Abstract
The development and regeneration of the pancreas is of considerable interest because of the role of these processes in pancreatic diseases, such as diabetes. Here, we sought to develop a large animal model in which the pancreatic cell lineage could be tracked. The pancreatic and duodenal homeobox-1 (Pdx1) gene promoter was conjugated to Venus, a green fluorescent protein, and introduced into 370 in vitro-matured porcine oocytes by intracytoplasmic sperm injection-mediated gene transfer. These oocytes were transferred into four recipient gilts, all of which became pregnant. Three gilts were sacrificed at 47–65 days of gestation, and the fourth was allowed to farrow. Seven of 16 fetuses obtained were transgenic (Tg) and exhibited pancreas-specific green fluorescence. The fourth recipient gilt produced a litter of six piglets, two of which were Tg. The founder Tg offspring matured normally and produced healthy first-generation (G1) progeny. A postweaning autopsy of four 27-day-old G1 Tg piglets confirmed the pancreas-specific Venus expression. Immunostaining of the pancreatic tissue indicated the transgene was expressed in β-cells. Pancreatic islets from Tg pigs were transplanted under the renal capsules of NOD/SCID mice and expressed fluorescence up to one month after transplantation. Tg G1 pigs developed normally and had blood glucose levels within the normal range. Insulin levels before and after sexual maturity were within normal ranges, as were other blood biochemistry parameters, indicating that pancreatic function was normal. We conclude that Pdx1-Venus Tg pigs represent a large animal model suitable for research on pancreatic development/regeneration and diabetes.
Keywords: ICSI-mediated gene transfer, Pancreas generation, Pdx1, Transgenic pig, Venus
The development and utilization of genetically modified pigs have contributed to the expansion of many biomedical research efforts. For example, genetically modified pigs serving as disease models for retinitis pigmentosa [1], diabetes [2, 3], and cystic fibrosis [4, 5] have been produced, and these models are expected to further the development of new drugs and treatment methods. In xenotransplantation research, α1,3-galactosyltransferase gene knockout pigs and pigs carrying human complement regulatory factor genes have been produced (for review, see [6, 7]) and are now being used in preclinical experiments, such as the transplantation of swine organs to monkeys. Additionally, pigs expressing fluorescent proteins are exceptionally useful in research on topics such as cell tracking [8, 9] and tissue regeneration [10]. Even more innovative and more widely applicable research results are likely to be obtained through the future use of genetically modified pigs. The aim of our research was to produce transgenic (Tg) pigs to advance research on pancreas generation.
Overcoming diabetes is a global challenge for modern society; thus, the production of Tg pigs that can be used to understand the mechanisms underlying pancreatic development and the control of pancreatic functions is of great value. We therefore embarked on a program to produce Tg pigs that express the Venus variant of green fluorescent protein (GFP) [11] under the control of the pancreatic duodenal homeobox-1 (Pdx1) gene promoter. Pdx1 functions as a master gene that induces the differentiation of β-cells from pancreatic stem cells, and research on Pdx1-positive cells is important for understanding the development of the pancreas and β-cell differentiation [12, 13]. The study of Pdx1-positive pancreatic stem cells may also lead to improved pathophysiological analysis and the development of treatments [13].
Methods for producing Tg pigs include pronuclear DNA injection [14], somatic cell nuclear transfer [15] and intracytoplasmic sperm injection-mediated gene transfer (ICSI-MGT) [16]. We chose ICSI-MGT for the present study, having previously confirmed that ICSI-MGT to in vitro-matured (IVM) porcine oocytes enables the production of Tg pigs with a high potential for reproducibility [2 , 17].
In the present study, we first produced several Tg pig fetuses by ICSI-MGT to confirm that the transferred Pdx1-Venus gene was expressed exclusively in the pancreas. Then, we produced Tg pigs and examined their progeny to determine whether the genes were transmitted to the succeeding generation, confirming the reproducibility of the pattern of pancreas-specific expression. We further transplanted pancreatic islets of the Pdx1-Venus Tg pig to immunodeficient mice to verify their in vivo traceability. The utility of the Pdx1-Venus Tg pigs as a model for research on pancreatic development is discussed.
Materials and Methods
Animal care
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Meiji University (IACUC-07-0005).
Chemicals
All chemicals were purchased from the Sigma Aldrich Chemical (St. Louis, MO, USA) unless otherwise indicated.
Construction of the Pdx1-Venus transgene
The Pdx1-Venus transgene construct (8.4 kb) consisted of the mouse Pdx1 promoter, Venus cDNA, and rabbit β-globin gene sequence (from partway through the second exon to the 3’ untranslated region), including a polyadenylation signal (pA) (Fig. 1). The transgene fragment was excised from the plasmid vector by enzymatic digestion using the BssHII restriction enzyme (Takara Bio, Shiga, Japan), separated by gel electrophoresis, and purified using the QIAquick® Gel Extraction Kit (QIAGEN, Hilden, Germany).
Fig. 1.
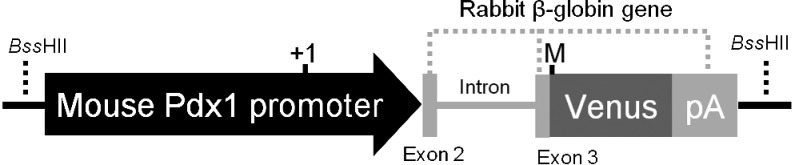
Structure of an expression vector for the Pdx1-Venus cDNA. A schematic presentation of the Pdx1-Venus transgene used to generate transgenic pigs. The fusion gene (8.4 kb) consists of 6.5 kb of the mouse Pdx1 promoter and a rabbit β-globin gene including an insertion of 0.72 kb Venus cDNA in the 3rd exon and a polyadenylation signal in the 3′ –flanking region. Transcription and translation start site are indicated by +1 and M, respectively.
