng/ml P + 500 fM GABA; P + 5 pM or 5e-12, Vehicle + 20 ng/ml P + 5 pM GABA; 5e-11, Vehicle + 20 ng/ml P + 50 pM GABA; 5e-10, Vehicle + 20 ng/ml P + 500 pM GABA; P + 5 nM or 5e-9, Vehicle + 20 ng/ml P + 5 nM GABA; 5e-8, Vehicle + 20 ng/ml P + 50 nM GABA; 5e-7, Vehicle + 20 ng/ml P + 500 nM GABA; P + 5 μM or 5e-6, Vehicle + 20 ng/ml P + 5 μM GABA; 5e-5, Vehicle + 20 ng/ml P + 50 μM GABA. a Significant difference compared with P (P < 0.05). b Significant difference compared with Vehicle (P < 0.05).
When spermatozoa were exposed to 20 ng/ml P for 5 min before exposure to GABA, GABA did not suppress P-enhanced hyperactivation at all (Fig. 6).
Suppression of P-enhanced hyperactivation by GABA through the GABAA receptor
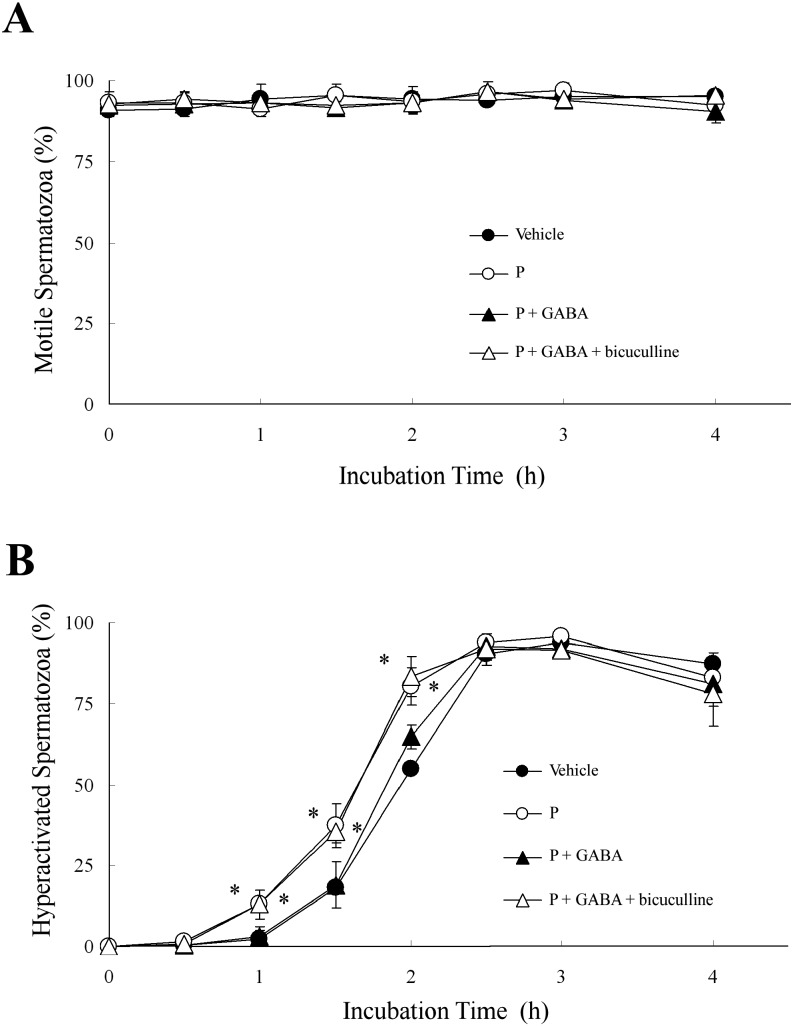
As shown in Fig. 7, we examined whether GABA suppressed P-enhanced hyperactivation through the GABAA receptor. As shown in Fig. 2, bicuculline did not affect the percentages of motile or hyperactivated spermatozoa with or without P. When hamster spermatozoa were exposed to 1 μM bicuculline before exposure to 5 μM GABA and 20 ng/ml P, bicuculline significantly inhibited the suppression of P-enhanced hyperactivation by GABA (Fig. 7B) but did not affect the percentage of motile spermatozoa (Fig. 7A).
