Abstract
Interferon regulatory factor (IRF) 7 has been demonstrated to be a master regulator of virus-induced type I interferon production (IFN), and it plays a central role in the innate immune response against viruses. Here, we identified death-associated protein kinase 1 (DAPK1) as an IRF7-interacting protein by tandem affinity purification (TAP). Viral infection induced DAPK1–IRF7 and DAPK1–IRF3 interactions and overexpression of DAPK1 enhanced virus-induced activation of the interferon-stimulated response element (ISRE) and IFN-β promoters and the expression of the IFNB1 gene. Knockdown of DAPK1 attenuated the induction of IFNB1 and RIG-I expression triggered by viral infection or IFN-β, and they were enhanced by viral replication. In addition, viral infection or IFN-β treatment induced the expression of DAPK1. IFN-β treatment also activated DAPK1 by decreasing its phosphorylation level at serine 308. Interestingly, the involvement of DAPK1 in virus-induced signaling was independent of its kinase activity. Therefore, our study identified DAPK1 as an important regulator of the cellular antiviral response.
Keywords: DAPK1, innate antiviral response, IRF3/7, type I interferon
Introduction
Upon viral infection, host cells activate a series of signaling cascades that lead to the production of type I interferons (IFNs). Type I IFNs are secreted by cells, and they bind to type I IFN receptors in a paracrine or autocrine manner, leading to the dimerization of IFN receptors. The dimerized IFN receptors recruit and activate the kinase JAK1, which phosphorylates STAT1 and STAT2, and they form a transcriptional complex together with interferon regulatory factor (IRF) 9. The STAT–IRF9 complex (also known as ISGF3) binds to conserved DNA sequences in the promoters of a wide range of IFN-stimulated genes (ISGs), and it activates the expression of these genes, eliciting an innate antiviral response. Thus, type I IFNs play a central role in innate antiviral immunity.1,2,3
The detection of invading viruses depends on the host germline-encoded pattern recognition receptors, which recognize pathogen-associated molecular patterns from viruses, such as double-stranded RNA. Of all the characterized pattern recognition receptors, Toll-like receptors and RIG-I-like receptors are well known for their ability to detect viral pathogen-associated molecular patterns and activate signal transduction pathways that lead to the expression of type I IFNs. For example, Toll-like receptor 3 binds to double-stranded RNA, and this binding triggers TRIF-dependent signaling.4,5,6 Additionally, RIG-I recognizes viral RNA and recruits VISA (also known as MAVS, IPS-1 and Cardif) to activate downstream signaling.2 TRIF and VISA are two critical adaptor proteins that act as platforms for the recruitment of downstream signaling complexes.5,7,8 TRAF6 and RIP1 promote the assembly and activation of the IKK complex, which leads to the phosphorylation and degradation of the inhibitor of NF-κB α and subsequent activation of NF-κB. Additionally, TRAF3, TBK1 and/or IKKε are recruited to form an IRF signaling complex that phosphorylates IRF3 or IRF7.9,10 Other components have been identified as necessary for the assembly of these signaling complexes, including TRADD, FADD, MITA and GSK3β.11,12,13,14,15
IRF3 and IRF7 are two transcription factors that bind to the interferon-stimulated response element (ISRE) that is located on the promoters of type I IFN genes.16 IRF3 is constitutively expressed and is responsible for the initial wave of virus-induced transcription of the IFNB1 gene, whereas IRF7 is induced by viral infection and is required for sustained transcriptional activation of IFNB1 and IFNA genes at later infection time points.17 Therefore, IRF7 is a master regulator of type I IFN production, and its activity is tightly controlled to prevent excessive type I IFN responses.18 It has been reported that IRF7 is regulated at three different levels, including the transcriptional, post-transcriptional and post-translational levels. Transcription of the IRF7 gene is induced by viral infection or type I IFN stimulation,19 while the stability of IRF7 mRNA is regulated by FoxO3.20 The post-translational regulation of the IRF7 protein includes phosphorylation, sumoylation and ubiquitination. It has been reported that TBK1, IKKε and IKKα phosphorylate IRF7, which results in its activation.10,21 TRAF6 catalyzes K63-linked ubiquitination of IRF7, which is required for full activation of IRF7,22 whereas another E3 ubiquitin ligase, RAUL, targets IRF7 for K48-linked ubiquitination and degradation.23 Although these mechanisms have been characterized, the regulation of IRF7 at different levels is still not fully understood, and whether other mechanisms are involved in the regulation of IRF7 is of great interest.
