Abstract
Amnestic mild cognitive impairment (aMCI) is recognized as the prodromal phase of Alzheimer’s disease (AD). Evidence showed that patients with multiple-domain (MD) aMCI were at higher risk of converting to dementia and exhibited more severe gray matter atrophy than single-domain (SD) aMCI. The investigation of the microstructural abnormalities of white matter (WM) among different subtypes of aMCI and their relations with cognitive performances can help to understand the variations among aMCI subtypes and to construct potential imaging based biomarkers to monitor the progression of aMCI. Diffusion-weighted MRI data were acquired from 40 patients with aMCI (aMCI-SD: n = 19; aMCI-MD: n= 21) and 37 healthy controls (HC). Voxel-wise and atlas-based analyses of whole-brain WM were performed among three groups. The correlations between the altered diffusion metrics of the WM tracts and the neuropsychological scores in each subtype of aMCI were assessed. The aMCI-MD patients showed disrupted integrity in multiple WM tracts across the whole-brain when compared with HCs or with aMCI-SD. In contrast, only few WM regions with diffusion changes were found in aMCI-SD as compared to HCs and with less significance. For neuropsychological correlations, only aMCI-MD patients exhibited significant associations between disrupted WM connectivity (in the body of the corpus callosum and the right anterior internal capsules) and cognitive impairments (MMSE and Digit Symb-Coding scores), whereas no such correlations were found in aMCI-SD. These findings indicate that the degeneration extensively exists in WM tracts in aMCI-MD that precedes the development of AD, whereas underlying WM pathology in aMCI-SD is imperceptible. The results are consistent with the view that aMCI is not a uniform disease entity and presents heterogeneity in the clinical progression.
Keywords: Amnestic mild cognitive impairment, diffusion tensor imaging, multiple-domain, single-domain, TBSS, white matter
INTRODUCTION
Amnestic mild cognitive impairment (aMCI) is considered as a transition between normal aging and Alzheimer’s disease (AD) [1]. Patients with aMCI are at greater risk for developing AD and convert at an annual rate of 10% to 15% [2, 3]. According to the number of affected cognitive domains, aMCI patients can be further categorized as single-domain (aMCI-SD) or multi-domain (aMCI-MD) aMCI. The aMCI-SD subtype indicates a selective episodic memory impairment. The aMCI-MD subtype, in contrast, indicates substantial deficits in at least one other cognitive domain [4]. There is evidence showed that aMCI-MD subjects were much more likely to convert to dementia (69% conversion) compared with aMCI-SD (32% conversion) [5]. In addition to the clinical evidence of higher conversion rates, previous structural magnetic resonance imaging (MRI) studies demonstrated that patients with aMCI-MD showed more diffuse and extensive gray matter atrophy than aMCI-SD [6, 7], suggesting aMCI-MD may be further along the path to AD.
It is known that abnormalities related to AD are not only limited to gray matter but also to white matter (WM). As reported previously using the diffusion tensor imaging (DTI) technique, abnormal diffusion changes in the WM tracts in patients with AD and aMCI were reported [8–15]. However, there were only a few investigations of WM disruption in aMCI subgroups. Recently, Haller and colleagues assessed the DTI based WM differences between aMCI-MD and aMCI-SD directly without recruiting normal controls [16]. The group-level analysis revealed that patients with aMCI-MD displayed a more widespread damage of long interhemispheric pathways, mainly in the right hemisphere compared with the aMCI-SD subgroup. Such investigation will not only be of research interest for better understanding the disease process, but more importantly in the search for better imaging-based biomarkers in the context of prevention and early treatment of AD.
Tract-based spatial statistics (TBSS) is a method for analyzing diffusion MRI data. TBSS can automatically perform voxel-wise statistics on diffusion measures while simultaneously minimizing the effects of misalignment [17]. It has been applied to investigate the WM changes in aMCI patients recently as a single group by different researchers [18–21]. However, the findings are to some extent discrepant, which may be due to different acquiring parameters and different sample size. Moreover, the heterogeneity in patients with MCI was also a potentially important reason. This study aimed to use TBSS to explore the diffusion changes in WM tracts in aMCI subtypes. We hypothesized that aMCI-MD patients would exhibit more diffuse and extensive WM structural alterations than aMCI-SD subjects. Furthermore, we examined the relationship between the altered diffusion measures and neuropsychological performances in aMCI subtypes, attempting to relate the WM abnormality with disease severity and cognitive changes.
METHODS
Participants
There were 77 participants (19 aMCI-SD, 21 aMCI-MD, and 37 socio-demographically matched HCs). Participants were recruited from three sources: the Clinic at Neurology Department of Beijing Hospital, the Clinic at China Academy of Chinese Medical, and the research center for cognitive aging and brain health at State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University. They were all right-handed and native Chinese speakers. All participants (1) were aged 50 to 85 years old; (2) had at least 6 years of education; (3) had no structural abnormalities other than cerebrovascular lesions—such as tumors, subdural hematomas, and contusions due to previous head trauma—that could impair cognitive function; (4) had no history of addictions, neurologic or psychiatric diseases, or treatments known to influence cerebral function including alcoholism, current depression, Parkinson’s disease, and epilepsy; (5) did not have large vessel disease, such as cortical or subcortical infarcts and watershed infarcts; and (6) did not have diseases with WM lesions, such as normal pressure hydrocephalus or multiple sclerosis. All aMCI subjects were diagnosed according to Petersen et al.’s criteria [3] for amnestic MCI, including subjective memory complaints, cognitive impairment in memory [scoring more than 1.5 standard deviations below the age- and education-adjusted norm on the Auditory Verbal Learning Test (AVLT) [22]], normal general cognitive function [scoring no less than 24 on the Mini-Mental-Status Examination-Chinese version (MMSE) [23], except two subjects scored 22 and 23], and preserved activities of daily living [scoring 0 on the Activities of Daily Living (ADL)] [24]. If episodic memory was the only area of impairment, the subject was considered to be aMCI-SD. If memory plus other cognitive domains assessed with neuropsychological testing were affected (1.5 standard deviations below age norms), the subject was considered to be aMCI-MD. The HCs had no evidence of cognitive deficits on neuropsychological tests. Demographic information of each group and between-group comparisons are presented in Table 1. The study was approved by the Institutional Review Board of the Beijing Normal University Imaging Center for Brain Research. Written informed consent was obtained from each participant. Ethical permission was obtained from the relevant Research Ethics Committees.