Fig. 7.
Effects of bicuculline on progesterone (P)-enhanced hyperactivation. The percentages of motile spermatozoa (A) and hyperactivated spermatozoa (B) are shown when 1 μM bicuculline, 5 μM γ-aminobutyric acid (GABA) and 20 ng/ml P were added to the mTALP medium. Spermatozoa were exposed to 5 μM GABA after exposure to 1 μM bicuculline for 5 min. After incubation for 5 min, spermatozoa were exposed to 20 ng/ml P. Data are expressed as means ± SD. Vehicle, mTALP + 0.1% (v/v) ethanol; P, Vehicle + 20 ng/ml P; P + GABA, Vehicle + 20 ng/ml P + 5 μM GABA; P + GABA + bicuculline, Vehicle + 20 ng/ml P + 5 μM GABA + 1 μM bicuculline. *Significant difference compared with Vehicle (P < 0.05).
Effects of GABA on binding of P on spermatozoa
Previous studies have shown that P binds to the head of the sperm [16, 18], so in the next step of our study, we examined the effects of GABA on the binding of P to the sperm head (Fig. 8).
Fig. 8.
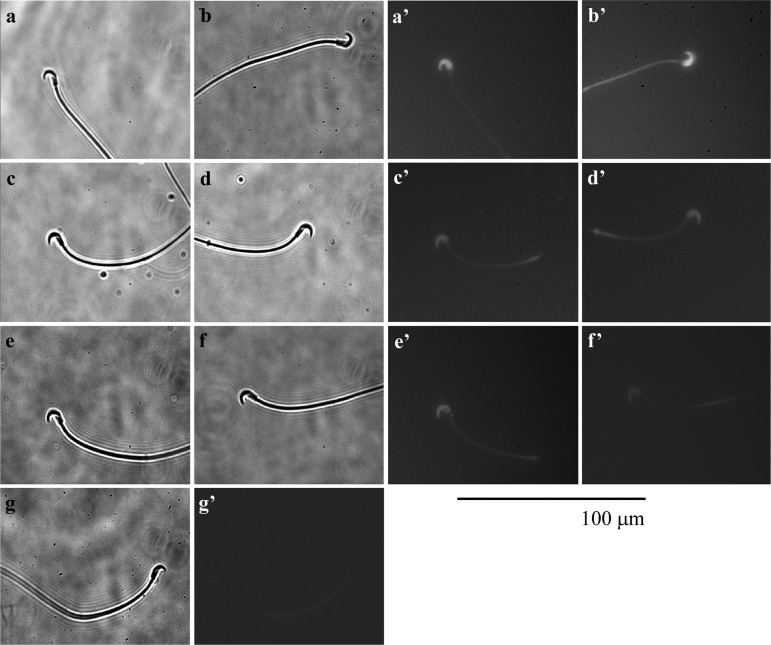
Effects of γ-aminobutyric acid (GABA) on binding of progesterone (P) to the sperm head. (a, a’, b, b’) Hamster spermatozoon incubated in mTALP medium with 12.6 nM FITC/BSA-P, which converted into approximately 20 ng/ml P, and 0.1% ethanol. (c, c’, d, d’) Hamster spermatozoon incubated in the medium with 12.6 nM FITC/BSA-P after being incubated in the medium with 23.4 μM RU486 and 0.1% ethanol for 5 min. (e, e’, f, f’) Hamster spermatozoon incubated in the medium with 12.6 nM FITC/BSA-P after being incubated in the medium with 5 μM GABA and 0.1% ethanol for 5 min. (g, g’) Hamster spermatozoon incubated in the medium with vehicle. (a–g) Observed under light field; (a’–g’) observed under fluorescent field. Fluorescence of the mitochondria sheath in the flagellum was autofluorescence. Bar=100 μm.
As shown in Fig. 8a, 8a’, 8b and 8b’, FITC/BSA-P is clearly bound to the sperm head, although the weak fluorescence of the middle piece of the sperm flagellum appears to be autofluorescence (Fig. 8g, 8g’). When spermatozoa were exposed to FITC/BSA-P after exposure to 23.4 μM RU486, which is an antagonist of the P receptor (PR), the binding of FITC/BSA-P to the sperm head was decreased (Fig. 8c, 8c’, 8d, 8d’). Moreover, GABA also decreased the binding of FITC/BSA-P on the sperm head when spermatozoa were exposed to FITC/BSA-P after exposure to 5 μM GABA (Fig. 8e, 8e’, 8f, 8f’).