In vitro maturation of oocytes
Porcine ovaries were collected at a local abattoir and transported to the laboratory in Dulbecco’s phosphate buffered saline (DPBS, Nissui Pharmaceutical, Tokyo, Japan) containing 75 μg/ml potassium penicillin G, 50 μg/ml streptomycin sulfate, 2.5 μg/ml amphotericin B, and 0.1% (w/v) polyvinyl alcohol (PVA). Cumulus-oocyte complexes were collected from the ovarian antral follicles (3.0 to 6.0 mm in diameter) by aspiration with a 10-ml syringe and a 20 G hypodermic needle, and those with at least three layers of compacted cumulus cells were selected and cultured in NCSU23 medium [18] supplemented with 0.6 mM cysteine, 10 ng/ml epidermal growth factor, 10% (v/v) porcine follicular fluid, 75 μg/ml potassium penicillin G, 50 μg/ml streptomycin sulfate, 10 IU/ml eCG (ASKA Pharmaceutica, Tokyo, Japan), and 10 IU/ml hCG (ASKA Pharmaceutical) at 38.5 C in a humidified atmosphere of 5% CO2 in air for 22 h. Then, the oocytes were cultured for an additional 21 h without eCG and hCG at 38.5 C in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 [19]. IVM oocytes with expanded cumulus cells were treated with 1 mg/ml hyaluronidase dissolved in Tyrode lactose medium containing 10 mM HEPES and 0.3% (w/v) polyvinylpyrrolidone (TL-HEPES-PVP) and separated from the cumulus cells by gentle pipetting. Oocytes with an evenly granulated ooplasm and an extruded first polar body were selected for the subsequent experiments.
Porcine sperm preparation for ICSI-MGT
Commercially available boar semen (Duroc) suitable for artificial insemination was used to prepare frozen sperm for ICSI-MGT. Beltsville thawing solution (BTS) was used as a freezing solution without cryoprotective agents [20]. The sperm were washed three times by centrifugation at 200 × g for 5 min in BTS to remove the extender. The sperm were then suspended in BTS (containing 5% (w/v) BSA) at a concentration of 3 × 107 cells/ml, placed in 0.25-ml plastic freezing straws (Fujihira Industry, Tokyo, Japan), and plunged into liquid nitrogen. The straws of frozen sperm were thawed by soaking in a 37 C water bath for 10 sec. The sperm recovered from the straws were washed twice in BTS (containing 0.1% (w/v) BSA), suspended in Nucleus Isolation Medium (NIM) [21], and used in ICSI-MGT within 60 min of thawing. For tail removal by sonication, an ultrasonic sonicator (Honda Electronics, Aichi, Japan) was used to apply ultrasonic vibrations (100 W, 28 kHz) for 9 sec to 300 μl of the sperm suspension (5 × 107 cells/ml) in a 1.5-ml microcentrifuge tube. This duration of sonication was determined to decapitate approximately 70% of the sperm. Sperm that had been subjected to tail removal by sonication were resuspended in NIM at a concentration of 2–5 × 104 cells/μl. Next, the DNA solution was added to the sperm suspension to yield a concentration of 2.5 ng/μl. The suspension was then gently mixed and incubated at room temperature for 5 min. The resulting sperm were stored on ice until use in ICSI-MGT.
Intracytoplasmic sperm injection
The IVM oocytes at 43–45 h after commencement of the maturation culture were activated by electrical stimulation before the injection of sperm heads. The oocytes were lined up between two wire electrodes (1.0 mm apart) of a fusion chamber (CUY500G1, Nepa Gene, Chiba, Japan) and overlaid with an activation solution, consisting of 0.28 M mannitol (Nacalai Tesque, Kyoto, Japan), 50 μM CaCl2, 100 μM MgSO4, and 0.01% (w/v) PVA. Activation was induced with one DC pulse of 150 V/mm for 100 μsec using an electric pulsing machine (ET-1, Fujihira Industry).
ICSI-MGT was performed in a 4-μl drop of TL-HEPES-PVP under mineral oil using an Nikon inverted microscope (TE-300, Nikon, Tokyo, Japan) as described previously [17]. Approximately 1 μl of sperm suspension that had been co-incubated with DNA was transferred to a 2-μl drop of 10% (w/v) PVP (in DPBS; Irvine Scientific, Sales, Santa Ana, CA, USA). Sperm heads were aspirated from the PVP drop using an injection pipette and moved to the drop containing the oocytes. An oocyte was first captured by a holding pipette. Next, with the oocyte immobilized with its polar body at either the 6- or 12-o’clock position, a sperm head was injected using the piezo-actuated microinjection unit (PMM-150FU, Prime Tech, Tsuchiura, Japan) and micromanipulators (MO-202U, Narishige, Tokyo, Japan). Sperm injection was carried out within 30 min of activation of the oocytes.
After ICSI-MGT, embryos to be transferred to recipients were cultured in Porcine Zygote Medium-5 (PZM-5, Research Institute for the Functional Peptides, Yamagata, Japan) for 1–3 days under a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 38.5 C.
Embryo transfer
Crossbred (Large White/Landrace × Duroc) prepubertal gilts weighing from 100 to 105 kg were used as recipients of the sperm-injected embryos. The gilts were treated with a single intramuscular injection of 1000 IU of eCG to induce estrus. Ovulation was induced by an intramuscular injection of 1500 IU of hCG (Kawasaki Pharmaceutical, Kanagawa, Japan) given 66 h after the injection of eCG. Sperm-injected embryos cultured for 1–3 days were surgically transferred into the oviducts of recipients approximately 48 h or 72 h after hCG injection.
All but one of the pregnant recipients were laparotomized to recover fetuses at 47–65 days of gestation, and the remaining recipient was allowed to farrow.
