Abstract
The estrogenic efficacy of topical vaginal application of Pueraria mirifica extract (PM) on the restoration of vaginal atrophy, and the presence of any systemic side effects, were investigated in postmenopausal cynomolgus macaques. Twelve postmenopausal cynomolgus macaques, with complete cessation of menstruation for at least 5 years before start of this experiment, were divided into three groups. They received a topical vaginal application daily of 0.1 or 1% (w/w) PM cream or a conjugated equine estrogen (CEE) cream (a mixture of estrone, equilin, 17β-dihydroequilin, 17α-estradiol and 17α-dihydroequilin at 0.625 mg total estrogen/g cream) for 28 days. Estrogenic efficacy was assessed weekly by vaginal cytology assay and vaginal pH measurement, whilst the plasma luteinizing hormone (LH) and sex skin coloration levels were determined at the end of each treatment period to evaluate the systemic side effects. PM significantly increased the proportion of superficial cells in a dose-dependent manner, with a similar efficacy between 1% (w/w) PM and CEE. Together with increased vaginal maturation, PM decreased the vaginal pH to acidic levels, as observed in the CEE group. PM induced no detected systemic side effects, whilst CEE decreased the plasma LH level and increased the reddish color of the sex skin during the posttreatment period. Topical vaginal treatment with PM stimulated the maturation of the vaginal epithelium without causing systemic side effects in postmenopausal monkeys. The implication is that PM could be a safer alternative to treat vaginal atrophy in postmenopausal women.
Keywords: Menopause, Phytoestrogens, Sex skin color, Vaginal dryness
Vaginal atrophy, a thinning and shrinking of the vaginal tissues and a decreasing in lubrication, is a common symptom found in postmenopausal women. Together with vaginal atrophy, a decrease in vaginal secretion and increase in vaginal pH also occurs, which leads to an increased incidence of vaginitis [1], vaginal dryness, itching, burning and irritation [1,2,3,4]. Most vaginal atrophic patients complain of dyspareunia during sexual intercourse. The etiology in most cases of vaginal atrophy is a decline in the circulating endogenous estrogen levels. Therefore, several estrogen formulations have been used to relieve the symptoms of vaginal atrophy, such as estradiol rings, tablets and creams [5]. Conjugated equine estrogens (CEE) cream, which contained a mixture of estrone, equilin, 17β-dihydroequilin, 17α-estradiol and 17α-dihydroequilin at 0.625 mg/g cream, is currently the most common choice of vaginal product for the treatment of vaginal atrophy [4,5,6]. However, the reported side effects of CEE cream include an increased occurrence of endometrial hyperplasia, endometrial stimulation, breast tenderness and uterine bleeding [5]. Therefore, the use of synthetic phytoestrogens or an extract of phytoestrogen containing plants for the treatment of vaginal atrophy has become attractive as a potentially safer alternative [7].
Pueraria mirifica (PM) is an endemic herb of Thailand, and its tuberous root contains a high amount of phytoestrogens [8]. The estrogenic activity of PM has been established in animal experiments and clinical trials, especially that association with vaginal proliferation [9,10,11,12,13,14]. Rats fed with PM at a dose of 50 to 1,000 mg/kg/day elicited a dose-dependent vaginal cornification [9,10,11,12,14]. Oral administration of 20 to 50 mg/day of PM for 24 weeks in healthy postmenopausal women resulted in increased vaginal proliferation, ablation of vaginal dryness symptoms and dyspareunia and a reduction in vaginal pH to acidic levels, but these also elicited adverse side effects, such as urticaria, in some patients [13].
It is well known that estrogens and phytoestrogens exhibit estrogenic activity in vaginal tissues after binding with estrogen receptors (ERs) [15, 16]. Although both the ERα and ERβ subtypes of estrogen receptors are expressed in vaginal tissues, a three-fold higher level of ERβ expression than ERα was noted in premenopausal or estrogen replacement therapy postmenopausal women [17]. Therefore, PM may be helpful in the management of postmenopausal vaginal atrophy in women because its phytoestrogens have a higher affinity to the ERβ subtype [18].
Since there is no information on the effect of vaginal application of PM on the restoration of vaginal atrophy and its systemic side effects, we performed this evaluation in postmenopausal monkeys. Cynomolgus macaques (Macaca fascicularis) were selected for this study because they have broadly similar reproductive organs and sex hormone profiles to those of humans, and they undergo natural menopause [19,20,21,22]. The advantage of using cynomolgus macaques over humans is that the systemic estrogenic activity of synthetic estrogens or phytoestrogens can be detected by the noninvasive method of visual observation of the external changes in the color of the sex skin, which is the skin at the anogenital and rump surrounding the ischial callosity [23,24,25,26]. Cynomolgus macaques exhibit cyclical changes in the red color of their sex skin in relation to the changes in their serum estrogen levels during the menstrual cycle. Thus, sex skin reddening was found to be greatest during the late follicular phase or before ovulation when the serum estrogen levels were highest [23, 25].
Here, we investigated (i) if daily topical application of a vaginal cream containing PM extract showed a similar efficacy to that of CEE cream using vaginal tissue proliferation and the vaginal pH as markers, (ii) if vaginally administered PM and CEE creams could be absorbed through the vaginal mucosa into the blood circulation and have a systemic side effect using the decrease in plasma luteinizing hormone (LH) as an indicator and (iii) if changes in sex skin reddening in postmenopausal cynomolgus macaques can reflect the systemic effects of vaginal application of CEE and PM.
Materials and Methods
Animals
Twelve postmenopausal cynomolgus macaques over 20 years of age and weighing 4.5 to 7.0 kg were selected from the colony of the Primate Research Unit, Chulalongkorn University, Bangkok, Thailand. All monkeys had exhibited a complete cessation of menstrual bleeding for at least 5 years before start of this study (Table 1). In humans, onset of vaginal atrophy occurs in postmenopausal women (progressive estrogen deficiency) approximately after 10 years of menopause, and this occurs later than other menopausal symptoms [27]. Thus, monkeys at least 5 years postmenopause, which are roughly equivalent to women 15 years postmenopause [28], were selected for this study, as they were thought to be a reasonable (representative) model for humans.