Discussion
Recent studies in the hamster have shown that sperm hyperactivation is enhanced by P, and that P-enhanced hyperactivation is competitively suppressed by E [16, 18]. In the present study, we showed that GABA also competitively suppressed P-enhanced hyperactivation of hamster spermatozoa through the GABAA receptor, similarly to E (Figs. 3–7). In humans and rats, on the other hand, it has been reported that GABA increases sperm hyperactivation through the GABAA receptor, similarly to P [19, 20]. The effect of P in human and rat spermatozoa is also mediated through the GABAA receptor [19, 20], whereas the effect of P in hamster spermatozoa is not related to the GABAA receptor (Fig. 2). Because the effect of P in hamster spermatozoa is mediated through the PR [16], we consider that our result is reasonable. In previous studies describing human and rat spermatozoa [19, 20], it was proposed that GABA and P increased sperm hyperactivation through activation of the Cl- channel and membrane hyperpolarization by Cl- influx, but neither study showed clear evidence, and there has been no debate about a signal pathway stimulated by both GABA and P. Moreover, the concentration of bicuculline used in those experiments [19, 20] was higher than the IC50 value (1.2 μM) [26]. At least, motility parameters and capacitation of rat spermatozoa were significantly suppressed by 10 μM bicuculline alone [19]. Because bicuculline alone also blocks Ca-activated potassium channels in addition to being a GABAA receptor antagonist and the IC50 value is 1–2 μM [27], we considered that the effects of 10 μM bicuculline alone on rat spermatozoa were not so much the effects of a GABAA receptor antagonist as other effects such as inhibition of Ca-activated potassium channels or toxic effects. Because 1 μM bicuculline did not have any effects on sperm hyperactivation and P-enhanced hyperactivation (Fig. 2), we used 1 μM bicuculline in the present study. We were subsequently able to observe effects of bicuculline as a GABAA receptor antagonist (Figs. 2 and 7). From results obtained in the present study and our previous study [16], it was suggested that GABA and P regulate sperm hyperactivation through the GABAA receptor and PR, respectively.
Generally, P regulates hyperactivation through two Ca2+ signals via the PR [6, 12]; one is the extracellular Ca2+ influx [16] associated with the CatSper, which is a voltage-dependent Ca2+ channel located in the principal piece of the flagellum [28, 29], and the other signal releases intracellular Ca2+ from an inositol 1,4,5-tris-phosphate receptor-gated Ca2+ store located at the base of the flagellum [12, 16, 30]. Furthermore, it has been shown that Ca2+ oscillation induced by P occurs on the flagellar side of the sperm head and extends to the sperm head and flagellum [31]. After Ca2+ oscillation, hyperactivation is also regulated through activation of protein kinase C, protein kinase A and calmodulin-dependent protein kinase [30, 32]. Activation of these kinases induces protein phosphorylations, especially tyrosine phosphorylations [5, 6, 8, 9]. At the very least, P enhances hamster sperm hyperactivation through both Ca2+ signals and protein phosphorylations [5, 6, 8, 9, 12, 16, 30]. Moreover, it has been suggested that GABA induces the AR and capacitation in human, ram, rat, guinea pig and bull spermatozoa [19, 21, 23, 33,34,35,36]. Although in these species it is suggested that the effects of GABA on the AR and capacitation are the same as those of P, the regulatory mechanisms vary. In bull [36], ram [21] and rat spermatozoa [19, 33], GABA increases the AR and capacitation through a GABAA receptor, whereas another study reported that in rat spermatozoa, GABA stimulates the AR through the GABAA receptor but inhibits it through the GABAB receptor [23]. In human spermatozoa, it has been suggested that both the GABAA and GABAB receptors block stimulation of the AR by follicular fluid [34]. In guinea pig spermatozoa, GABA regulates the AR through activation of phospholipase A2, protein kinase C and the mitogen-activated protein kinase cascade [35]. Therefore, it is not possible to propose a universal GABA signal pathway regulating sperm function.