PCR and Southern blot analyses
Genomic DNA was extracted from tail biopsies of fetuses and newborn piglets using proteinase K (Life Technologies Corporation, Carlsbad, CA, USA) and purified by the phenol-chloroform method. To identify Tg pigs, DNA samples were analyzed by PCR using the following primers: 5’-caatgatggctccagggtaa (forward) and 5’-ctccttgaagtcgatgccctt (reverse).
For Southern blot analysis, genomic DNA extracted as described above was digested with the PstI restriction enzyme (Takara Bio), separated by gel electrophoresis, and transferred onto a nylon membrane (GE Healthcare, Buckinghamshire, UK), which was then hybridized with the DIG-labeled probes prepared by PCR using the following primers: 5’-caatgatggctccagggtaa (forward) and 5’-ggtggtgcagatcagcttca (reverse). The signal (i.e., binding of the probe) was detected by chromogenic methods. The number of transgene copies integrated into the porcine genome was determined by comparison of the hybridization signal with that of the copy-number control, which was diluted to make a standard series (1–100 copies per diploid genome).
Pancreas-specific fluorescence expression in Tg fetuses and G1 offspring
The tails of the fetuses (day 47–65) obtained from autopsies of the sacrificed pregnant pigs were used to extract genomic DNA. Tg fetuses were identified by PCR. Fetal viscera were also removed, and the expression of green fluorescence in the organs was analyzed by fluorescence stereomicroscopy (MVX10, Olympus, Tokyo, Japan; excitation wavelength of 460–480 nm; absorption filter of 495–540 nm). Pancreatic tissue samples from fetuses were fixed in 4% paraformaldehyde and used to prepare paraffin-embedded sections (hematoxylin/eosin stain). The paraffin-embedded sections were also analyzed by fluorescence microscopy (Olympus BX52; excitation wavelength of 460–480 nm; absorption filter of 495–540 nm).
A subset of the founder Tg pigs was allowed to grow to maturity and was mated with wild-type pigs. The offspring (G1) obtained were sacrificed when they reached the age of 27 days to examine pancreas-specific fluorescence expression by fluorescence stereomicroscopy.
Pancreatic tissue samples of the founder Tg pig (G0) were double-stained using anti-insulin (1:500; LS-C24686, LifeSpan BioSciences, Seattle, WA, USA) and anti-GFP (1:500-1:1000; #598, MBL, Nagoya, Japan) antibodies to determine the Venus-expressing cells in the pancreatic islets. Alexa Fluor® 594 goat anti-guinea pig IgG (A11076, Life Technologies) and Alexa Fluor® 488 donkey anti-rabbit IgG (A21206, Life Technologies) were used as the secondary antibodies. The tissue sections were also double-stained for glucagon and Venus. For glucagon staining, anti-glucagon antibody (1:500; G2654) and Alexa Fluor®594 goat anti-mouse IgG (A11020, Life Technologies) were employed. After antibody treatments, the sections were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) containing 4′,6-diamidino-2-phenylindole (DAPI) for nuclear counterstaining and observed by confocal laser scanning microscopy (FV1000-D; Olympus, Tokyo, Japan).
Fluorescence in situ hybridization (FISH)
Peripheral blood cells derived from the two Tg founder pigs (male and female) were cultured in RPMI1640 containing 20% (v/v) FBS for 3 days. The cells were then cultured with 30 μg/ml BrdU for 5 h, followed by incubation with 0.02 μg/ml colcemide for 1 h. After fixation with methanol-acetic acid (3:1 ratio), the cells were spread on slides and air-dried. The cells were then stained with Hoechst 33258 and treated with UV light for G-banding. Pdx1-Venus DNA was labeled with Cy3 as a probe and hybridized at 37 C overnight. After stringent washing, the bound label was detected with anti–Dig-Cy3 using Leica DRAM2 and CW4000 FISH software.
Tracing of pancreatic islets by fluorescence after ectopic transplantation
Pancreatic islets were isolated from a 4.5-month-old Tg pig using a conventional method. The pancreas collected from a Tg pig was distended by infusion with Liberase DL (Roche Diagnostics, Indianapolis, IN, USA) suspended in Hank’s balanced salt solution (HBSS; Life Technologies), followed by a static incubation in an empty 125 ml storage bottle for 30 min at 37 C. Then the digesting pancreatic tissue was gently shaken with 7 mm Teflon® beads in RPMI 1640 (Life Technologies). Digestion was terminated by the addition of cold HBSS containing 10% (v/v) FBS, 100 IU/ml of penicillin, 100 mg/ml of streptomycin, and 2.5 μg/ml amphotericin B. The digested tissue was passed through a 500 μm stainless steel mesh screen. The tissue effluent was collected in 50 ml conical tubes and centrifuged for 2 min at 155 × g at 4 C. The islets were purified using a Histopaque®-1.077 gradient with RPMI 1640. Following centrifugation at 1700 × g for 17 min at 4 C, the islets were collected from the interface between the RPMI 1640 and Histopaque®-1.077. Purified islets were washed by centrifugation at 155 × g for 2 min at 4 C in RPMI 1640 supplemented with 10% (v/v) FBS. The purity of the isolated islets was confirmed to be over 90% by microscopic inspection after Dithizone (5 mg/ml, in DPBS) staining.
Fluorescence in the isolated islets was observed by fluorescence stereoscopic microscopy (MVX10, Olympus). Isolated islets were then transplanted under the renal capsules of anesthetized NOD/SCID mice (CLEA Japan, Tokyo, Japan). Kidneys were removed either immediately or at one month after transplantation and analyzed by fluorescence stereomicroscopy (MVX10, Olympus) to determine whether the islets could be traced using Venus fluorescence as an indicator.
Results
Efficiency of production of Pdx1-Venus Tg pigs by ICSI-MGT
The ICSI-MGT method was selected for creating Pdx1-Venus Tg pigs. In total, 370 sperm-injected embryos were transferred into four recipients, all of which became pregnant.