Table 1.
Demographics of all participants and neuropsychological test results
| HCs (n = 37) | Range | aMCI-SD (n = 19) | Range | aMCI-MD (n = 21) | Range | F-value (χ2) | p-value | |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 62.9 ± 5.4 | 51–73 | 64.3 ± 7.4 | 54–76 | 65.6 ±7.2 | 55–81 | 1.19 | 0.31 |
| Education (y) | 11.2 ±2.4 | 7–16 | 11.3± 2.9 | 6–17 | 11.4 ±2.8 | 8–17 | 0.04 | 0.96 |
| Gender (M/F) | 18/19 | 10/9 | 9/12 | 0.39 | 0.82* | |||
| General mental status | ||||||||
| MMSE | 28.3 ±1.7 | 22–30 | 26.8± 1.3 | 25–30 | 25.7 ±1.7 | 22–28 | 17.74 | <0.001a,b,c |
| Episodic memory | ||||||||
| AVLT | 6.3 ±2.1 | 3–11 | 1.8± 1.6 | 0–4 | 2.0 ±1.3 | 0–4 | 57.72 | <0.001a,b |
| ROCF Recall | 15.5 ±7.4 | 6–48 | 10.8± 7.0 | 0–26 | 8.3 ±6.0 | 0–19 | 7.72 | 0.001a,b |
| Visual-spatial ability | ||||||||
| ROCF Copy | 33.4 ±4.3 | 11–36 | 33.9± 1.7 | 31–36 | 32.2 ±3.2 | 25–36 | 1.35 | 0.266 |
| Working memory | ||||||||
| Digit Span | 12.8 ±2.0 | 9–17 | 11.2± 2.0 | 8–15 | 10.9 ±2.9 | 7–17 | 5.63 | 0.005a,b |
| Processing speed | ||||||||
| Symb-Coding | 36.6 ±9.6 | 19–56 | 34.0± 6.7 | 26–53 | 25.3 ±9.6 | 11–50 | 10.85 | <0.001b,c |
| Executive function | ||||||||
| TMT-A time (s) | 55.0 ±19.4 | 25–138 | 53.9± 14.1 | 34–95 | 80.5 ±34.8 | 49–199 | 9.13 | <0.001b,c |
| TMT-B time (s) | 156.8 ±55.2 | 85–310 | 161.6± 32.0 | 109–230 | 230.6 ±72.5 | 104–427 | 12.64 | <0.001b,c |
| Reasoning | ||||||||
| Similarities | 17.1 ±4.0 | 7–23 | 15.8± 3.3 | 8–21 | 13.2 ±4.5 | 6–21 | 6.31 | 0.003b,c |
| Language ability | ||||||||
| BNT | 25.2 ±2.8 | 18–29 | 23.8± 3.1 | 19–28 | 21.5 ±3.7 | 16–28 | 9.27 | <0.001b,c |
All subjects (HCs, aMCI-SD, aMCI-MD) were matched for age, gender, and education. Values are mean ± standard deviation. The comparisons of demographics and neuorpsychological scores among three groups (aMCI-SD, aMCI-MD, and HCs) were performed with separate one-way analysis of variance (ANOVA). Post-hoc pair-wise comparisons were then performed using t-tests. P< 0.05 was considered significant.
The p value for gender distribution in the three groups was obtained using a Chi-square test.
Post-hoc paired comparisons showed significant group differences between HCs and aMCI-SD.
Post-hoc paired comparisons showed significant group differences between HCs and aMCI-MD.
Post-hoc paired comparisons showed significant group differences between aMCI-SD and aMCI-MD.
Neuropsychological testing
All participants received 8 neuropsychological tests assessing general mental status and other cognitive domains such as processing speed, verbal and nonverbal episodic memory, visual-spatial ability, working memory, executive function, reasoning and language ability. General mental status was assessed with MMSE. Processing speed was assessed with Digit Symb-Coding subtest of Wechsler Adults Intelligence Scale-Chinese revision (WAIS-RC) [25]. Verbal and nonverbal episodic memory tests included AVLT and Recall component of Rey-Osterrieth Complex Figure Test (ROCF) [26]. Visual-spatial ability was assessed with Copy component of ROCF [26]. Verbal working memory was assessed with Digit Span scores of WAIS-RC. Executive function was assessed with Trail Making Test (TMT) [27]. Verbal reasoning and abstract thinking was assessed with Similarities subtest of WAIS-RC. Finally, language ability was assessed with Boston Naming Test (BNT) [28]. Neuropsychological characterizations for each group are presented in Table 1.
Image acquisition
MRI data were acquired using a SIEMENS TRIO-3T scanner in the Imaging Center for Brain Research, Beijing Normal University. Participants lay supine with their head snugly fixed by straps and foam pads to minimize head movement. Diffusion tensor images were acquired using a single-shot echoplanar imaging sequence [coverage of the whole brain, 2 mm slice thickness with no interslice gap, 70 axial slices, repetition time = 9500 ms, echo time = 92 ms, flip angle = 90°, 30 diffusion directions with b = 1000 s/mm2, and an additional image without diffusion weighting (i.e., b =0 s/mm2), acquisition matrix=128×128, filed of view=256×256 mm2, Averages = 3]. Conventional axial T1-weighted, T2-weighted, fluid attenuated inversion recovery (FLAIR) were also acquired to exclude brain pathology and cerebrovascular disease based on the evaluation of WM hyperintensities.
Imaging preprocessing
Preprocessing of imaging data consisted of three steps. First, eddy current distortions and motion artifacts were corrected by applying affine alignment of each diffusion-weighted image to the b = 0 image using FMRIB’s Diffusion Toolbox (FDT) (FSL 4.1.4; http://www.fmrib.ox.ac.uk/fsl). The first volume of the diffusion data without a gradient applied (i.e., the b = 0 image) was then used to generate a binary brain mask using the Brain Extraction Tool. Finally, DTIfit was used to independently fit diffusion tensor to each voxel. The output of DTIfit yielded voxelwise maps of fractional anisotropy, radial diffusivity, axial diffusivity and mean diffusivity.