To search for potential IRF7-interacting proteins, we performed tandem-affinity purification (TAP) with IRF7 as the bait. This effort led to the identification of death-associated protein kinase 1 (DAPK1) as an IRF7-interacting protein. Viral infection resulted in an endogenous interaction between DAPK1 and IRF3/7. Overexpression of DAPK1 resulted in virus-induced activation of the IFN-β promoter and expression of the IFNB1 gene. In contrast, knockdown of DAPK1 abrogated virus-induced expression of downstream genes. In addition, DAPK1 was induced by viral infection and was required for the cellular antiviral response. Therefore, our findings demonstrate that DAPK1 is an IRF3/7-associated protein that is required for virus-triggered type I IFN induction and the innate antiviral response.
Materials and methods
Reagents and antibodies
Recombinant TNF-α (R&D Systems, Minneapolis, MN, USA), recombinant IFN-β (Peprotech, Rocky Hill, NJ, USA) and mouse monoclonal antibodies against FLAG (Sigma, St. Louis, MO, USA), HA (Roche, Basel, Switzerland), β-actin (Sigma), IRF3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-IRF3(S396) (CST, Boston, MA, USA), DAPK1 (Sigma, Cat. no. SAB4500620) and phospho(S308)-DAPK1 (Sigma) were purchased from the indicated manufacturers. The viruses Sendai virus (SeV), vesicular stomatitis virus (VSV) and GFP-Newcastle disease virus (NDV), and the antibodies for RIG-I and ISG56, have been previously described.14,24,25 A mouse anti-IRF7 antibody was raised against recombinant human full-length IRF7.
Plasmid constructs
NF-κB, ISRE and IFN-β promoter luciferase reporter plasmids, mammalian expression plasmids for HA- or FLAG-tagged IRF7, RIG-I, MDA5, VISA, TRAF3, TRAF6, TBK1, IKKε and IRF3 have been previously described.7,24,26,27 Mammalian expression plasmids for human HA- or FLAG-DAPK1 and its mutants, and HA- or FLAG-STAT1 were constructed by standard molecular biology techniques.
Protein purification and mass spectrometry analysis
We first made a pCTAP-IRF7 construct, in which the IRF7 cDNA was fused in frame to the C-terminal calmodulin-binding peptide and streptavidin-binding peptide tags in the pCTAP-A plasmid (Stratagene, Santa Clara, CA, USA). In total, 293 cells (1×109) were stably transfected with pCTAP-IRF7 then stimulated with SeV for 3 h. The cells were then collected, and the cell lysate was subjected to tandem affinity purification procedures with the interplay Mammalian TAP System (Stratagene, Cat. no. 240107). The purified IRF7-interacting proteins were digested by trypsin in solution. The tryptic peptides were analyzed by HPLC-ES/MS with a Thermo Finnigan LTQ adapted for nanospray ionization. The tandem spectra were searched against the Homo Sapiens National Center for Biotechnology Information reference database using SEQUEST. The results was filtered by Xcorr +1 >1.9, +2 >2.2, +3 >3.5, sp>500, Deltcn>0.1, Rsp≤5.
Transfection and reporter assays
The 293 cells (1×105) were seeded in 24-well plates and were transfected the following day by the standard calcium phosphate precipitation method. An empty control plasmid was added to ensure that each transfection received the same amount of total DNA. To normalize for transfection efficiency, 0.01 µg of pRL-TK (Renilla luciferase) reporter plasmid was added to each transfection. Approximately 18 h after the transfections, luciferase assays were performed using a dual-specificity luciferase assay kit (Promega, Madison, WI, USA).