Three of the recipient pigs were autopsied at 47–65 days of gestation, and 16 fetuses were recovered for analysis (Table 1). The production efficiency of fetuses was between 4 and 8%, as each recipient received approximately 80 embryos. Seven of the 16 fetuses were Tg (43.8%), including approximately 30% of the fetuses in two of the recipients and all three fetuses in one recipient. Overall, 2.4–3.7% of the transferred embryos produced Tg fetuses.
Table 1. Efficiency of the ICSI-MGT method for the production of Tg pig fetuses and offspring carrying the Pdx1-Venus gene.
| Recipient | No. of embryos transferred | Production efficiency of fetuses or offspring (%)*1 |
Production efficiency of Tg fetuses or offspring (%)*2 |
|
| Fetus | W8 | 83 | 8.4 [7/83] | 28.6 [2/7] |
| W9 | 81 | 3.7 [3/81] | 100 [3/3] | |
| W11 | 79 | 7.6 [6/79] | 33.3 [2/6] | |
| Offspring | W10 | 127 | 4.7 [6/127] | 33.3 [2/6] |
*1 No. of fetuses or piglets / No. of embryos transferred × 100. *2 No. of Tg fetuses or piglets / No. of fetuses or piglets obtained × 100.
The fourth pregnant pig, which received 127 embryos, was allowed to farrow and produced six (4.7%) piglets, two of which were Tg (one female and one male).
Pancreas-specific expression of Venus in Tg fetuses and offspring
The viscera of the seven Tg fetuses obtained were examined by fluorescence stereomicroscopy, and we found that all the fetuses had pancreas-specific expression of Venus fluorescence (Fig. 2A and B). The Southern blot analysis of genomic DNAs indicated an integration of 5 to 100 copies of the gene. Although the fluorescence intensity tended to be greater in fetuses with higher copy numbers (≥15), except for a female fetus (W8-1) harboring 30 copies of the gene, pancreas-specific expression was clear in all fetuses regardless of the copy number (Table 2).
Fig. 2.
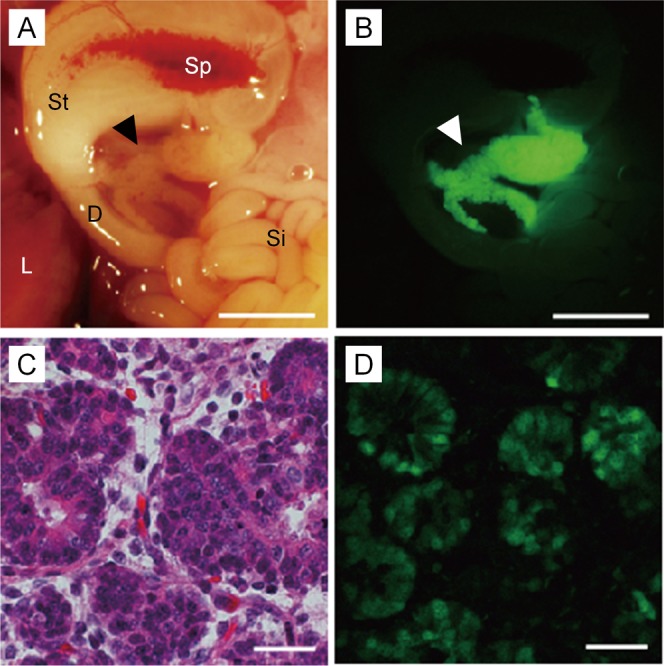
Pancreas-specific expression of the Pdx1-Venus gene in the Tg pig fetus. Bright-field (A) and fluorescence microscopic (B) observation of the pancreas (arrowheads). Acinar cells (C, HE stain) showed prominent Venus expression (D). D, duodenum; L, liver; Si, small intestine; Sp, spleen; St, stomach. Scale bars = 5 mm (A, B); 50 μm (C, D).
Table 2. Expression of the Pdx1-Venus gene in Tg pig fetuses produced by the ICSI-MGT method.
| Fetus | Fetal age | Fetal sex | Fluorescence intensity |
Transgene copy number |
| W8-1 | Day 48 | F | + | 30 |
| W8-5 | Day 48 | F | + | 5 |
| W9-1 | Day 47 | F | + | 5 |
| W9-2 | Day 47 | M | ++ | 15 |
| W9-3 | Day 47 | M | ++ | 70 |
| W11-2 | Day 65 | F | + | 5 |
| W11-5 | Day 65 | F | ++ | 100≤ |
A histological analysis of pancreatic tissues of four Tg fetuses showed that Venus fluorescence was present in cells determined to be acinar cells based on their appearance. This expression pattern was consistent among all fetuses analyzed (Fig. 2C and D).
The two founder (G0; male and female) Tg pigs grew normally to adulthood and were crossed with wild-type pigs to produce G1 offspring of six litters. Of the 22 G1 pigs obtained from the male founder and the 28 G1 pigs derived from the female founder, the transgene was transmitted to 10 (45.5%) and 16 pigs (57.1%), respectively, indicating that the transgene was transmitted in the Mendelian fashion. It was found that 10 and 30 transgene copies were integrated into the genomes of the male and female founder pigs, respectively. FISH analysis of these founder Tg pigs revealed that concatemerized transgenes were integrated into a single site on the chromosomes (Suppl. Fig. 1 : on-line only).
Four 27-day-old G1 piglets (Tg female and male, non-Tg female and male) were autopsied to examine fluorescence expression in their viscera. The pancreas, duodenum, small intestine, liver, spleen, kidneys, skin, heart, lungs, and stomach were observed under a fluorescence stereomicroscope. This analysis confirmed the retention of pancreas-specific fluorescence expression (Fig. 3A and Suppl. Fig. 2 : on-line only) as in the founder Tg fetuses. Green fluorescence was not detected in the viscera of non-Tg pigs.
Fig. 3.