Tract-Based Spatial Statistics (TBSS)
TBSS of fractional anisotropy, radial diffusivity, axial diffusivity, and mean diffusivity images were carried out using TBSS in the FMRIB software library (FSL 4.1.4; http://www.fmrib.ox.ac.uk/fsl for a detailed description of the methods, see [17]). Briefly, the TBSS analyses are comprised of the following steps: 1) Each subject’s fractional anisotropy image was aligned to a pre-defined target fractional anisotropy image (FMRIB58 FA) by non-linear registrations; 2)All the aligned fractional anisotropy images were transformed into the MNI152 template (1 mm × 1 mm × 1 mm) by affine registrations; 3) The mean fractional anisotropy image and its skeleton were created from all subjects; 4) Individual subjects’ fractional anisotropy images were projected onto the skeleton; and 5) Voxel-wise statistics were performed over the common skeleton.
Then, data for radial diffusivity, axial diffusivity, and mean diffusivity were generated by applying the above fractional anisotropy transformations to the diffusivity maps and projecting them onto the skeleton using identical projection vectors to those inferred from the original fractional anisotropy data.
Atlas-based quantification at the tract level
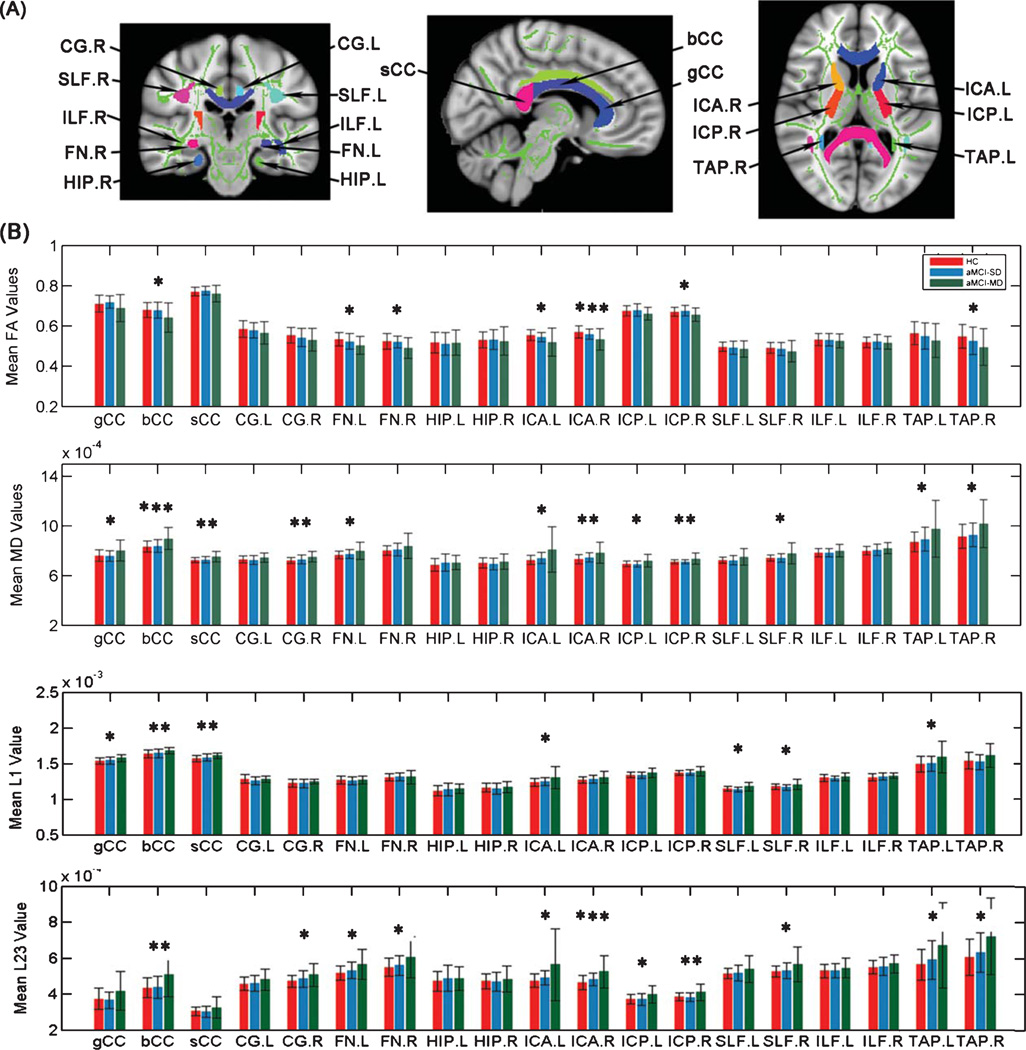
To investigate the diffusion changes in several specific tracts, we used the digital WM atlas JHU ICBM-DTI-81 (http://cmrm.med.jhmi.edu/), a probabilistic atlas generated by mapping DTI data of 81 subjects to a template image. As shown in Fig. 2A, the JHU-WM atlas was overlaid on the WM skeleton of each subject in the ICBM-DTI-81 space, such that each skeleton voxel could be categorized into one of the major tracts. Then fractional anisotropy, radial diffusivity, axial diffusivity, and mean diffusivity at the skeleton voxels within each tract can be calculated. We examined the following tracts: the genu, body and splenium of the corpus callosum (CC), the bilateral cingulum bundles (CG), fornices (FN), hippocampus (HIP), anterior (ICA) and posterior (ICP) internal capsules, superior (SLF) and inferior (ILF) longitudinal fascicule, and taptum (TAP).
Fig. 2.