Co-immunoprecipitation, immunoblot analysis and VSV plaque assays
These experiments were performed as previously described.7,24,26,27
Virus manipulation
Two hundred and ninety-three cells grown in media containing 1% fetal bovine serum were incubated with VSV at a multiplicity of infection (MOI) of 0.1 for 1 h before the medium was replaced with complete media containing 10% Fetal Bovine Serum (FBS, fetal bovine serum). Twenty-four hours later, the supernatant was harvested and diluted to infect confluent BHK21 cells that were cultured in 24-well dishes. One hour post-infection, the cell culture medium was removed and 2% methylcellulose was overlaid on the cells. On day 3 post-infection, the overlay was removed and the cells were fixed with 0.5% glutaraldehyde for 30 min and stained with 1% crystal violet in 70% methanol for 15 min. The plaques were counted, averaged and multiplied by a dilution factor to determine the viral titer as a log10 (pfu/ml).
The viral infection was performed when the cells were 70% confluent. The culture medium was replaced by serum-free DMEM, and NDV-GFP was added into the medium at various MOIs, which were determined by the specific experiments. After 1 h, the medium was removed and replaced with DMEM containing 2% methylcellulose. NDV-GFP replication in 293 cells was visualized by fluorescence microscopy.
RNAi experiments
Double-stranded oligonucleotides corresponding to target sequences were cloned into the pSuper.Retro RNAi plasmid (Oligoengine Inc., Seattle, WA, USA). The following sequences were used for the human DAPK1 cDNA:28 DAPK1-RNAi #1: CAAGAAACGTTAGCAAATG; RNAi #2: GGTCAAGGATCCAAAGAAG.
Real-time PCR
Total RNA was isolated from cells using Trizol reagent (TAKARA, Japan, Dalian, China) and subjected to real-time PCR analysis to measure mRNA expression. The following gene-specific primer sequences were used:
IFNB1: TTGTTGAGAACCTCCTGGCT (forward),
TGACTATGGTCCAGGCACAG (reverse);
RIG-I: ACGCAGCCTGCAAGCCTTCC (forward);
TGTGGCAGCCTCCATTGGGC (reverse);
ISG56: tcatcaggtcaaggatagtc (forward);
CCACACTGTATTTGGTGTCTAGG (reverse);
IFNA1: TGGTCCTGGTGGTGCTCAGC (forward);
CCAGGCTGTGGGTCTCAGGG (reverse);
IRF7: CCCCCATCTTCGACTTCAGA (forward);
CAGGACCAGGCTCTTCTCCTT (reverse);
DAPK1: AATGGAGTTGGCGATTTCAGCGTG (forward);
AAGGGACTTCAGGAAACTGAGCCA (reverse);
GAPDH: GAGTCAACGGATTTGGTCGT (forward),
GACAAGCTTCCCGTTCTCAG (reverse).
Results
Identification of DAPK1 as an IRF7-interacting protein
Previous studies have demonstrated that IRF7 is a master regulator of type I IFN-dependent immune responses. To investigate how IRF7 is regulated in virus-induced signaling events, we attempted to identify IRF7-interacting proteins by TAP as previously described.29 An expression plasmid for IRF7 tagged with calmodulin-binding peptide and streptavidin-binding peptide was stably transfected into 293 cells, and the IRF7-interacting proteins were purified by using the TAP purification system. The eluted proteins were identified through a shot-gun mass spectometry analysis method. By comparing the experimental purification with other non-related purifications using the same method, we identified DAPK1 in the purified proteins as specifically interacting with IRF7. DAPK1 is a Ser/Thr protein kinase that has been shown to regulate IFN-γ-induced apoptosis.30 Recent studies have also shown that DAPK1 is involved in a diverse range of signaling pathways and that it functions as a tumor suppressor.31,32,33,34,35,36 However, whether and how DAPK1 is involved in virus-triggered type I IFN induction is unknown.
To validate the DAPK1-IRF7 association, we generated FLAG- or HA-tagged DAPK1 expression plasmids and performed transient transfections and co-immunoprecipitation experiments. The results suggest that DAPK1 interacted with IRF7, IRF3 and VISA but not with IRF9, RIG-I or MDA5 (Figure 1a and Supplementary Figure 1). We next examined the endogenous association between DAPK1 and IRF7 or IRF3. As shown in Figure 1b, DAPK1 did not interact with, or interacted weakly with, IRF7 or IRF3 in resting cells, and these associations were enhanced by SeV infection. These data suggest that DAPK1 interacts with IRF7 and IRF3 after viral infection.