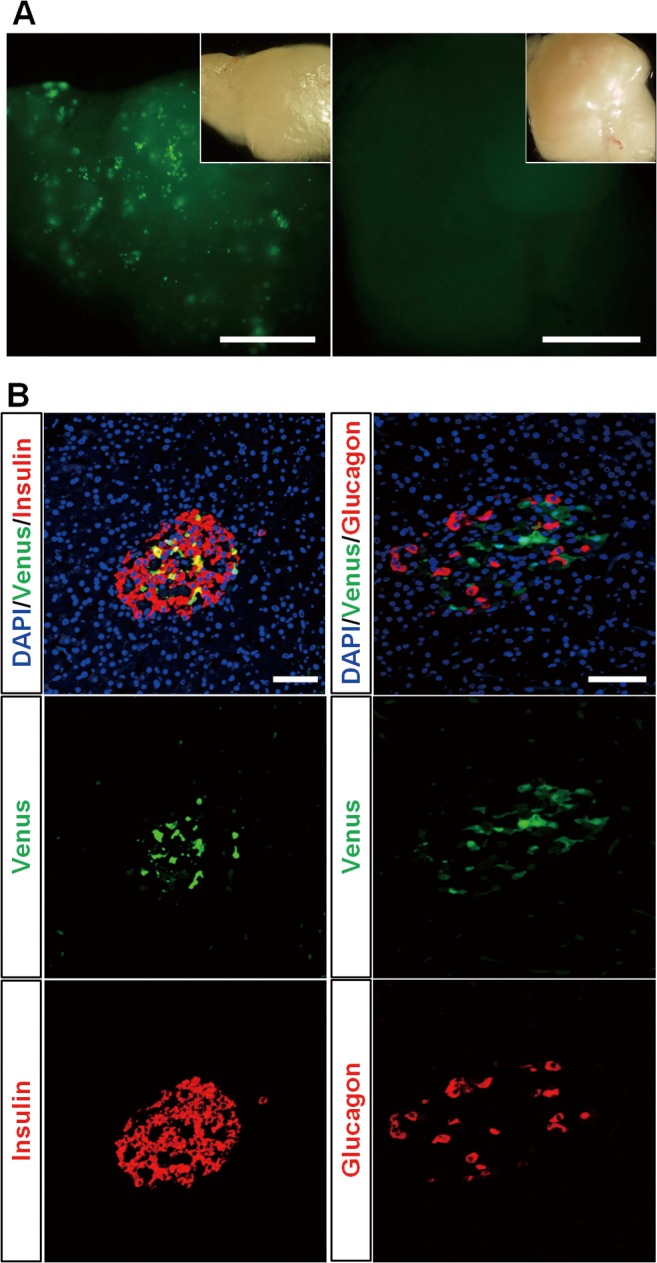
Expression of the Pdx1-Venus gene in the pancreas of a Tg pig. (A) Green fluorescent spots were observed by fluorescence stereomicroscopy throughout the pancreatic tissue of the Tg pigs (left panel), indicating Pdx1-Venus expression in islets. Right panel: pancreatic tissue of a control wild-type pig. The inset in each panel presents a bright-field image of the tissue. Scale bars = 2.5 mm. (B) Immunohistochemical staining of pancreatic islets of a Pdx1-Venus Tg pig. Merged images of the Tg pig islet demonstrated that the expression of the Pdx1-Venus gene was confined to β-cells (top left), whereas this gene was not expressed in glucagon-producing cells (top right). Scale bars = 50 μm.
The pancreatic tissue of the G1 Tg pigs showed green fluorescent spots throughout (Fig. 3A), indicating Pdx1-Venus expression in islets. Venus expression was found to be confined to β-cells in the pancreatic tissue after double staining with anti-insulin and anti-GFP antibodies (Fig. 3B).
Tracing of the fluorescence expression of pancreatic islets
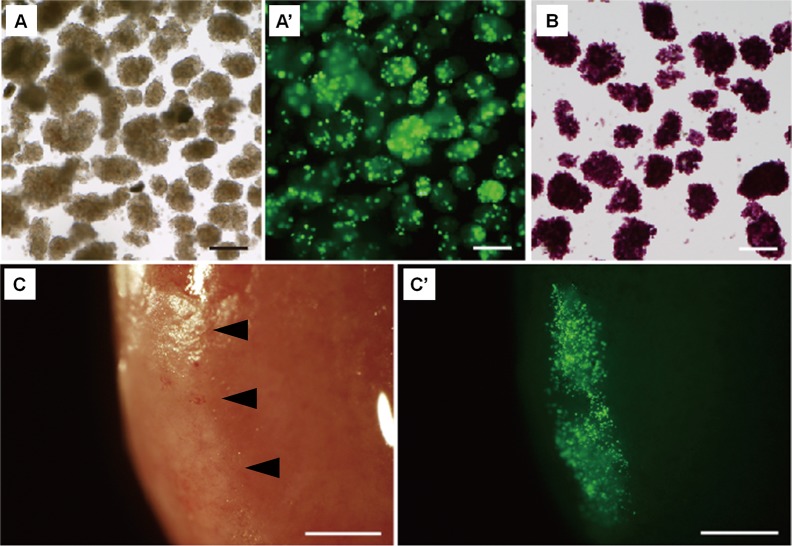
To further examine the potential of Pdx1-Venus Tg pigs for future use in pancreatic islet research, we investigated the traceability of the pancreatic islets using their fluorescence as an indicator. As shown in Fig. 4, Venus fluorescence expression patterns were clearly observed under a fluorescence stereomicroscope, which confirmed clear fluorescence spots in the islets (Fig. 4A and A’).
Fig. 4.
Fluorescence of pancreatic islets isolated from a Pdx1-Venus Tg pig. (A) Pancreatic islets isolated from a Tg pig. (A’) Fluorescent spots were observed in the islets of a Tg pig. (B) Dithizone-stained islets of a Tg pig. (C, C’) Pancreatic islets of a Pdx1-Venus Tg pig transplanted into the kidney capsule of NOD/SCID mice (arrowheads). Bright-field (C) and fluorescence (C’) observation by fluorescence stereomicroscopy showed that the fluorescence of the transplanted islets was clear at 30 days after transplantation (A’). Scale bars = 200 μm (A–C); 1 mm (C, C’).