Table 1. Age and menopausal period in each monkey used in the three treatment groups.
| Treatment | Monkey no. | Age (years) | Menopausal period (years) |
| 0.1% (w/w) PM | 77 | 29 | 5.16 |
| 612 | 27 | 6 | |
| 801 | 24 | 7.66 | |
| 104 | 29 | 8.16 | |
| 1% (w/w) PM | 624 | 23 | 5 |
| 102 | 29 | 5.41 | |
| 616 | 27 | 7.50 | |
| 99 | 29 | 9.50 | |
| CEE | 627 | 21 | 6.66 |
| 70 | 30 | 7.91 | |
| 610 | 28 | 10 | |
| 628 | 21 | 11.25 | |
The animals were trained to turn their back, bend their body and show their rump for topical vaginal treatment and vaginal smears. Monkeys were housed in individual cages under standard housing conditions of controlled lighting (12 h light/12 h dark cycle). They were fed daily a monkey chow (Perfect Companion Group, Samut Prakarn, Thailand) in the morning (0900−1000 h) and given fresh fruits in the afternoon (1400−1500 h). The experimental protocol was approved by the Animal Care and Use Committee of the Faculty of Science in accordance with the guide for the care and use of laboratory animals prepared by Chulalongkorn University (Protocol Review No. 0923002).
Experimental design
The twelve monkeys were divided into three groups (four monkeys/group) that were topically treated once daily with 0.5 g of 0.1 or 1% (w/w) PM extract or CEE vaginal cream (Premarin Vaginal Cream®, Wyeth, Montreal, Canada), respectively. The treatment schedule was separated into the three periods, the pretreatment, treatment and posttreatment periods, each with a duration of 28 days. Monkeys were assessed weekly for vaginal cytology and vaginal pH. Blood samples were collected from the femoral vein under ketamine hydrochloride anesthesia (10 mg/kg BW, i.m.) between 0830–0930 h for the LH assay at the end of the pretreatment, treatment and posttreatment periods. Collected blood samples were immediately centrifuged (1,700 × g, 30 min, 4 C), and the plasma was harvested and stored at –20 C until used for the LH assay. The sex skin color was also determined after blood collection (see below).
Vaginal cytology
A sterile cotton swab soaked in sterile normal saline was introduced into the posterior vagina [29]. The material collected from the vaginal wall was immediately smeared on a glass slide, which was then wet fixed in 95% (v/v) ethanol and stained using Papanicolaou’s method [30]. The vaginal smears were collected between 0900–1000 h weekly. The smears were examined under an Olympus compound light microscope, and 100 epithelial cells were randomly counted to determine the maturation index. The epithelial cells were classified by the following morphological criteria: (i) superficial cells containing a pyknotic nucleus of less than 5 μm in diameter and an orange-red (or eosinophilic) cytoplasm, (ii) intermediate cells having vesicular nuclei and a pale blue cytoplasm, and (iii) parabasal cells having a nuclear diameter of greater than one-third the diameter of the cell and a blue-green cytoplasm [29].
Vaginal fluid pH
Vaginal fluid pH measurements were taken once a week before vaginal smears throughout the study. Vaginal pH was determined using a pH indicator strip (Whatman® Panpeha™; Sigma-Aldrich, St. Louis, MO, USA), which was inserted into the vagina and left for 10 sec (until moistened). The pH was read after the color stabilized by comparison with a standard colorimetric chart (sensitivity of 0.5 pH) ranging from pH 0 to 14. If the color fell between two values on the chart, the average of the two pH readings was used.
Plasma LH levels
Plasma LH levels were measured using the heterologous radioimmunoassay system described previously [20] using iodinated rat NIDDK-rat LH-I-5 and anti-ovine LH antiserum (YM#18; NIH, Torrance, CA, USA). The results are expressed in terms of NIDDK rat LH-RP-2 start (NIH, Torrance, CA, USA), and the intra- and inter-assay coefficients of variation were 5.7% and 7.1%.
Sex skin coloration
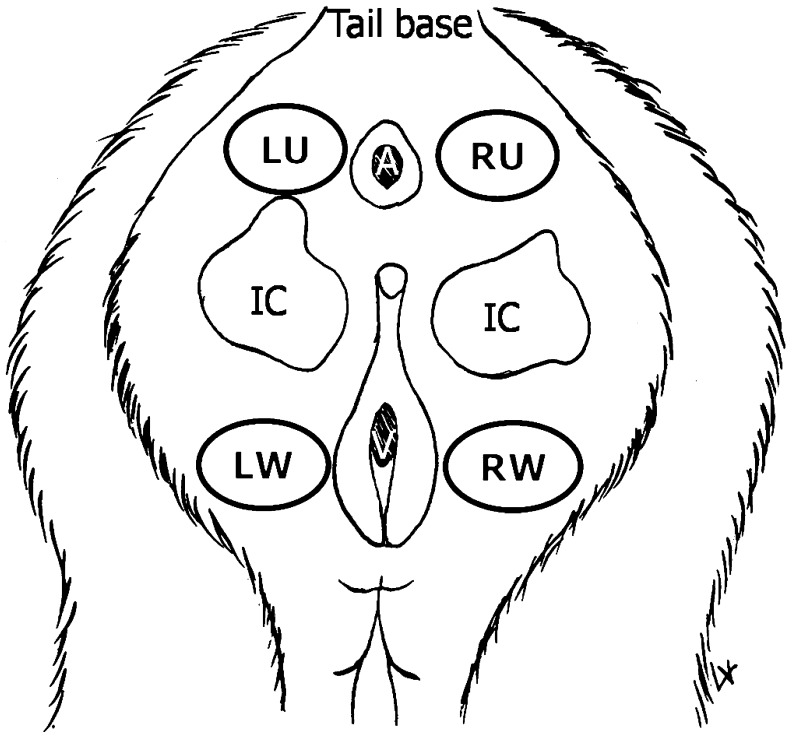
Sex skin color was quantitatively determined using a color reflectometer (Color Analyzer Model CR-200, Minolta, Japan) and expressed by the three parameters, L* (lightness, ranging from darkest (value 0) to lightest (value 100)), a* (the hue of green ranging from (–60) to red (+60)) and b* (the hue of blue ranging from (–60) to yellow (+60)) [31]. Since the sex skin reddening indicated the serum estrogen levels, only the a* value was analyzed in this study. The color of the sex skin around the vagina was measured at four areas, the upper and lower areas of the ischial callosities on both the left and right sides (Fig. 1), after blood collection at the end of the pretreatment, treatment and posttreatment periods.
Fig. 1.
Approximate location of the four areas of the sex skin around the vagina where the color was measured in each postmenopausal monkey: left side upper ischial callosity (LU), right side upper ischial callosity (RU), left side lower ischial callosity (LW), and right side lower ischial callosity (RW). A, V and the grey areas indicate the anus, vagina and ischial callosities, respectively.