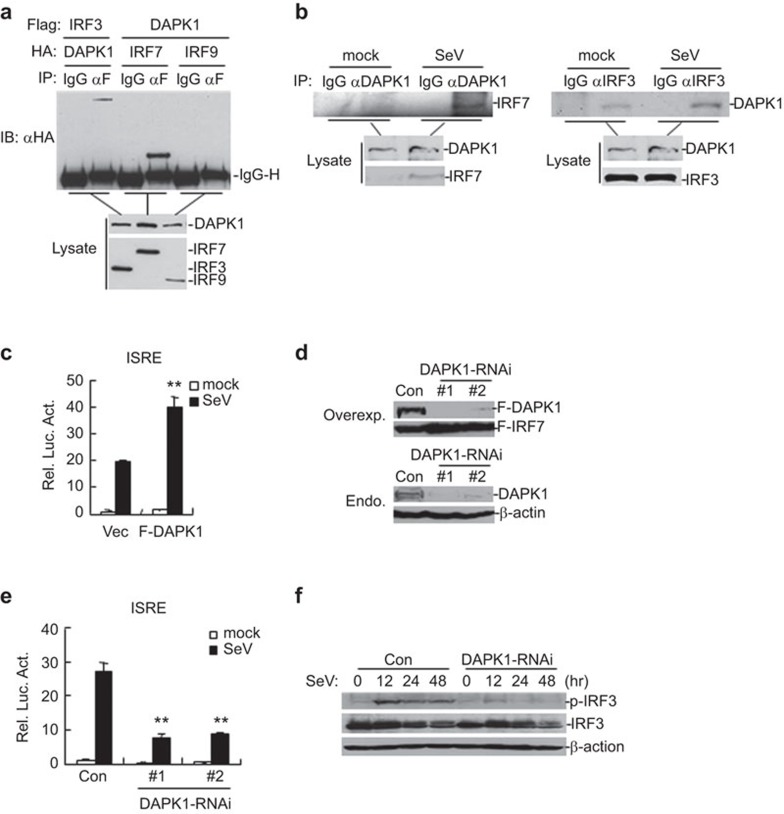
Figure 1.
DAPK1 interacts with IRF7 and IRF3. (a) DAPK1 interacts with IRF7 and IRF3 in a mammalian overexpression system. Two hundred and ninety-three cells (1×107) were transfected with the indicated plasmids (8 µg each). Co-immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. (b) Endogenous association between DAPK1 and IRF7/IRF3 was investigated. In total, 293 cells (8×107) were left uninfected or infected with SeV for 8 h prior to co-immunoprecipitation. An immunoblot analysis was performed with the indicated antibodies. (c) Overexpression of DAPK1 enhances SeV-induced ISRE activation in 293 cells. These 293 cells (1×105) were transfected with an ISRE reporter and a DAPK1 expression plasmid. Eighteen hours after transfection, the cells were infected with SeV or left untreated. Luciferase assays were performed 12 h after infection. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (d) Effects of DAPK1-RNAi plasmids on the expression of transfected and endogenous DAPK1. In the upper panels, 293 cells (2×105) were transfected with expression plasmids for FLAG-DAPK1, FLAG-IRF7 (0.5 µg each) and the indicated RNAi plasmids (1 µg each). Twenty-four hours after transfection, the cell lysates were analyzed by immunoblot with anti-FLAG. In the lower panels, 293 cells (1×107) were transfected with a control or the indicated DAPK1-RNAi plasmids (8 µg each) for 36 h. The lysates were analyzed by immunoblot with the indicated antibodies. (e) Effects of DAPK1-RNAi on SeV-induced ISRE activation in 293 cells. In total, 293 cells (2×105) were transfected with the indicated RNAi plasmids (1 µg each) and an ISRE reporter plasmid (0.1 µg). Twenty-four hours after transfection, the cells were left untreated or were infected with SeV for 12 h before the luciferase assays were performed. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (f) The 293 cells (1×106) were transfected with the indicated RNAi plasmids (2 µg each). Twelve hours after transfection, the cells were selected with puromycin (1 µg/ml) for 24 h, then infected with SeV or left uninfected for the indicated time points. The cell lysates were analyzed by immunoblot with the indicated antibodies. DAPK, death-associated protein kinase; IRF, interferon regulatory factor; ISRE, interferon-stimulated response element; SeV, Sendai virus.