The isolated islets were transplanted under the renal capsules of NOD/SCID mice, and the transplanted islets could clearly be identified by their fluorescence. The fluorescence of the transplanted pancreatic islets was still clear at 30 days after transplantation (Fig. 4C and C’).
Discussion
This report describes the production of the first Pdx1-Venus Tg pig expressing green fluorescent protein specifically in the pancreas, particularly in β-cells. Pdx1 is a key molecule with an important role in pancreatic stem cell differentiation into β-cells [12, 13, 22, 23]. In fact, Pdx1 knockout mice reportedly suffer impaired pancreatic development [12, 24]. The identification and separation of Pdx1-positive cells is therefore expected to stimulate new developments in research on islet architecture during the ontogeny and differentiation of β-cells from precursors [13, 25, 26]. Research on pancreas development and β-cell differentiation is also expected to lead to the pathophysiological analysis of diabetes and the development of new therapeutic methods [27]. In particular, the neogenesis of β-cells has been a recent focus in diabetes research [28,29,30,31].
In research using laboratory rodents, Pdx1GFP/w mice [32] and mouse insulin I gene promoter (MIP)-GFP Tg mice [33] have been created and used to conduct research on pancreatic development and differentiation. However, in research using pigs, a Tg model that is useful for the study of β-cell biology, including the identification of progenitor cells, has not been available. Considering that the importance of pigs, as a large laboratory animal with several similarities to humans, in translational research is now recognized and that research is being undertaken on the clinical applications of porcine islet transplantation [34], the Pdx1-Venus pig we have produced has strong potential for use as an effective research tool. The Expression pattern of the Pdx1-Venus in the islet of our transgenic pigs was similar to that reported previously in the Pdx1GFP/w mice [32].
In the present study, we employed the mouse Pdx1 promoter to drive the Venus expression in the transgenic pigs. However the transgene was expressed in a highly tissue-specific manner. In fact, Pdx1-Venus expression was confined to the pancreas during the early fetal stage (day 47) and at the adult stage. Pdx1 is also known to be expressed in the duodenum at the fetal stage [13]. Further studies need to be undertaken to examine the expression of the Pdx1-Venus in the early stages of pancreatogenesis in the transgenic pig fetuses.
Concerning Pdx1-Venus expression in the islets, we observed that cells that were Venus positive were also insulin-positive cells. This pig is, accordingly, very useful for tracking the behavior of pancreatic progenitor cells and β-cells.
Pdx1-Venus is also useful as a cell marker following islet transplantation. The clinical application of islet transplantation using human islets has been hampered, as is the case with other transplants, by the shortage of donor organs. However, if xenogeneic pancreas transplantation—more specifically, the transplantation of pig islets to humans—becomes possible, substantial advances will be made in treatments for diabetes patients [35]. Xenogeneic transplantation will require further basic studies, including a long-term follow-up of islets transplanted to animals. Pdx1-Venus Tg pig islets will serve as a very useful tool in such research. For example, production of insulin or C-peptide from the transplanted islets may be correlated with the Pdx1-Venus expression that indicates the viability of β-cells. We have already produced diabetic model Tg pigs by mutant hepatocyte nuclear factor-1α gene transfer [2]. Transplanting islets from Pdx1-Venus Tg pigs using such diabetic models should provide knowledge that can be extrapolated from large animals to humans.
Pdx1-Venus Tg pigs were observed to show a high level of green fluorescence expression in the pancreas (β-cells) with normal pancreas function. This finding was confirmed by the pigs’ physiological characteristics, including growth, casual blood glucose levels, postprandial blood glucose and insulin levels, and blood biochemical parameters, which were measured during the period from the postweaning through the growth stages (Suppl. Text, Suppl. Fig. 3 , and Suppl. Table 1: on-line only). Based on these results, we hypothesize that Pdx1-Venus Tg pigs may also be suitable as donor animals in studies of islet transplantation.
In this study, we introduced transgenes using the ICSI-MGT method. We previously reported that the application of ICSI-MGT is highly effective for introducing exogenous genes to porcine IVM oocytes [2, 17]. In this study, approximately 30–100% of the fetuses/piglets obtained in each litter were Tg, once more demonstrating the high efficiency of the ICSI-MGT method. The production efficiency of Tg fetuses or piglets obtained in this study was equal or rather higher compared with our previous studies, probably due to lower detrimental effect of the transgene expression [2, 36, 37]. In vitro maturation of pig oocytes is now an established method, and the combination of IVM oocytes and the ICSI-MGT method can accordingly be considered a practical method for generating Tg pigs.
Our previous research confirmed that transgenes introduced by the ICSI-MGT method generally insert into a single site on the host genome as concatemers [17, 38]. In the founder Tg pigs used for generating G1 offspring in this study, it was shown that the transgenes did concatamerize and integrated into a single site of the chromosome as shown in our previous studies [17, 38]. No significant differences in growth were observed in fetuses with transgene copy numbers between 5 and 100. The level of transgene expression is considered to be more readily influenced by the integration site on the chromosome than by the integrated copy number [39, 40]. Even so, in the case of Tg individuals with an exceptionally high number of integrated transgenes, it is possible that high-level transgene expression may influence normality in piglets and affect their long-term survival. Because the copy number of the integrated genes is affected by various factors related to the binding of DNA to sperm [38, 41, 42], the preliminary optimization of the transgene-sperm co-incubation will be critical for the efficient production of Tg pigs using the ICSI-MGT method.