Mean diffusion metrics of the atlas-based tracts in the aMCI-SD, aMCI-MD, and control groups. A) The JHU-white matter atlas was overlaid on the mean WM skeleton in the ICBM-DTI-81 space. Colored regions indicate major WM tracts. The skeleton from averaged fractional anisotropy maps is shown as green solid curve. FA, fractional anisotropy; MD, mean diffusivity; L1, axial diffusivity; L23, radial diffusivity; gCC, genu of corpus callosum; bCC, body of corpus callosum; sCC, splenium of corpus callosum; CG, cingulum bundle at cingulate gyrus; FN: fornix; HIP: hippocampus; ICA: anterior internal capsule; ICP: posterior internal capsule; SLF: superior longitudinal fasciculus; ILF: inferior longitudinal fasciculus; TAP: taptum. B) Group differences of the mean diffusion metrics of the atlas-based tracts among the aMCI-SD, aMCI-MD, and HC groups. *p < 0.05; **p < 0.01; ***p < 0.05 after Bonferroni correction.
Statistical analysis
Group differences in age, education, and neuropsychological scores were examined by one-way analysis of variance (ANOVA). Post-hoc pair-wise t-tests were performed if ANOVA is significant (p < 0.05). Gender data were analyzed using a Chi-square test. Voxelwise TBSS were carried out using a permutation-based inference tool for nonparametric statistical thresholding (“randomize”, part of FSL [17]). In this study, voxel-wise group comparisons were performed using non-parametric, two-sample t-tests in: aMCI-SD versus HC, aMCI-MD versus HC, and aMCI-MD versus aMCI-SD. The mean fractional anisotropy skeleton was used as a mask (thresholded at a mean fractional anisotropy value of 0.2), and the number of permutations was set to 5,000. The significance threshold for between-group differences was set at p < 0.05 [family-wise error (FWE) correction for multiple comparisons] using the threshold-free cluster enhancement (TFCE) option in the “randomize” permutation-testing tool in FSL [29]. Third, for the diffusion alterations in the atlas-based tract regions of interest (ROIs), we performed one-way ANOVA to compare fractional anisotropy, radial diffusivity, axial diffusivity, and mean diffusivity among three groups for each ROI. Tukey’s post hoc test was performed if ANOVA is significant (p < 0.05). To adjust for possible spurious findings due to multiple testing, Bonferroni correction was conducted. These analyses included 19 comparisons of tract ROIs, thus p value of < 0.0026 (0.05/19) was considered statistically significant for these comparisons. Once significant between-group differences were observed in any diffusion metric of the atlas-based ROIs (p < 0.05 after Bonferroni correction), we assessed the relationship between the altered diffusion metrics of the tract ROIs and the neuropsychological scores in each subtype of aMCI, performed by Pearson’s correlation analysis.
RESULTS
Demographics and neuropsychological testing
There were no significant differences in age, gender, or education among the three groups (p > 0.30, Table 1). As expected, the MMSE scores were significantly higher in HCs compared to aMCI-SD and aMCI-MD patients, although aMCI-SD patients performed better than aMCI-MD patients. For neuropsychological scores, the group effects were significant for all the cognitive domains except visual-spatial ability (p = 0.266), with the best performance in HCs, intermediate in aMCI-SD patients, and the worst in aMCI-MD patients. Comparing the neuropsychological test scores between the aMCI-SD and aMCI-MD groups revealed significant differences in the processing speed, executive function, reasoning, and language ability, but no differences in episodic or working memory.
Whole-brain voxelwise TBSS comparisons
Group comparisons of diffusion metrics between aMCI-SD and HCs
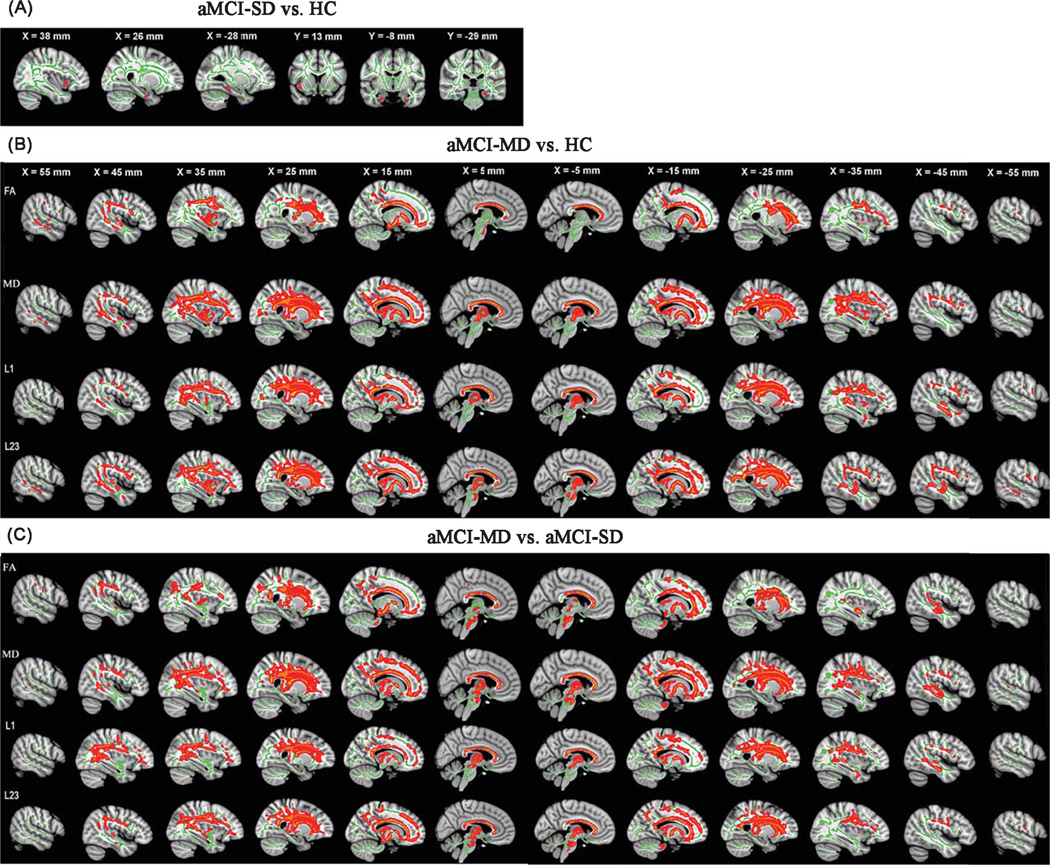
Direct comparison between aMCI-SD patients and HCs did not reveal any significant difference in diffusion metrics after multiple comparison corrections. However, at p< 0.05 (uncorrected), aMCI-SD patients showed increased mean diffusivity in the WM of bilateral parahippocampus and right insula as compared to HCs (Fig. 1A).