Preparation of PM extract and phytoestrogen analysis
The PM cultivar SARDI 190, from Kasetsart University, Kampangsan campus (lot no. 0070317), was used as the source material. Tuberous roots of 3-year-old plants were harvested in March 2007. The fresh tubers were chopped into small pieces and dried at 60 ± 5 C. The dried material (100 g) was ground and macerated three times in 300 ml of 70% (v/v) ethanol each [14]. The ethanol extracts were pooled, filtered through filter paper (Whatman No. 4) and evaporated in a rotary evaporator to produce 17.8 g ethanol extract of PM. The dried extract was stored at –20 C until used, at which time it was first homogeneously mixed at 0.1 or 1% (w/w) into K-Y jelly (Johnson & Johnson, Bangkok, Thailand).
To determine the phytoestrogen content in the PM extract, the extract was analyzed using HPLC as previously described [14] with minor modifications. Briefly, 1 mg of dried extract was dissolved in 1.5 ml of absolute ethanol and diluted with 0.5 ml of 0.1% (v/v) phosphoric acid in deionized water. The extract was injected (10 μl) into an HPLC system (model Agilent 1000; Agilent, Waldbronn, Germany) equipped with a reverse phase Symmetry C18 column (250 mm × 4.6 mm, 5 μm; Phenomenex). The mobile phase consisted of 0.1% (v/v) phosphoric acid in deionized water and 0.1% (v/v) phosphoric acid in acetonitrile with gradient elution at flow rate of 1 ml/min. The isoflavone contents in the sample were identified and quantified by comparison of their retention time and peak areas with reference to known standards and concentrations, respectively, resolved under the same conditions. The isoflavone standards used were puerarin (LKT Laboratories, Inc., St. Paul, MN, USA), daidzin (Sigma-Aldrich), genistin (Fluka, Buchs, Switzerland), daidzein (Sigma-Aldrich) and genistein (LC Laboratories, Woburn, MA, USA), each calibrated at four different concentrations over the range of 0.05–50 μg/ml for daidzin, genistin and genistein and 0.07–70 μg/ml for puerarin and daidzein.
Statistical analysis
The results are expressed as the mean ± 1 standard error (SE). Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS, Chicago, IL, USA). Differences among the treatment periods were evaluated by one-way analyses of variance (ANOVA) followed by the least significant difference post hoc test when the distribution of the variables was normal. Statistical significance was accepted at a P value of less than 0.05.
Results
Isoflavone contents in PM
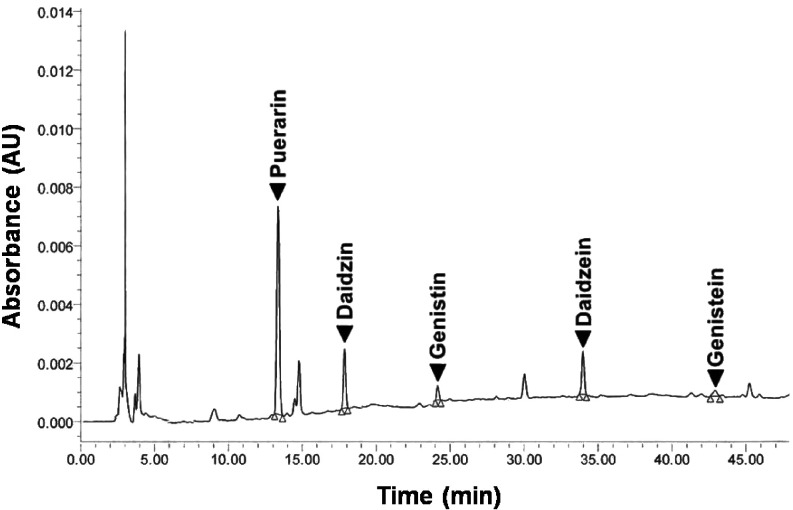
The retention time of the five isoflavone standards, puerarin, daidzin, genistin, daidzein and genistein, were 13.38, 17.88, 24.17, 33.98 and 42.92 min, respectively (Fig. 2). The obtained calibration curve for each standard isoflavone had a high linearity (R2 = 1.000) over the assayed range, whilst the detection sensitivity for the established HPLC analysis of isoflavones in the PM sample was approximately 0.005 μg. The total concentrations of the isoflavones in the PM cultivar SARDI 190 extract, as analyzed by HPLC, were found to be 76.27, 14.71, 17.44, 3.23 and 12.01 mg/100 g of PM dry powder for puerarin, daidzin, genistin, daidzein and genistein, respectively.
Fig. 2.
HPLC chromatogram of the P. mirifica extract revealing its principal isoflavone contents as puerarin, daidzin, genistin, daidzein and genistein.
Vaginal cytology
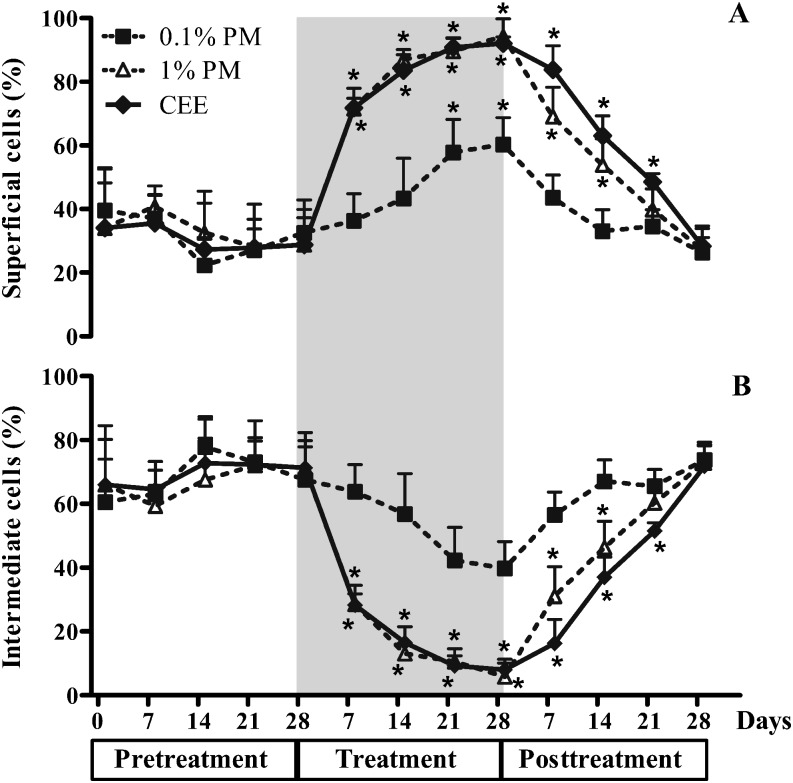
During the pretreatment period, the proportion of superficial cells in all monkeys remained at levels of 10–55% (31.7 ± 1.5%), with the majority being intermediate cells (range 45–89%; 68.3 ± 1.5%) and very few parabasal cells (Figs. 3 and 4). Although the monkeys had different durations for the postmenopausal period (Table 1), the patterns of response to the treatments were essentially the same. Topical vaginal treatment with the 0.1% (w/w) PM cream stimulated a slight proliferation of vaginal epithelium cells to superficial cells in postmenopausal monkeys, but this was not significantly higher than that in the pretreatment period until day 21 of the treatment period (Fig. 4A). In contrast, treatment with the 1% (w/w) PM or CEE both markedly (~1.8- to 2-fold) and significantly increased the proportion of superficial cells above that in the pretreatment period from day 7 of the treatment period, and the proportions remained higher than those in the untreated monkeys until the end of the treatment period. The proportion of the superficial cells then declined to the pretreatment levels within 7, 21 and 28 days after withdrawal of the 0.1% (w/w) PM, 1% (w/w) PM and CEE treatment groups, respectively (Fig. 4A). Congruent with the increased proportion of superficial cells in the 1% (w/w) PM and CEE groups, the levels of intermediate cells in these groups were significantly decreased from day 7 of the treatment period and returned to the pretreatment levels within 21 and 28 days of the posttreatment period, respectively (Fig. 4B). Although the proportion (%) of intermediate cells in the 0.1% (w/w) PM treated group decreased by 21 to 28 days of the treatment period, the proportion was not significantly lower than the pretreatment level.