In the present study using hamster spermatozoa, GABA suppressed P-enhanced hyperactivation through the GABAA receptor/Cl- channel (Fig. 7). As for the regulatory mechanism of suppression of P-enhanced hyperactivation by GABA, we can assume two types. One is suppression of P-enhanced hyperactivation by GABA through inactivation of the CatSper via hyperpolarization by the GABAA receptor/Cl- channel, because it has been suggested that P regulates hyperactivation through Ca2+ influx associated with the CatSper and intracellular Ca2+ release associated with phospholipase C [6, 16, 28,29,30]. The other type of regulatory mechanism of the suppression of P-enhanced hyperactivation by GABA is inhibition of the binding of P to the PR in the sperm head. Although we could not examine the first assumption, we examined the second and observed that GABA suppressed the binding of P to the sperm head, similarly to RU486 (Fig. 8). Therefore, it is likely that suppression of P-enhanced hyperactivation by GABA occurs through a decrease in the binding of P to the PR in the sperm head. But the mechanism regulating the suppression of the binding of P to sperm head is still unclear.
In the rat, GABA is found in the oviduct at over 2.5-fold the amount present in the brain [37]. Moreover, the concentration of GABA changes in the female genital tract of the rat with the estrous cycle [38]. The concentrations of P and E also change with the estrous cycle. From the results of both the present study (Figs. 3–6) and previous studies [16, 18], we propose that P increases and enhances hyperactivation and that E and GABA suppress the effects of P on hyperactivation in the hamster at least. Moreover, it seems that regulation of hyperactivation by P, E and GABA is associated with the estrous cycle.
References
- 1.Chebib M, Johnston GA. GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem 2000; 43: 1427–1447 [DOI] [PubMed] [Google Scholar]
- 2.Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci 1989; 10: 401–407 [DOI] [PubMed] [Google Scholar]
- 3.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951; 168: 697–698 [DOI] [PubMed] [Google Scholar]
- 4.Austin CR. The capacitation of the mammalian sperm. Nature 1952; 170: 326 [DOI] [PubMed] [Google Scholar]
- 5.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (ed.), The Physiology of Reproduction Vol. 2, 2nd ed. New York: Raven Press; 1994: 189-317.
- 6.Fujinoki M. Non-genomic regulation of mammalian sperm hyperactivation. Reprod Med Biol 2009; 8: 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langlais J, Roberts KD. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 1985; 12: 183–224 [Google Scholar]
- 8.Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59: 1–6 [DOI] [PubMed] [Google Scholar]
- 9. Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS. The molecular basis of sperm capacitation. J Androl 1998; 19: 242–248 [PubMed] [Google Scholar]
- 10.Fujinoki M, Suzuki T, Takayama T, Shibahara H, Ohtake H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation via activation on hamster spermatozoa. Reprod Med Biol 2006; 5: 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujinoki M, Ohtake H, Okuno M. Serine phosphorylation of flagellar proteins associated with the motility activation of hamster spermatozoa. Biomed Res 2001; 22: 45–58 [Google Scholar]
- 12.Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Domest Anim 2003; 38: 119–124 [DOI] [PubMed] [Google Scholar]
- 13.du Plessis SS, Hagenaar K, Lampiao F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010; 42: 112–116 [DOI] [PubMed] [Google Scholar]
- 14.Armon L, Eisenbach M. Behavioral mechanism during human sperm chemotaxis: involvement of hyperactivation. PLoS ONE 2011; 6: e28359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujinoki M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008; 136: 533–541 [DOI] [PubMed] [Google Scholar]