Fig. 1.
TBSS results of the diffusion metrics between the aMCI-SD versus HC, aMCI-MD versus HC, and aMCI-MD versus aMCI-SD groups. Green represents the mean white matter skeleton of all subjects. A) Red-yellow voxels (thickened for better visibility) represent the white matter regions with increased mean diffusivity in the aMCI-SD patients compared with HCs (p < 0.05, uncorrected). B) Red-yellow voxels represent the WM regions with reduced fractional anisotropy (FA, first row), increased mean diffusivity (MD, second row), increased axial diffusivity (L1, third row), and increased radial diffusivity (L23, fourth row) in the aMCI-MD patients compared with HCs (p < 0.05, FWE corrected). C) Red-yellow voxels represent the WM regions with reduced FA (first row), increased MD (second row), increased L1 (third row), and increased L23 (fourth row) in the aMCI-MD patients compared with aMCI-SD patients (p < 0.05, FWE corrected).
Group comparisons of diffusion metrics between aMCI-MD and HCs
Compared to the HCs, aMCI-MD patients showed reduced fractional anisotropy in distributed WM regions (Fig. 1B), including the right inferior, middle and superior temporal gyrus, the right medial temporal lobe, the right supramarginal gyrus and angular gyrus, the bilateral precentral and postcentral gyrus, the right insula and precuneus, the bilateral posterior cingulate cortex, superior parietal lobe, medial and orbital frontal gyrus, as well as the whole CC, SLF, inferior fronto-occipital fasciculus, internal capsule, external capsule, and corticospinal tract. For aMCI-MD patients, mean diffusivity increases were also widespread (Fig. 1B), overlapping with the regions showing decreased fractional anisotropy. Additionally, increased mean diffusivity were also found in the bilateral superior and inferior frontal gyrus, lateral occipital lobe, frontal pole and thalamus, the left supramarginal gyrus, angular gyrus, insula, precuneus, and cerebellum. The similar patterns were observed when studying radial and axial diffusivity indices (Fig. 1B).
Group comparisons of diffusion metrics between aMCI-SD and aMCI-MD
Compared to aMCI-SD, aMCI-MD patients showed significantly reduced fractional anisotropy across several WM regions (Fig. 1C), including the left medial and superior frontal gyrus, the right triangle part of inferior frontal gyrus, the bilateral superior temporal gyrus, the left middle and medial temporal lobe, the right angular gyrus, supramarginal gyrus, precuneus, the right lateral occipital lobe and postcentral gyrus, the bilateral insula, precentral gyrus, posterior cingulate cortex, and the whole CC. Increased mean diffusivity, radial diffusivity, and axial diffusivity in aMCI-MD patients were also identified in almost all WM tracts across the brain (Fig. 1C).
Group comparisons of atlas-based tract ROIs
Among three groups, significant differences in fractional anisotropy were found in the body of CC, the bilateral FN and ICA, as well as the right ICP and TAP (Fig. 2B). Post hoc analyses found that patients with aMCI-MD had significantly lower fractional anisotropy in these fibers than HCs. The regions showing fractional anisotropy decreases among aMCI-MD patients showed corresponding increases in radial diffusivity. They also had additional increments of radial diffusivity than HCs in the right CG, the bilateral ICP, the right SLF, and the left TAP. Compared with aMCI-SD, aMCI-MD had higher radial diffusivity in the body of CC and the ICP (Fig. 2B). In addition, significant changes in axial diffusivity for aMCI-MD were found in the body, genu, splenium of CC, the left ICA, and TAP as well as the bilateral SLF (Fig. 2B). In general, mean diffusivity changes in aMCI-MD were widespread, including the regions exhibiting radial diffusivity increases in aMCI-MD as well as the genu and splenium of CC (Fig. 2B). No significant differences in any tract were found between aMCI-SD and HCs (all p > 0.1). Group differences in mean diffusivity of the body of CC as well as fractional anisotropy and radial diffusivity of the right ICA remained significant after Bonferroni correction made for multiple comparisons (p < 0.05) (Table 2).
Table 2.
Group comparisons of mean DTI indices of each ROI in aMCI-SD, aMCI-MD, and HCs
| WM tracts | Side | Group |
One-way ANOVA |
Tukey’s Post Hoc Test |
|||||
|---|---|---|---|---|---|---|---|---|---|
| aMCI-SD (n = 19) |
aMCI-MD (n = 21) |
HC (n = 37) |
F | p value | aMCI-SD versus aMCI-MD |
aMCI-SD versus HC |
aMCI-MD versus HC |
||
|
bCC MD |
- | 0.84 ±0.05 | 0.90 ±0.09 | 0.84 ±0.04 | 8.00 | 0.001***a | 0.009** | NS | 0.001*** |
|
ICA FA |
right | 0.56 ±0.03 | 0.53 ±0.05 | 0.57 ±0.03 | 6.80 | 0.002***a | NS | NS | 0.001*** |
| L23 | right | 0.48 ±0.03 | 0.53 ±0.09 | 0.47 ± 0.04 | 7.47 | 0.001***a | 0.050* | NS | 0.001*** |
Values are mean ± standard deviation.
p < 0.005; bCC, body of corpus callosum; ICA, anterior internal capsule; NS, not significant; FA, fractional anisotropy; L23, radial diffusivity; MD, mean diffusivity;
significant at p < 0.05 after Bonferroni correction.
As the TAP is located adjacent to the lateral ventricle, we confirmed that the results by “deprojecting” all voxels in the TAP from the WM skeleton-space back into each subject’s native diffusion space. Same results of the diffusion changes in TAP among three groups were found (data not shown). This confirmed that the tract difference identified on the skeletonized maps corresponded well to WM disruption in TAP.