Fig. 3.
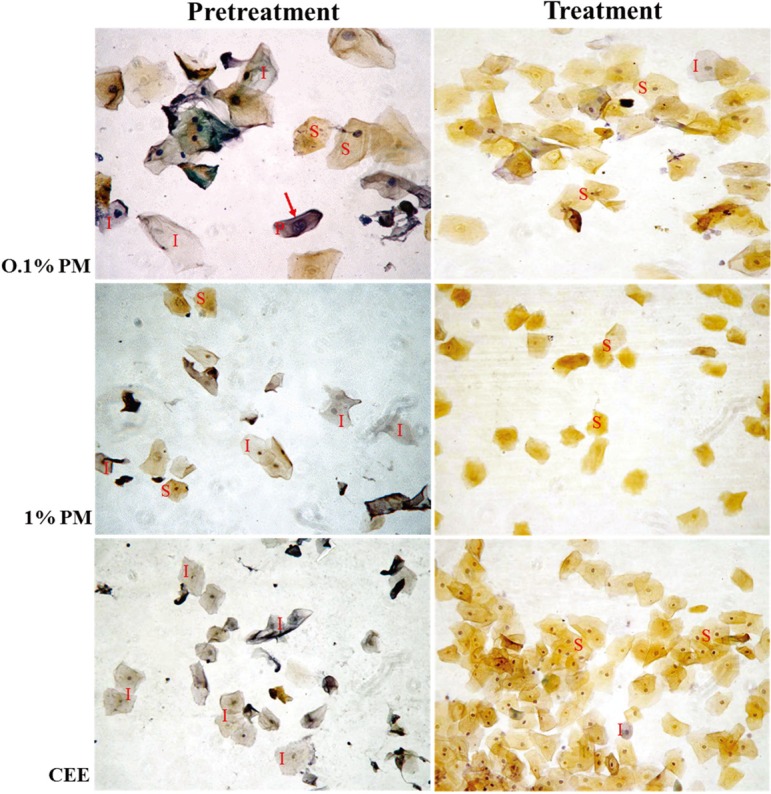
Vaginal cytology for postmenopausal macaques before and after treatment with 0.1 or 1% (w/w) P. mirifica (PM) or conjugated equine estrogens (CEE) vaginal cream for 28 days. Pretreatment vaginal cytology: a low proportion of superficial cells (S; orange-red-stained cytoplasm), a high proportion of intermediate cells (I; pale blue-stained cytoplasm) and very few parabasal cells (P; a nuclear diameter (arrow) of greater than one-third the diameter of the cell). Posttreatment vaginal cytology: an increased proportion of superficial cells (S) in all treatment groups. Images shown are representative fields of vaginal smears from the four monkeys. 200 × magnification, Papanicolau’s stain.
Fig. 4.
The proportion (%) of vaginal (A) superficial cells and (B) intermediate cells in postmenopausal monkeys topically treated daily with 0.1 or 1% (w/w) P. mirifica (PM) or conjugated equine estrogens (CEE) vaginal cream during the pretreatment (day 0, 7, 14, 21, and 28), treatment (grey area; day 7, 14, 21, and 28) and posttreatment (day 7, 14, 21, and 28) periods. Data are shown as the mean ± 1 SE of the four animals in each treatment. *P< 0.05 vs. pretreatment period.
Vaginal fluid pH
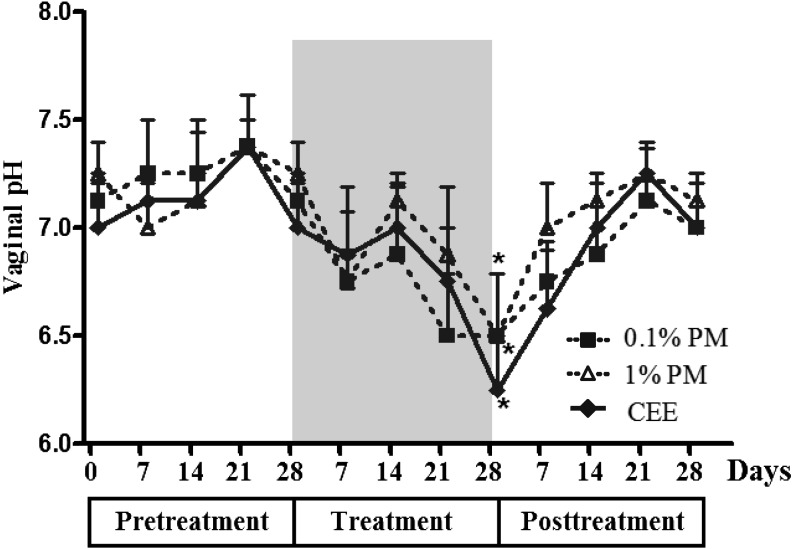
All monkeys had a slightly basic vaginal pH of 7–7.5 (7.2 ± 0.05) throughout the pretreatment period (Fig. 5). Topical treatment with PM and CEE significantly decreased the vaginal pH to slightly acidic levels of 6–7 (6.4 ± 0.14) after 28 days of treatment in a potentially dose-independent manner. The vaginal pH increased to the pretreatment levels within 7 days after withdrawal of the respective PM or CEE treatment.
Fig. 5.
The vaginal pH in postmenopausal monkeys topically treated daily with 0.1 or 1% (w/w) P. mirifica (PM) or conjugated equine estrogen (CEE) vaginal cream during the pretreatment (day 0, 7, 14, 21, and 28), treatment (grey area; day 7, 14, 21, and 28) and posttreatment (day 7, 14, 21, and 28) periods. Data are shown as the mean ± 1 SE of four animals. *P< 0.05 vs. pretreatment period.