- 16.Noguchi T, Fujinoki M, Kitazawa M, Inaba N. Regulation of hyperactivation of hamster spermatozoa by progesterone. Reprod Med Biol 2008; 7: 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujinoki M. Serotonin-enhanced hyperactivation of hamster sperm. Reproduction 2011; 142: 255–266 [DOI] [PubMed] [Google Scholar]
- 18.Fujinoki M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by estrogen. Reproduction 2010; 140: 453–464 [DOI] [PubMed] [Google Scholar]
- 19.Jin J-Y, Chen W-Y, Zhou CX, Chen Z-H, Yu-Ying Y, Ni Y, Chan HC, Shi Q-X. Activation of GABAA receptor/Cl- channel and capacitation in rat spermatozoa: HCO3- and Cl- are essential. Syst Biol Reprod Med 2009; 55: 97–108 [DOI] [PubMed] [Google Scholar]
- 20.Calogero AE, Hall J, Fishel S, Green S, Hunter A, D’Agata R. Effects of γ-aminobutyric acid on human sperm motility and hyperactivation. Mol Hum Reprod 1996; 2: 733–738 [DOI] [PubMed] [Google Scholar]
- 21.de las Heras MA, Valcarcel A, Perez LJ. In vitro capacitating effect of gamma-aminobutyric acid in ram spermatozoa. Biol Reprod 1997; 56: 964–968 [DOI] [PubMed] [Google Scholar]
- 22.Ritta MN, Calamera JC, Bas DE. Occurrence of GABA and GABA receptors in human spermatozoa. Mol Hum Reprod 1998; 4: 769–773 [DOI] [PubMed] [Google Scholar]
- 23.Hu JH, He XB, Wu Q, Yan YC, Koide SS. Biphasic effect of GABA on rat sperm acrosome reaction: involvement of GABA(A) and GABA(B) receptors. Arch Androl 2002; 48: 369–378 [DOI] [PubMed] [Google Scholar]
- 24.He X, Zhang Y, Yan Y, Li Y, Koide SS. Identification of GABABR2 in rat testis and sperm. J Reprod Dev 2003; 49: 397–402 [DOI] [PubMed] [Google Scholar]
- 25.Kanbara K, Okamoto K, Nomura S, Kaneko T, Shigemoto R, Azuma H, Katsuoka Y, Watanabe M. Cellular localization of GABA and GABAB receptor subunit proteins during spermiogenesis in rat testis. J Androl 2005; 26: 485–493 [DOI] [PubMed] [Google Scholar]
- 26.Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci 2003; 23: 11158–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pfügers Arch–Eur J Physiol 1999; 438: 314–321 [DOI] [PubMed] [Google Scholar]
- 28.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011; 471: 387–391 [DOI] [PubMed] [Google Scholar]
- 29.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011; 471: 382–386 [DOI] [PubMed] [Google Scholar]
- 30.Fujinoki M. Progesterone-enhanced sperm hyperactivation through IP3-PKC and PKA signals. Reprod Med Biol 2013; 12: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol 2003; 161: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ignotz GG, Suarez SS. Calcium/calmodulin and calmodulin kinase II stimulate hyperactivation in demembranated bovine sperm. Biol Reprod 2005; 73: 519–526 [DOI] [PubMed] [Google Scholar]
- 33.Hu JH, He XB, Wu Q, Yan YC, Koide SS. Subunit composition and function of GABAA receptors of rat spermatozoa. Neurochem Res 2002; 27: 195–199 [DOI] [PubMed] [Google Scholar]
- 34.Burrello N, Vicari E, D’Amico L, Satta A, D’Agata R, Calogero AE. Human follicular fluid stimulates the sperm acrosome reaction by interacting with the γ-aminobutyric acid receptors. Fertil Steril 2004; 82(Suppl 3): 1086–1090 [DOI] [PubMed] [Google Scholar]
- 35.Chen WY, Ni Y, Pan YM, Shi QX, Yuan YY, Chen AJ, Mao LZ, Yu SQ, Roldan ERS. GABA, progesterone and zona pellucida activation of PLA2 and regulation by MEK-ERK1/2 during acrosomal exocytosis in guinea pig spermatozoa. FEBS Lett 2005; 579: 4692–4700 [DOI] [PubMed] [Google Scholar]
- 36.Puente MA, Tartaglione CM, Ritta MN. Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci 2011; 127: 31–37 [DOI] [PubMed] [Google Scholar]
- 37.Martín del Rio R. γ-aminobutyric acid system in rat oviduct. J Biol Chem 1981; 256: 9816–9819 [PubMed] [Google Scholar]
- 38.Louzan P, Gallardo MGP, Tramezzani JH. Gamma-aminobutyric acid in the genital tract of the rat during the oestrous cycle. J Reprod Fertil 1986; 77: 499–504 [DOI] [PubMed] [Google Scholar]