In conclusion, building on our current knowledge, this study verifies that using IVM oocytes and ICSI-MGT together is an effective method for producing Tg pigs. Additionally, because the Pdx1-Venus Tg pigs produced in this study express green fluorescent protein specifically in the pancreas (β-cells) and maintain normal physiological function, we can conclude that this large animal model is suitable for research on pancreatic development and regeneration as well as diabetes.
Supplementary Text
Physiological characteristics of Pdx1-Venus Tg pigs
G1 offspring were obtained by breeding the founder Tg pigs with wild-type pigs. The weights of G1 Tg (one female and one male) offspring and a non-Tg (one male) littermate were assessed until the pigs were three months old. The postweaning blood glucose levels of these pigs were measured weekly until the same age. Blood samples collected from the ear vein were analyzed using a human blood glucose meter (Glucocard G+ meter, GT-1820; Arkray, Inc., Kyoto, Japan). At five months old, the fasting and postprandial blood insulin levels of the animals were measured.
Various aspects of blood biochemical parameters were analyzed in three G1 pigs aged between 5 and 15 months of age to determine whether the Pdx1-Venus Tg pigs had a normal physiology before and after sexual maturity. As a control, female and male non-Tg pigs, aged between 7 and 8 months of age, from the same litter as the Tg pigs were used. Blood urea nitrogen (BUN), glucose (GLU), creatinine (CRE), total protein (TP), total cholesterol (TCHO), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), sodium (Na), potassium (K), and chloride (Cl) were measured using an auto analyzer (DRI-CHEM, FDC-700, Fujifilm, Tokyo, Japan). Insulin concentrations were measured using ELISA (Pig Insulin ELISA KIT (TMB), AKRIN-013T; Shibayagi, Gunma, Japan), and the concentrations of 1,5-anhydroglucitol (1,5-AG) were determined using the standard enzymatic method (SRL, Tokyo, Japan).
Suppl. Fig. 3 shows the physiological features of the Tg pigs and their non-Tg siblings. The G1 Tg piglets grew at the same rate as their non-Tg siblings (Suppl. Fig. 3A).
The non-fasting blood glucose levels of the Tg pigs, which were monitored consecutively after weaning until the pigs were 3 months old, were within the normal physiological range for blood glucose for pigs (Suppl. Fig. 3B). With regard to postprandial blood glucose and insulin levels, the Tg pigs showed similar reactions to those of non-Tg pigs (Suppl. Fig. 3C, D), indicating that the pancreatic functions of Pdx1-Venus Tg pigs were normal.
All 13 blood biochemical parameters measured were found to be within the normal ranges in the Tg pigs and their non-Tg control siblings (Suppl. Table 1). Blood 1,5-anhydroglucitol (1,5-AG) levels, indicators of glycemic control during the previous days, were also within the normal ranges in the Tg pigs.
Acknowledgments
This work was supported by the Japan Science and Technology Agency, ERATO, Nakauchi Stem Cell and Organ Regeneration Project, JSPS KAKENHI Grant Number 24659596, and the Meiji University International Institute for Bio-Resource Research (MUIIBR).
References
- 1.Petters RM, Alexander CA, Wells KD, Collins EB, Sommer JR, Blanton MR, Rojas G, Hao Y, Flowers WL, Banin E, Cideciyan AV, Jacobson SG, Wong F. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat Biotechnol 1997; 15: 965–970 [DOI] [PubMed] [Google Scholar]
- 2.Umeyama K, Watanabe M, Saito H, Kurome M, Tohi S, Matsunari H, Miki K, Nagashima H. Dominant-negative mutant hepatocyte nuclear factor 1alpha induces diabetes in transgenic-cloned pigs. Transgenic Res 2009; 18: 697–706 [DOI] [PubMed] [Google Scholar]
- 3.Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W, Göke B, Pfeifer A, Wanke R, Wolf E. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes 2010; 59: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008; 321: 1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klymiuk N, Mundhenk L, Kraehe K, Wuensch A, Plog S, Emrich D, Langenmayer MC, Stehr M, Holzinger A, Kröner C, Richter A, Kessler B, Kurome M, Eddicks M, Nagashima H, Heinritzi K, Gruber AD, Wolf E. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J Mol Med (Berl) 2012; 90: 597–608 [DOI] [PubMed] [Google Scholar]
- 6.Miyagawa S, Yamamoto A, Matsunami K, Wang D, Takama Y, Ueno T, Okabe M, Nagashima H, Fukuzawa M. Complement regulation in the GalT KO era. Xenotransplantation 2010; 17: 11–25 [DOI] [PubMed] [Google Scholar]
- 7.Matsunari H, Watanabe M, Umeyama K, Nakano K, Kurome M, Kessler B, Wolf E, Miyagawa S, Nagashima H. Cloning of homozygous α1,3-galactosyltransferase gene knock-out pigs by somatic cell nuclear transfer. In: Miyagawa S (ed.), Xenotransplantation. Rijeka, Croatia: InTech; 2012: 37-54.