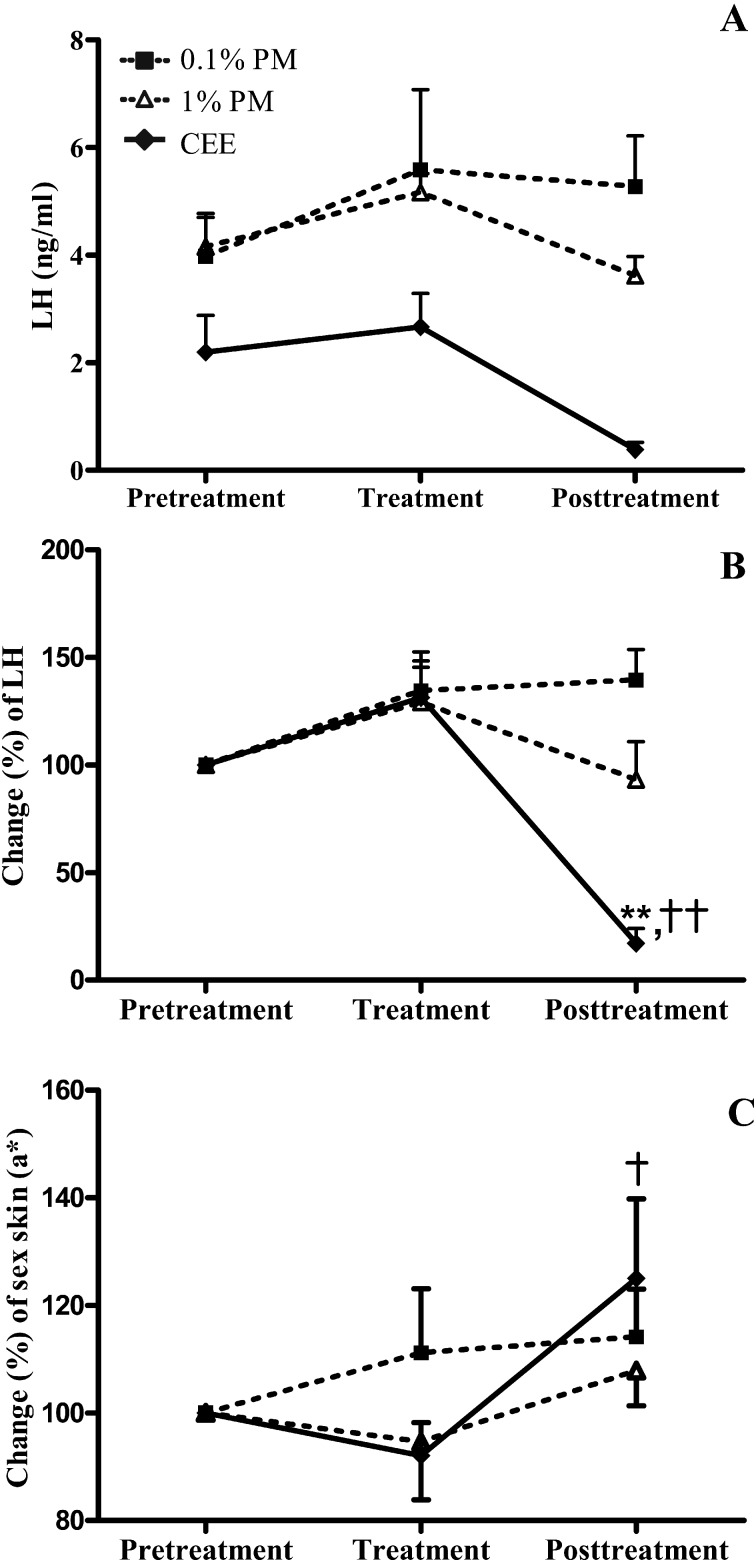
Plasma LH levels
Due to the magnitude of the interindividual variation in the pretreatment levels of LH in plasma (Fig. 6A), in addition to the limitation of a small sample size (four monkeys per treatment group), the plasma LH levels at the end of the treatment and posttreatment periods were adjusted to percentage changes of the pretreatment levels (Fig. 6B). Compared with the pretreatment levels, the plasma LH levels did not significantly change throughout the treatment and posttreatment periods in either PM-treated group of monkeys (0.1% (w/w) and 1% (w/w) PM). Although the plasma LH levels were unchanged at the end of the treatment period in the CEE group, a large significant decrease was found (P <0.01) at the end of the posttreatment period.
Fig. 6.
The levels (A) and (B) change (%) in plasma LH and (C) a* values (the hue of green (–60) to red (+60)) for the sex skin color in postmenopausal monkeys topically treated daily with 0.1 or 1% (w/w) P. mirifica (PM) or conjugated equine estrogen (CEE) vaginal cream at the end of the 28-day pretreatment, treatment and posttreatment periods. The values for sex skin color were expressed only for areas below the ischial callosities. Data are shown as the mean ± 1 SE of four animals. **P< 0.01 vs. pretreatment period. †,†† P< 0.05 and 0.01 vs. treatment period, respectively.
Sex skin color
Because the sex skin color (a* value) was measured in four areas, the upper and lower areas of the ischial callosities on the left and right sides (separate analyses) were compared to determine whether or not they were statistically equivalent. No significant differences between the left and right areas were detected during the three treatment periods in each of the three treatment groups, and so the values for the left and right upper areas, and separately those of the lower areas, of the ischial region were pooled. As expected, the intensity of the red hue in the lower ischial region was numerically higher than in the upper region, with statistically significant differences being found in two (the 0.1% (w/w) PM posttreatment and CEE pretreatment groups) treatment groups (Table 2). Given the small sample size and relatively large variance within each group, it is plausible that other numerical differences between groups could be significant, and so the values for the colors of the upper and lower ischial regions were not pooled but compared separately between the treatment periods and groups.