Relationship between diffusion metrics and neuropsychological scores
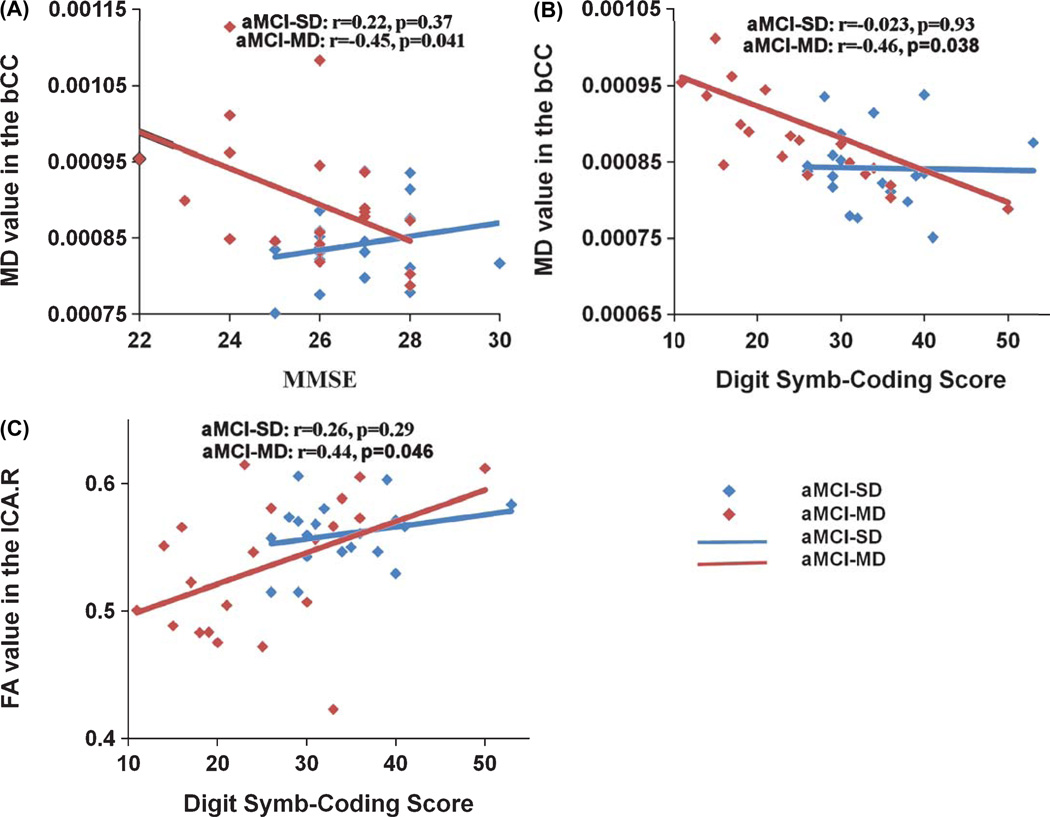
Then, the relationship between regional diffusion metrics of ROIs with significant group effects (p < 0.05 after Bonferroni correction) and neuropsychological scores was examined. For aMCI-MD group, mean diffusivity of the body of CC was negatively correlated with MMSE (r = −0.45, p = 0.041) and Digit Symb-Coding scores (r = −0.46, p = 0.038) (Fig. 3). Fractional anisotropy of the right ICA was positively correlated with Digit Symb-Coding scores (r = 0.44, p = 0.046). No significant correlation was found in aMCI-SD (all p> 0.1).
Fig. 3.
The correlations between the diffusion metrics of atlas-based ROIs and neuropsychological scores in aMCI-SD and aMCI-MD. A) Plots showing the significant decrease of mean diffusivity in the body of CC with MMSE scores in aMCI-MD (in red), whereas no significant correlation was found in aMCI-SD (in blue). B) Plots showing the significant decrease of mean diffusivity in the body of CC with the Digit Symb-Coding score in aMCI-MD (in red), whereas no significant correlation was found in aMCI-SD (in blue). C) Plots showing the significant increase of FA in the right ICA with the Digit Symb-Coding score in aMCI-MD (in red), whereas no significant correlation was found in aMCI-SD (in blue).
DISCUSSION
This DTI study investigated the WM alteration patterns in patients with different subtypes of aMCI. We found that aMCI-MD patients showed reduced fractional anisotropy and increased axial diffusivity, radial diffusivity, and mean diffusivity in multiple WM tracts across the whole-brain compared to HCs or to aMCI-SD. In contrast, only a few WM regions showed diffusion changes in aMCI-SD as compared to HCs and with reduced statistical significances. These findings indicate that the degeneration extensively exists in WM tracts in aMCI-MD that precedes the development of AD. The results are consistent with the view that aMCI is not a uniform disease entity and presents heterogeneity in the clinical progression.
In addition to the demonstrated susceptibility of the medial temporal lobe structures, posterior cingulate, and CC to the MCI syndrome, our aMCI-MD subjects also showed abnormality in frontal, temporal, parietal, and occipital WM, together with several commissural, association and projection fibers. The characteristics of the WM pathological changes in aMCI-MD are more “AD-like” [30–32]. The anatomical locations of WM abnormalities are mostly consistent with a number of previous DTI studies in AD and MCI patients [8–10, 12, 15, 21, 31, 33–38]. In addition, patients with aMCI-MD showed changes in the integrity of the corticospinal tract. This result was somewhat unexpected. The corticospinal tract is a bundle of motor neurons that extend from the cerebral cortex of the brain and the spinal cord. Although it is widely accepted that disability in motor function is a late symptom in AD, the presence of motor dysfunction in mildly AD [39] and even MCI [40] has been reported. It is possible the disruption of the corticospinal tract is responsible for the motor impairment. Some other DTI studies also reported microstructural abnormality in the corticospinal tract in patients with AD[31], MCI, and aMCI-MD [19]. Furthermore, the DTI indices of the callosal body and the ICA remained significant after Bonferroni correction in atlas-based analyses. For aMCI-MD, mean diffusivity is higher in the body of the CC when compared with healthy elders. This finding was consistent with a recent report reporting mean diffusivity increases in aMCI using whole-brain TBSS analyses [32], as well as previous findings in MCI and AD [37, 41, 42]. However, some other studies found no microstructural abnormality in the callosal body in patients with aMCI [12, 43]. The discrepancy might be due to the heterogeneity in aMCI group and the fact that those investigations did not distinguish single-domain and multi-domain aMCI. In this regard, it is also interesting to note that the aMCI-MD subjects, not the aMCI-SD subjects, exhibited reduced WM integrity in the ICA. Finally, our current findings of ICA fiber disruption was also demonstrated in MCI and AD patients in several previous DTI investigations [9, 44].