- 8.Lai L, Park KW, Cheong HT, Kühholzer B, Samuel M, Bonk A, Im GS, Rieke A, Day BN, Murphy CN, Carter DB, Prather RS. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol Reprod Dev 2002; 62: 300–306 [DOI] [PubMed] [Google Scholar]
- 9.Matsunari H, Onodera M, Tada N, Mochizuki H, Karasawa S, Haruyama E, Nakayama N, Saito H, Ueno S, Kurome M, Miyawaki A, Nagashima H. Transgenic-cloned pigs systemically expressing red fluorescent protein, Kusabira-Orange. Cloning Stem Cells 2008; 10: 313–323 [DOI] [PubMed] [Google Scholar]
- 10.Shigeta T, Hsu HC, Enosawa S, Matsuno N, Kasahara M, Matsunari H, Umeyama K, Watanabe M, Nagashima H. Transgenic pig expressing the red fluorescent protein kusabira-orange as a novel tool for preclinical studies on hepatocyte transplantation. Transplant Proc 2013; 45: 1808–1810 [DOI] [PubMed] [Google Scholar]
- 11.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 2002; 20: 87–90 [DOI] [PubMed] [Google Scholar]
- 12.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994; 371: 606–609 [DOI] [PubMed] [Google Scholar]
- 13.Bonal C, Herrera PL. Genes controlling pancreas ontogeny. Int J Dev Biol 2008; 52: 823–835 [DOI] [PubMed] [Google Scholar]
- 14.Hammer RE, Pursel VG, Rexroad CEJ, Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature 1985; 315: 680–683 [DOI] [PubMed] [Google Scholar]
- 15.Park KW, Cheong HT, Lai L, Im GS, Kühholzer B, Bonk A, Samuel M, Rieke A, Day BN, Murphy CN, Carter DB, Prather RS. Production of nuclear transfer-derived swine that express the enhanced green fluorescent protein. Anim Biotechnol 2001; 12: 173–181 [DOI] [PubMed] [Google Scholar]
- 16.Yong HY, Hao Y, Lai L, Li R, Murphy CN, Rieke A, Wax D, Samuel M, Prather RS. Production of a transgenic piglet by a sperm injection technique in which no chemical or physical treatments were used for oocytes or sperm. Mol Reprod Dev 2006; 73: 595–599 [DOI] [PubMed] [Google Scholar]
- 17.Kurome M, Ueda H, Tomii R, Naruse K, Nagashima H. Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res 2006; 15: 229–240 [DOI] [PubMed] [Google Scholar]
- 18.Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl 1993; 48: 61–73 [PubMed] [Google Scholar]
- 19.Funahashi H, Day BN. Effects of the duration of exposure to hormone supplements on cytoplasmic maturation of pig oocytes in vitro. J Reprod Fertil 1993; 98: 179–185 [DOI] [PubMed] [Google Scholar]
- 20.Pursel VG, Johnson LA. Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J Anim Sci 1975; 40: 99–102 [DOI] [PubMed] [Google Scholar]
- 21.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod 1996; 55: 789–795 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem 2001; 276: 25279–25286 [DOI] [PubMed] [Google Scholar]
- 23.Lottmann H, Vanselow J, Hessabi B, Walther R. The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med (Berl) 2001; 79: 321–328 [DOI] [PubMed] [Google Scholar]
- 24.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development 1996; 122: 1409–1416 [DOI] [PubMed] [Google Scholar]
- 25.Holland AM, Hale MA, Kagami H, Hammer RE, MacDonald RJ. Experimental control of pancreatic development and maintenance. Proc Natl Acad Sci USA 2002; 99: 12236–12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000; 127: 2317–2322 [DOI] [PubMed] [Google Scholar]
- 27.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of ß-cell regeneration. Islets 2010; 2: 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010; 464: 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung C-H, Levine F. Adult pancreatic alpha-cells: a new source of cells for beta-cell regeneration. Rev Diabet Stud 2010; 7: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianani R. Beta cell regeneration in human pancreas. Semin Immunopathol 2011; 33: 23–27 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lanzoni G, Carpino G, Cui CB, Dominguez-Bendala J, Wauthier E, Cardinale V, Oikawa T, Pileggi A, Gerber D, Furth ME, Alvaro D, Gaudio E, Inverardi L, Reid LM. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells 2013; 31: 1966–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland AM, Micallef SJ, Li X, Elefanty AG, Stanley EG. A mouse carrying the green fluorescent protein gene targeted to the Pdx1 locus facilitates the study of pancreas development and function. Genesis 2006; 44: 304–307 [DOI] [PubMed] [Google Scholar]
- 33.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab 2003; 284: E177–E183 [DOI] [PubMed] [Google Scholar]
- 34.Elliott RB, Escobar L, Tan PLJ, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007; 14: 157–161 [DOI] [PubMed] [Google Scholar]
- 35.Orive G, Hernández RM, Gascón AR, Igartua M, Pedraz JL. Encapsulated cell technology: from research to market. Trends Biotechnol 2002; 20: 382–387 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe M, Kurome M, Matsunari H, Nakano K, Umeyema K, Shiota A, Nakauchi H, Nagashima H. The creation of transgenic pigs expressing human proteins using BAC-derived, full-length genes and intracytoplasmic sperm injection-mediated gene transfer. Transgenic Res 2012; 21: 605–618 [DOI] [PubMed] [Google Scholar]
- 37.Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, Nakauchi H. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA 2013; 110: 4557–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umeyama K, Saito H, Kurome M, Matsunari H, Watanabe M, Nakauchi H, Nagashima H. Characterization of the ICSI-mediated gene transfer method in the production of transgenic pigs. Mol Reprod Dev 2012; 79: 218–228 [DOI] [PubMed] [Google Scholar]
- 39.Clark AJ, Bissinger P, Bullock DW, Damak S, Wallace R, Whitelaw CBA, Yull F. Chromosomal position effects and the modulation of transgene expression. Reprod Fertil Dev 1994; 6: 589–598 [DOI] [PubMed] [Google Scholar]
- 40.Kong Q, Wu M, Huan Y, Zhang L, Liu H, Bou G, Luo Y, Mu Y, Liu Z. Transgene expression is associated with copy number and cytomegalovirus promoter methylation in transgenic pigs. PLoS ONE 2009; 4: e6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirabayashi M, Kato M, Ishikawa A, Kaneko R, Yagi T, Hochi S. Factors affecting production of transgenic rats by ICSI-mediated DNA transfer: effects of sonication and freeze-thawing of spermatozoa, rat strains for sperm and oocyte donors, and different constructs of exogenous DNA. Mol Reprod Dev 2005; 70: 422–428 [DOI] [PubMed] [Google Scholar]
- 42.Li C, Mizutani E, Ono T, Wakayama T. An efficient method for generating transgenic mice using NaOH-treated spermatozoa. Biol Reprod 2010; 82: 331–340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.