Table 2. The sex skin color (a* value) in the upper and lower areas of ischial callosities in each monkey used in the three treatment groups.
| Treatment1 | Pretreatment2,3 | Treatment2,3 | Posttreatment2,3 |
| 0.1% (w/w) PM | |||
| Upper (U) | 20.23 ± 1.59 | 20.96 ± 0.87 | 18.68 ± 2.67 |
| Lower (W) | 24.85 ± 2.27 | 25.90 ± 1.75 | 28.81 ± 0.65a |
| 1% (w/w) PM | |||
| Upper (U) | 12.24 ± 1.55 | 12.65 ± 1.20 | 13.19 ± 1.80 |
| Lower (W) | 16.51 ± 1.68 | 15.96 ± 2.71 | 17.50 ± 1.66 |
| CEE | |||
| Upper (U) | 15.28 ± 2.09 | 16.75 ± 1.04 | 19.78 ± 2.24 |
| Lower (W) | 21.16 ± 1.95a | 18.91 ± 1.46 | 24.27 ± 0.82† |
1 The locations of the areas used to measure the color of the upper (U) and lower (W) areas of the ischial are shown in Fig. 1. 2 Data are shown as the mean ± 1 SE of the two upper or lower areas of the ischial in the four monkeys in each group. a P<0.05 vs. upper. † P<0.05 vs. treatment period. 3 At the each end of the 28-day pretreatment, treatment and posttreatment periods.
Similar to the plasma LH levels, the sex skin color of the macaques varied between individuals in the pretreatment period. Therefore, to validate the results, the a* values were adjusted to the percentage change relative to those of the pretreatment values. The only significant increase (P <0.05) in the a* value of the upper ischial region in the CEE group was observed during the posttreatment period (Fig. 6C).
Discussion
The present study demonstrated that daily topical vaginal treatment of the PM extract significantly improved the vaginal atrophy of postmenopausal cynomolgus macaques with efficacy comparable to that of the CEE, as shown by the increased ratio of superficial cells to intermediate cells in the vaginal cytology smears. These vaginal applications of PM extract and CEE creams elicited similar effects to those found with oral administration of PM in humans, cynomolgus monkeys and rodents [9,10,11,12,13,14, 32, 33]. In general, the increased proportion of superficial cells (karyopyknotic or eosinophilic index) correlated well with the estrogen peak before the time of ovulation in women and female cynomolgus macaques with regular menstrual cycles [13, 34]. The vagina of premenopausal and postmenopausal women and cynomolgus macaques is covered by a mucosa with a pluristratified Malpighian epithelium [33, 35] that expresses both ERα and ERβ [15, 17, 36] and is markedly sensitive to sex steroids, particularly to estrogens [37]. As expected, genistein and puerarin, the key phytoestrogen components in PM, were reported to stimulate vaginal proliferation in postmenopausal women and ovariectomized rats [12, 38, 39]. Regarding the comparable recovery times between the 1% (w/w) PM and CEE treatments, this suggested that a 1% (w/w) PM extract cream (~4.69 μg of puerarin plus genistin per application; 6.18 μg of total phytoestrogens per application) could be used as an alternative to the CEE cream (313 μg mixed estrogens per application) in postmenopausal women.
An important rationale for the use of the macaque model in this study is the high degree of similarity in the pathophysiology and responses to hormonal agents between the human and macaque vaginal tissues [17, 32,33,34, 40]. Together with the increased proportion of vaginal superficial cells, treatment with the PM extract also resulted in a decrease in vaginal pH to a slightly acidic level similar to that observed in the CEE-treated group. Indeed, estrogens and phytoestrogens stimulate vaginal epithelial maturation and subsequent glycogen production. Glycogen-consuming Lactobacilli can then colonize the vagina and lower the vaginal pH by catabolism of glycogen into lactic acid [41, 42]. With a decline in estrogen levels, as found in menopause, the pH in the vagina rises because of a loss of Lactobacilli and overgrowth of other pathogenic bacteria [42, 43]. With respect to the finding that estrogen replacement therapy can restore the Lactobacillus microbiota in the vagina of postmenopausal women [44], the decreased vaginal pH after the PM and CEE treatments observed in this study (–1 to –1.5 pH) would potentially be indirectly due to the increase in Lactobacilli numbers. Judging from the steps required for the formation and maintenance of an acidic environment in the vagina, it is not surprising that the significant decrease in vaginal pH after topical application of the PM extract and CEE occurred beginning about three weeks after the vaginal proliferation (based upon the observed vaginal keratinization and pH drop from day 7 to day 28 of the treatment period, respectively). In agreement with the results of this study, postmenopausal women vaginally treated with 0.3 mg CEE cream were observed to exhibit a decrease in vaginal pH (–1.6 pH) from the baseline after 12 weeks of treatment [40].
Estrogen induces feedback inhibition of pituitary synthesis and secretion of LH [45]. The decline in estrogen levels in menopause is the predominant cause for the increase in serum LH levels, and so the decline in LH levels observed after oral administration of PM in ovariectomized rats [10] and postmenopausal monkeys [20] represents the systemic estrogenic activity of PM. Although intravaginal formulations were developed to avoid systemic exposure to estrogens, several studies have demonstrated that all intravaginal estrogen formulations led to increased serum estrogen levels [6, 46]. Thus, lack of an increase in serum LH levels after vaginal CEE or PM treatment could be used to indicate the resultant systemic estrogenic activity of the topically applied chemicals. With regards to the present study, significant suppression of the plasma LH levels was found in the CEE-treated group but not in either of the PM-treated (0.1% (w/w) or 1% (w/w) PM) groups. In addition, suppression of the plasma LH levels in the CEE-treated group was observed later than expected at the end of the posttreatment period and not at the end of the treatment period. One possible explanation for this is that the localized topical application of CEE to the vagina resulted in slow absorption through the atrophic vaginal mucosa into the blood circulation [40, 47, 48], and so it took some time to reach the threshold level for the suppression of pituitary LH synthesis and secretion. In the present study, suppression of the plasma LH levels in the CEE-treated group was observed only at the end of the posttreatment period; however, the vaginal cytology and pH were similar to those in the pretreatment state. These different responses may result from a reduced sensitivity of vaginal tissue due to prolonged estrogen exposure in the postmenopausal monkey. Taken together, the present findings indicate that systemic absorption is greater with the CEE cream compared with the PM cream.
Systematic changes in the sex skin color have been found in several species of Old World nonhuman primates, such as Japanese, rhesus and cynomolgus macaques, which all have a multi-male/multi-female social systems in which one male can copulate with many females and vice versa [24, 49, 50]. Generally, these color changes can be discriminated with the naked eye. Sex skin reddening during the ovulation period, induced by a high serum estrogen level, is used as an external sign for male monkeys to approach females for copulation [23, 49]. For example, ovariectomized rhesus macaques injected daily with estradiol benzoate or estrone for 10 days showed a significant increase in the intensity of their sex skin reddening and were then approached and mounted by males and received ejaculates from them more frequently than females not treated with hormones (control) [51]. However, use of seasonal breeding animals, such as rhesus or Japanese macaques, to detect the estrogenic activity of exogenous chemical treatments on sex skin reddening could be difficult because the changes are subtle during the nonbreeding season [50, 52]. Thus, cynomolgus macaques, nonseasonal breeders, should be a better nonhuman primate model in this sense [21, 24]. Interestingly, congruent with the reduction in the plasma LH levels, topical vaginal treatment with CEE increased the intensity of the redness of the sex skin, while topical vaginal treatment with either dose of PM did not. This is consistent with previously reported results in which oral administration of 100 to 1,000 mg/day of PM increased the reddish color of the sex skin of postmenopausal cynomolgus macaques due to the lack of an increase in serum LH levels [20, 25]. Thus, measurement of the intensity of sex skin reddening, which is a noninvasive, inexpensive, convenient and quick method, may provide a simple and reliable way of estimating the likely short-term systemic estrogen effect of topical application of vaginal estrogenic chemicals.