The present study demonstrated that there were distributed WM tracts that exhibited differences between aMCI-MD and aMCI-SD, similar to the regional differences between a MCI-MD and HCs. Most previous DTI studies of MCI patients did not compare WM integrity patterns between aMCI subtypes except one recent study [16]. Similar to the findings in our study, Haller et al. reported that, compared with aMCI-SD, aMCI-MD had significantly reduced fractional anisotropy in a bilateral, right-dominant network, including right uncinate fasciculus, forceps minor, and internal capsule as well as bilateral inferior fronto-occipital fasciculus, anterior thalamic radiation, SLF, ILF, and corticospinal tract. Our findings in the current study, on the large part, reconfirmed theirs, probably the first study of this type. We noted that our study also enrolled normal controls and explored the differences of each of the aMCI sub-types to them. In addition, besides the fractional anisotropy changes, in the present study we also identified the widespread alterations of mean diffusivity in aMCI-MD compared with HCs and aMCI-SD. The findings of our study are also consistent with previous ones regarding grey matter loss in these two aMCI subgroups: patients with aMCI-MD/MCI-MD showed more diffuse and extensive pattern of brain atrophy compared with aMCI-SD/MCI-SD [7, 45, 46]. Given the more severe and more widespread damage within brain tissues and the cognitive impairment in multiple domains, the aMCI-MD patients are likely “closer” to clinical AD. In this regard, some researchers viewed aMCI-MD as transitional between aMCI-SD and AD [47]. Furthermore, our results support the notion that aMCI subtyping is meaningful and more informative to analyze the changes of WM fiber tracts. A careful distinction among MCI subtypes may be important for our understanding of the neuropathological states of the disease.
Our current study found that aMCI-SD patients showed disrupted WM in the bilateral parahippocampus and right insula (p < 0.05, uncorrected), which are vulnerable to AD and aMCI [30, 31, 34]. As episodic memory is selectively impaired in aMCI-SD, it is not surprising that WM disruption occurred in the medial temporal. However the differences were not significant after multiple comparison corrections. We have found no prior reports about the abnormality in WM integrity in aMCI-SD as compared to HCs partly due to the fact that most of the previous studies did not consider the aMCI subtypes. We cannot exclude the possibility that the sensitivity of whole-brain voxelwise TBSS analysis might be limited in particular brain regions (such as HIP).
When examining the relationship of WM alterations with the clinical and cognitive performances in each subgroup of aMCI, mean diffusivity of the body of the CC was found to be associated with MMSE and Digit Symb-Coding scores only in aMCI-MD. The significant correlation between the DTI indices of the splenium of CC and the MMSE scores in AD and MCI have been previously published [48, 50], whereas in those studies the body of CC was not selected to research on the relationship with cognitive function. It is well known that the CC links the cerebral cortices of the left and right hemispheres and is the largest fiber pathway in the brain. The anterior part of the body of the CC is responsible for the interhemispheric connection between the prefrontal association cortices and premotor cortical connections are located in the mid body region [43, 51]. Although there is no direct evidence supporting the association of the cognitive dysfunction with reduced integrity of WM tracts in the body of the CC, the anatomical connection is nevertheless supportive for the correlation results. In the current study, we also found fractional anisotropy of the right ICA was correlated with Digit Symb-Coding score which was a measure of information processing speed in aMCI-MD. The anterior limb of internal capsule contains the anterior thalamic peduncle, which connects the dorsomedial and anterior thalamic nuclei with the prefrontal cortex and the cingulate gyrus [52, 53]. A similar finding was reported in another study that the best predictor of reaction time in a visual target detection task for older adults was FA in the anterior limb of the internal capsule [54]. Furthermore, we did not find any relationship between DTI indices and cognitive performance in the aMCI-SD group. It might be due to the fact that WM pathology in aMCI-SD was mild and the cognitive function was relatively intact except memory.
Mean diffusivity is the average amount of water diffusion and fractional anisotropy refers to the coherence of the orientation of water diffusion. Related to the neurodegeneration in AD, several pieces of evidence are implicative about the effects of the mean diffusivity and fractional anisotropy variation, such as axonal damage related to Wallerian degeneration [15, 32, 55], myelin breakdown [32, 56], and other neuropathologic processes. Recent DTI studies have also employed axial diffusivity and radial diffusivity to reveal the normal and pathological changes of WM [31, 32, 57]. Axial and radial diffusivity provide information on magnitude of water diffusion either parallel or perpendicular to the principal direction of the tensor [58]. They could reflect the degree of myelin breakdown or axonal damage selectively [32, 57]. With all of the arguments supportive to the simultaneous use of multi-indices, research using them together is still at an early stage. Further studies are needed to reveal the mechanisms underlying these diffusion indices.
We are aware of several methodological limitations in our current study. First, we examined the WM alterations in aMCI patients cross-sectionally and with unknown rates of conversion to AD. Thus, the assertion that aMCI-MD patients had a higher risk of converting AD is primarily based on reported findings in the literature [2] and the correlation with neuropsychological and clinical tests. Longitudinal studies are needed to confirm these findings and to assess longitudinal changes. Second, the automated TBSS methodology is subject to bias, especially in areas of tract junctions or crossing fibers, such as the SLF and ILF. Therefore, the interpretations of the diffusion changes in these regions must be with caution. Third, we only focused on the WM changes in aMCI patients in this study. The relationship of these structural changes to the functional ones, however, is still unclear. Finally, the exact histopathological processes leading to changes in diffusion measures are complex, and appropriate animal models where MRI DTI assessments can be directly correlated with morphometric histology will be very useful.
In conclusion, our study illustrated aMCI heterogeneity. For aMCI-MD, WM abnormality was more anatomically widespread. The altered diffusion measures were related to neuropsychological changes in aMCI-MD patients. TBSS analysis of WM integrity can serve as a potential biomarker of aMCI subtypes. Longitudinal studies are needed to investigate the conversions of aMCI subtypes and to evaluate the clinical values of DTI technique to predict clinical progression.