A 28-day topical vaginal treatment with PM extract could stimulate maturation of the vaginal epithelium and lead to an acidic vaginal pH in cynomolgus macaques at least 5 years postmenopause. By using topical vaginal application, PM did not induce any discernible systemic side effects, as indicated by the absence of detected changes in the plasma LH levels and sex skin coloration. With respect to previous findings in postmenopausal women, the oral consumption of PM was reported to also ameliorate other postmenopausal symptoms, such as hot flushes, frustration, sleep disorder, skin dryness, high blood cholesterol levels and dyspareunia [13, 53]. In addition, oral administration of PM also maintained the bone mass [54] in ovariectomized rats and had beneficial cardiovascular effects in ovariectomized rabbits [55]. Taken together, these results suggest that PM should be a safer alternative choice for treatment of vaginal atrophy in postmenopausal women. However, to ensure the absence of systemic side effects in long-term use, suitable studies on long-term vaginal treatment with PM need to be performed. In addition, since a daily vaginal application of PM cream might result in low patient compliance, especially for postmenopausal women with busy daily live or reduced memory levels, slow release and better vaginal adherence forms of PM extract should be developed.
In conclusion, these results clearly demonstrate that topical vaginal treatment with PM plays a key role in the maturation of the vaginal epithelium in postmenopausal monkeys. Additionally, PM should be applicable to treatment of vaginal atrophy and reduce the incidence of related symptoms in menopausal women.
Acknowledgments
The authors thank Dr Robert Butcher, Faculty of Science, Chulalongkorn University for proofreading of the manuscript, Mr Lerson Vasinopas for providing the drawing in Fig. 1 and Professor Yuzuru Hamada for a color reflectometer. This study was supported in part by a Grant for Development of New Faculty Staff, Chulalongkorn University, and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530191-AS).
References
- 1.Hextall A. Oestrogens and lower urinary tract function. Maturitas 2000; 36: 83–92 [DOI] [PubMed] [Google Scholar]
- 2.Stenberg A, Heimer G, Ulmsten U, Cnattingius S. Prevalence of genitourinary and other climacteric symptoms in 61-year-old women. Maturitas 1996; 24: 31–36 [DOI] [PubMed] [Google Scholar]
- 3.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61: 3090–3096 [PubMed] [Google Scholar]
- 4.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 18: CD001500 [DOI] [PubMed] [Google Scholar]
- 5.Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. J Womens Health (Larchmt) 2009; 18: 1595–1606 [DOI] [PubMed] [Google Scholar]
- 6.Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009; 12: 91–105 [DOI] [PubMed] [Google Scholar]
- 7.Geller SE, Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt) 2005; 14: 634–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Fr Medecine 2012; 6: 8–21 [DOI] [PubMed] [Google Scholar]
- 9.Cherdshewasart W, Kitsamai Y, Malaivijitnond S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J Reprod Dev 2007; 53: 385–393 [DOI] [PubMed] [Google Scholar]
- 10.Malaivijitnond S, Kiatthaipipat P, Cherdshewasart W, Watanabe G, Taya K. Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J Pharmacol Sci 2004; 96: 428–435 [DOI] [PubMed] [Google Scholar]
- 11.Malaivijitnond S, Chansri K, Kijkuokul P, Urasopon N, Cherdshewasart W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J Ethnopharmacol 2006; 107: 354–360 [DOI] [PubMed] [Google Scholar]
- 12.Malaivijitnond S, Tungmunnithum D, Gittarasanee S, Kawin K, Limjunyawong N. Puerarin exhibits weak estrogenic activity in female rats. Fitoterapia 2010; 81: 569–576 [DOI] [PubMed] [Google Scholar]
- 13.Manonai J, Chittacharoen A, Theppisai U, Theppisai H. Effect of Pueraria mirifica on vaginal health. Menopause 2007; 14: 919–924 [DOI] [PubMed] [Google Scholar]
- 14.Urasopon N, Hamada Y, Asaoka K, Poungmali U, Malaivijitnond S. Isoflavone content of rodent diets and its estrogenic effect on vaginal cornification in Pueraria mirifica-treated rats. Sci Asia 2008; 34: 371–376 [Google Scholar]
- 15.Robinson D, Register TC, Carter LR. The effects of delayed hormone replacement therapy on estrogen receptors of the cynomolgus monkey bladder and vagina. Neurourol Urodyn 1998; 17: 241–247 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt S, Degen GH, Seibel J, Hertrampf T, Vollmer G, Diel P. Hormonal activity of combinations of genistein, bisphenol A and 17β-estradiol in the female Wistar rat. Arch Toxicol 2006; 80: 839–845 [DOI] [PubMed] [Google Scholar]
- 17.Skala CE, Petry IB, Albrich SB, Puhl A, Naumann G, Koelbl H. The effect of hormonal status on the expression of estrogen and progesterone receptor in vaginal wall and periurethral tissue in urogynecological patients. Eur J Obstet Gynecol Reprod Biol 2010; 153: 99–103 [DOI] [PubMed] [Google Scholar]
- 18.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252–4263 [DOI] [PubMed] [Google Scholar]
- 19.Trisomboon H, Malaivijitnond S, Watanabe G, Taya K. Estrogenic effects of Pueraria mirifica on the menstrual cycle and hormone-related ovarian functions in cyclic female cynomolgus monkeys. J Pharmacol Sci 2004; 94: 51–59 [DOI] [PubMed] [Google Scholar]
- 20.Trisomboon H, Malaivijitnond S, Watanabe G, Cherdshewasart W, Taya K. The estrogenic effect of Pueraria mirifica on gonadotrophin levels in aged monkeys. Endocrine 2006; 29: 129–134 [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh K, Koudy Williams J, Wagner JD. Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol 2005; 34: 171–177 [DOI] [PubMed] [Google Scholar]
- 22.Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, Cline JM. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod 2006; 75: 477–486 [DOI] [PubMed] [Google Scholar]
- 23.Dixson AF. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes and Human Beings. New York: Oxford University Press; 1998 [Google Scholar]
- 24.Engelhardt A, Hodges JK, Niemitz C, Heistermann M. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm Behav 2005; 47: 195–204 [DOI] [PubMed] [Google Scholar]
- 25.Trisomboon H, Malaivijitnond S, Cherdshewasart W, Watanabe G, Taya K. Effect of Pueraria mirifica on the sexual skin coloration of aged menopausal cynomolgus monkeys. J Reprod Dev 2006; 52: 537–542 [DOI] [PubMed] [Google Scholar]
- 26.Malaivijitnond S, Hamada Y, Suryobroto B, Takenaka O. Female long-tailed macaques with scrotum-like structure. Am J Primatol 2007; 69: 721–735 [DOI] [PubMed] [Google Scholar]
- 27.Samsioe G. Urogenital aging—a hidden problem. Am J Obstet Gynecol 1998; 178: S245–S249 [DOI] [PubMed] [Google Scholar]
- 28.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science 2004; 305: 1423–1426 [DOI] [PubMed] [Google Scholar]
- 29.Mohanty D, Das KC. Effect of folate deficiency on the reproductive organs of female rhesus monkeys: a cytomorphological and cytokinetic study. J Nutr 1982; 112: 1565–1576 [DOI] [PubMed] [Google Scholar]
- 30.Lillie RD. Histologie Technic and Practical Histochemistry, 3rd ed. New York: McGraw-Hill Book; 1965: 561–564. [Google Scholar]
- 31.Hamada Y, Suryobroto B, Goto S, Malaivijitnond S. Morphological and body color variation in Thai Macaca fascicularis fascicularis north and south of the Isthmus of Kra. Int J Primatol 2008; 29: 1271–1294 [Google Scholar]
- 32.Kaari C, Haidar MA, Júnior JM, Nunes MG, Quadros LG, Kemp C, Stavale JN, Baracat EC. Randomized clinical trial comparing conjugated equine estrogens and isoflavones in postmenopausal women: a pilot study. Maturitas 2006; 53: 49–58 [DOI] [PubMed] [Google Scholar]
- 33.Cline JM, Botts S, Lees CJ, Brommage R. Effects of lasofoxifene on the uterus, vagina and breast in ovariectomized cynomolgus monkeys (Macacafascicularis). Am J Obstet Gynecol 2008; 199: 158.e1–158.e8. [DOI] [PubMed] [Google Scholar]
- 34.Mehta RR, Jenco JM, Gaynor LV, Chatterton RT., JrRelationships between ovarian morphology, vaginal cytology, serum progesterone, and urinary immunoreactive pregnanediol during the menstrual cycle of the cynomolgus monkey. Biol Reprod 1986; 35: 981–986 [DOI] [PubMed] [Google Scholar]
- 35.Chen GD, Oliver RH, Leung BS, Lin LY, Yeh J. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril 1999; 71: 1099–1102 [DOI] [PubMed] [Google Scholar]
- 36.Flickinger GL, Elsner C, Illingworth DV, Muechler EK, Mikhail G. Estrogen and progesterone receptors in the female genital tract of humans and monkeys. Ann N Y Acad Sci 1977; 286: 180–189 [DOI] [PubMed] [Google Scholar]
- 37.Jensen EV, Suzuki T, Numata M, Smith S, DeSombre ER. Estrogen-binding substances of target tissues. Steroids 1969; 13: 417–427 [DOI] [PubMed] [Google Scholar]
- 38.Rimoldi G, Christoffel J, Seidlova-Wuttke D, Jarry H, Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect 2007; 115(Suppl 1): 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Donne M, Caruso C, Mancuso A, Costa G, Iemmo R, Pizzimenti G, Cavallari V. The effect of vaginally administered genistein in comparison with hyaluronic acid on atrophic epithelium in postmenopause. Arch Gynecol Obstet 2011; 283: 1319–1323 [DOI] [PubMed] [Google Scholar]
- 40.Bachmann G, Bouchard C, Hoppe D, Ranganath R, Altomare C, Vieweg A, Graepel J, Helzner E. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009; 16: 719–727 [DOI] [PubMed] [Google Scholar]
- 41.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun 1999; 67: 5170–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis 2003; 188: 1054–1058 [DOI] [PubMed] [Google Scholar]
- 44.Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can J Microbiol 2005; 51: 777–781 [DOI] [PubMed] [Google Scholar]
- 45.Johnson MH, Everitt BJ. Essential Reproduction, 6th ed. Massachusetts: Blackwell Publishing; 2007: 102–132. [Google Scholar]
- 46.Labrie F, Cusan L, Gomez JL, Côté I, Bérubé R, Bélanger P, Martel C, Labrie C. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 2009; 16: 30–36 [DOI] [PubMed] [Google Scholar]
- 47.Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency—a pharmacokinetic and pharmacodynamic study. Maturitas 1992; 15: 121–127 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt G, Andersson SB, Nordle O, Johansson CJ, Gunnarsson PO. Release of 17-beta-oestradiol from a vaginal ring in postmenopausal women: pharmacokinetic evaluation. Gynecol Obstet Invest 1994; 38: 253–260 [DOI] [PubMed] [Google Scholar]
- 49.Czaja JA, Robinson JA, Eisele SG, Scheffler G, Goy RW. Relationship between sexual skin colour of female rhesus monkeys and midcycle plasma levels of oestradiol and progesterone. J Reprod Fertil 1977; 49: 147–150 [DOI] [PubMed] [Google Scholar]
- 50.Wallner B, Aspernig D, Millesi E, Machatschke IH. Non-lactating versus lactating females: a comparison of sex steroids, sexual coloration, and sexual behavior in Japanese macaques. Primates 2011; 52: 69–75 [DOI] [PubMed] [Google Scholar]
- 51.Wallen K, Goy RW. Effects of estradiol benzoate, estrone, and propionates of testosterone or dihydrotestosterone on sexual and related behaviors of ovariectomized Rhesus monkeys. Horm Behav 1977; 9: 228–248 [DOI] [PubMed] [Google Scholar]
- 52.Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm Behav 1976; 7: 481–494 [DOI] [PubMed] [Google Scholar]
- 53.Muangman V, Cherdshewasart W. Clinical trial of the phytoestrogen rich herb, Pueraria mirifica as a crude drug in the treatment of symptoms in menopausal woman. Siriraj Hosp Gaz 2001; 53: 300–309 [Google Scholar]
- 54.Urasopon N, Hamada Y, Cherdshewasart W, Malaivijitnond S. Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas 2008; 59: 137–148 [DOI] [PubMed] [Google Scholar]
- 55.Wattanapitayakul SK, Chularojmontri L, Srichirat S. Effects of Pueraria mirifica on vascular function of ovariectomized rabbits. J Med Assoc Thai 2005; 88(Suppl 1): S21–S29 [PubMed] [Google Scholar]