ACKNOWLEDGMENTS
This work is supported by the National Science Foundation of China (Nos. 30873458, 81173460, and 81000633), Project of Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences (Z0175), the Fundamental Research Funds for the Central Universities (248-105102), Program for New Century Excellent Talents in University (NCET-10), and Program for Excellent Doctoral Dissertation Foundation (2007B7).
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1727).
REFERENCES
- 1.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 2.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as adiagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment-beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: Relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- 6.Becker JT, Davis SW, Hayashi KM, Meltzer CC, Toga AW, Lopez OL, Thompson PM. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 7.Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 8.Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, Qian Y, Jia J. Abnormal integrity of association fiber tracts in amnestic mild cognitive impairment. J Neurol Sci. 2009;278:102–106. doi: 10.1016/j.jns.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ. Abnormal integrity of corticocortical tracts in mild cognitive impairment: A diffusion tensor imaging study. J Korean Med Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensorimaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 12.Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: Assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- 14.Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller S, Missonnier P, Herrmann FR, Rodriguez C, Deiber MP, Nguyen D, Gold G, Lovblad KO, Giannakopoulos P. Individual classification of mild cognitive impairment subtypes by support vector machine analysis of white matter DTI. AJNR Am J Neuroradiol. 2013;34:283–291. doi: 10.3174/ajnr.A3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA. White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller S, Nguyen D, Rodriguez C, Emch J, Gold G, Bartsch A, Lovblad KO, Giannakopoulos P. Individual prediction of cognitive decline in mild cognitive impairment using support vector machine-based analysis of diffusion tensor imaging data. J Alzheimers Dis. 2010;22:315–327. doi: 10.3233/JAD-2010-100840. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Kivipelto M, Hallikainen M, Vanninen R, Soininen H. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2011;32:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, Reppermund S, Brodaty H, Sachdev P. White matter integrity in mild cognitive impairment: A tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009;23:253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 23.Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, Qu GY, Grant I, Yu E, Levy P, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: Impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 24.Zhang MY, Elena Y, He YL. Activities of daily living scale. Shanghai Arch Psychiatr. 1995;7:3. [Google Scholar]
- 25.Gong YX. Wechsler Adult Intelligence Scale–Revised in China Version. Changsha, Hunan/China: Hunan Map Press; 1992. [Google Scholar]
- 26.Zhou Y, Lu JC, Guo QH, Hong Z. Rey-Osterriche complex figure test used to identify mild Alzheimer’s disease. Chin J Clin Neurosci. 2006;14:501–504. [Google Scholar]
- 27.Lu JC, C GQ, Hong Z, Shi WX, Lv CZ. Trail making test used by Chinese elderly patients with mild cognitive impairment and mild Alzheimer dementia. Chin J Clin Psychol. 2006;4:118–121. [Google Scholar]
- 28.Guo QH, Hong Z, Shi WX, Sun YM, Lv CZ. Boston naming test using by Chinese elderly, patient with mild cognitive impairment and Alzheimer’s dementia. Chin Ment Health J. 2006;20:81–85. [Google Scholar]
- 29.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 31.Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258:853–863. doi: 10.1148/radiol.10101284. [DOI] [PubMed] [Google Scholar]
- 32.Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normalaging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer’s disease. Alzheimers Dement. 2010;6:394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua TC, Wen W, Chen X, Kochan N, Slavin MJ, Trollor JN, Brodaty H, Sachdev PS. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- 35.Parente DB, Gasparetto EL, da Cruz LC, Jr., Domingues RC, Baptista AC, Carvalho AC. Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer’s disease. AJR Am J Roentgenol. 2008;190:1369–1374. doi: 10.2214/AJR.07.2617. [DOI] [PubMed] [Google Scholar]
- 36.Stenset V, Bjornerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tonnessen P, Gjerstad L, Fladby T. Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging. 2011;32:581–589. doi: 10.1016/j.neurobiolaging.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: A DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. AJNR Am J Neuroradiol. 2009;30:893–899. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology. 1999;53:956–962. doi: 10.1212/wnl.53.5.956. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 41.Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer’s disease and mild cognitive impairment using different MRI techniques: A review. J Alzheimers Dis. 2010;20:67–95. doi: 10.3233/JAD-2010-1370. [DOI] [PubMed] [Google Scholar]
- 42.Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- 43.Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini AR, Sancesario G, Passafiume D, Caltagirone C, Spalletta G. When, where, and how the corpus callosum changes in MCI and AD: A multimodal MRI study. Neurology. 2010;74:1136–1142. doi: 10.1212/WNL.0b013e3181d7d8cb. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Goldstein FC, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. White matter hyperintensities and changes in white matter integrity in patients with Alzheimer’s disease. Neuroradiology. 2011;53:373–381. doi: 10.1007/s00234-010-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mielke MM, Kozauer NA, Chan KC, George M, Toroney J, Zerrate M, Bandeen-Roche K, Wang MC, Vanzijl P, Pekar JJ, Mori S, Lyketsos CG, Albert M. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, Yang DW, Kim SI, Cho YS, Na DL. Cortical thickness in singleversus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007;36:289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 48.Duan JH, Wang HQ, Xu J, Lin X, Chen SQ, Kang Z, Yao ZB. White matter damage of patients with Alzheimer’s disease correlated with the decreased cognitive function. Surg Radiol Anat. 2006;28:150–156. doi: 10.1007/s00276-006-0111-2. [DOI] [PubMed] [Google Scholar]
- 49.Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM. Loss of connectivity in Alzheimer’s disease: An evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000;69:528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer’s disease and in patients with mild cognitive impairment by using diffusion tensor imaging. Radiol Med. 2008;113:915–922. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- 51.Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki M, Zhou SY, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Matsui M, Seto H, Kurachi M. Volume reduction of the right anterior limb of the internal capsule in patients with schizotypal disorder. Psychiatry Res. 2004;130:213–225. doi: 10.1016/j.pscychresns.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 54.Madden DJ, Whiting WL, Huettel SA. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- 56.Bartzokis G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta. 2012;1822:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]