Because DAPK1 interacted with IRF7 and IRF3, we examined whether DAPK1 was involved in virus-induced ISRE activation. We observed that overexpression of DAPK1 enhanced SeV-induced activation of ISRE in 293 cell reporter assays (Figure 1c). We next constructed two human DAPK1-RNAi plasmids (DAPK1-RNAi#1 and DAPK1-RNAi#2), both of which could markedly inhibit the expression of transfected and endogenous DAPK1 in 293 cells (Figure 1d). We found that knockdown of DAPK1 inhibited SeV-induced ISRE activation (Figure 1e). We selected the DAPK1-RNAi#1 plasmid for the experiments described below, and similar results were obtained with the #2 RNAi plasmid. Consistent with the observations made in the reporter assays, knockdown of DAPK1 inhibited SeV-induced phosphorylation of IRF3 (Figure 1f). These data suggest that DAPK1 is important for SeV-induced ISRE activation.
DAPK1 is involved in virus-triggered type I IFN signaling
We next examined the effect of DAPK1 and DAPK1-RNAi on virus-induced activation of the IFN-β promoter. As shown in Figure 2, overexpression of DAPK1 enhanced SeV-induced activation of the IFN-β promoter (Figure 2a), while knockdown of DAPK1 attenuated SeV-induced activation of the IFN-β promoter in 293 cell reporter assays (Figure 2b), indicating that DAPK1 is important for virus-induced type I IFN induction. Because DAPK1 interacted with IRF3 and IRF7, we hypothesized that DAPK1 functioned at the level of IRF3 or IRF7. The reporter assay results indicated that knockdown of DAPK1 inhibited IRF3- and IRF7-mediated activation of ISRE (Figure 2c). It has been reported that IRF3 is constitutively expressed in cells and is responsible for the initial wave of virus-induced transcription from the IFNB1 gene, whereas viral infection or type I IFNs induced the expression of IRF7. IRF7 is required for sustained transcriptional activation of the IFNB1 and IFNA genes following viral infection.17 To determine whether DAPK1 mediated SeV-triggered IRF3-dependent signaling, we treated 293 cells that were transfected with a control or a DAPK1-RNAi plasmid with the JAK inhibitor Ruxolitinib, then infected the cells with SeV and performed reporter assays. The results suggested that SeV-induced activation of ISRE was inhibited by the knockdown of DAPK1 in the presence or absence of Ruxolitinib (Figure 2d), indicating that DAPK1 regulates a SeV-triggered IRF3-dependent signaling event. Consistent with these observations, the results from the quantitative real-time PCR analysis showed that knockdown of DAPK1 inhibited SeV-induced transcription of IFNB1, RIG-I and ISG56 in 293 and HeLa cells (Figure 2e and f). In addition, knockdown of DAPK1 inhibited SeV-induced expression of the RIG-I and ISG56 proteins (Figure 2g). Collectively, these data suggest that DAPK1 is important for virus-induced expression of downstream genes.
Figure 2.
Effects of DAPK1 on viral induction of downstream genes. (a) Overexpression of DAPK1 enhances SeV-induced activation of the IFN-β promoter in 293 cells. Luciferase assays were performed as in Figure 1c. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (b) Effects of DAPK1-RNAi on SeV-induced activation of the IFN-β promoter in 293 cells. Reporter assays were performed as in Figure 1e. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (c) Effects of DAPK1-RNAi on IRF3/7-mediated activation of the IFN-β promoter in 293 cells. DAKP1-RNAi-expressing cells (2×105) were transfected with an ISRE reporter (0.05 µg each) and a FLAG-IRF3/7 expression plasmid (0.1 µg each). Luciferase assays were performed 18 h after infection. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (d) Effects of DAPK1-RNAi on IRF3-dependent signaling. In the left panels, 293 cells (2×105) were transfected with an ISRE reporter (0.05 µg each). Eighteen hours after transfection, the cells were treated with or left untreated with the indicated concentrations of the JAK inhibitor Ruxolitinib for 4 h. Reporter assays were performed after the cells were infected with SeV for 12 h. The cell lysates were analyzed by immunoblot with the indicated antibodies. In the right panels, 293 cells (2×105) were transfected with the indicated RNAi plasmids (1 µg each). Twelve hours after transfection, the cells were selected with puromycin (1 µg/ml) for 36 h, then treated with or left untreated with the JAK inhibitor Ruxolitinib (0.05 M) for 4 h. Reporter assays were performed after the cells were infected with SeV for 12 h. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (e) Effects of DAPK1-RNAi on SeV-induced transcription of RIG-I, ISG56 and IFNB1 genes in 293 cells. In total, 293 cells (2×105) were transfected with the indicated RNAi plasmids (2 µg each). Twelve hours after transfection, the cells were selected with puromycin (1 µg/ml) for 24 h, then infected with SeV or left uninfected for 12 h before real-time PCR was performed. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (f) Effects of DAPK1-RNAi on SeV-induced transcription of RIG-I, ISG56 and IFNB1 genes in HeLa cells. Real-time PCR was performed as in Figure 1e. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (g) Knockdown of DAPK1 inhibited SeV-induced expression of RIG-I and ISG56 proteins. In total, 293 cells (2×105) were transfected with a DAPK1-RNAi plasmid for 12 h, selected with puromycin (1 µg/ml) for 24 h and then infected with SeV or left uninfected for the indicated time points. The cell lysates were analyzed by an immunoblot with the indicated antibodies. *P<0.05; **P<0.01. DAPK, death-associated protein kinase; IFN, interferon; IRF, interferon regulatory factor; ISRE, interferon-stimulated response element; SeV, Sendai virus.
DAPK1 is involved in IFN-β-triggered signaling
Because both viral infection and type I IFNs induce expression of RIG-I, ISG56 and IFNB1 genes, and because treatment with Ruxolitinib partially restored the DAPK1-RNAi-mediated inhibition of SeV-induced IFN-β activation, we hypothesized that DAPK1 mediated type I IFN signaling. The results of the real-time PCR analysis indicated that knockdown of DAPK1 inhibited the IFN-β-induced transcription of downstream genes, such as ISG56, RIG-I, IRF7, IFNA1 and IFNB1, in 293 cells (Figure 3a). Consistent with this hypothesis, the results from an immunoblot analysis indicated that knockdown of DAPK1 attenuated IFN-β-induced RIG-I and ISG56 protein expression (Figure 3b). In the transient transfection and co-immunoprecipitation experiments, DAPK1 interacted with STAT1 and IKKε, but not with IRF9 or TBK1 (Figure 3c). In addition, we found that the knockdown of DAPK1 inhibited TBK1- or IKKε-mediated activation of ISRE (Supplementary Figure 3). It has previously been demonstrated that TBK1 and IKKε phosphorylate IRF3/7 after virus infection to induce the transcription of the IFNB1 gene,9,37 while IKKε phosphorylates STAT1 to mediate IFN-β-dependent signaling.38 These findings indicate that DAPK1 interacts with IKKε and is involved in both virus- and IFN-β-induced signaling.
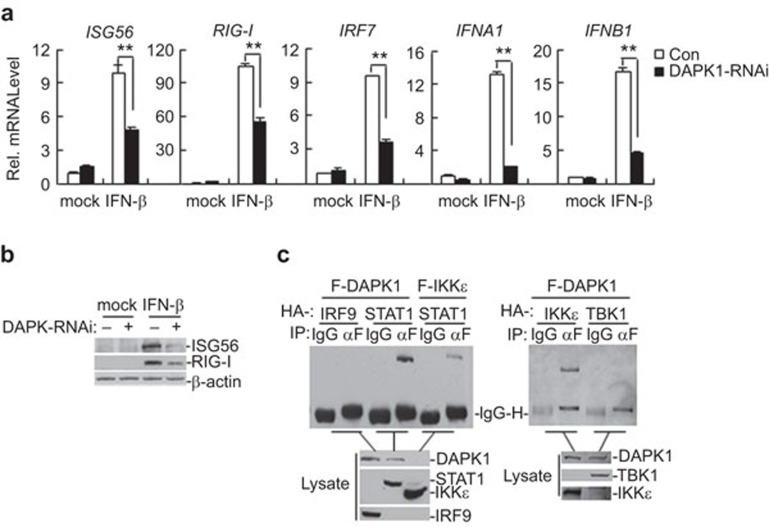
Figure 3.
Effects of DAPK1 on IFN-β-induced signaling. (a) Effects of DAPK1-RNAi on IFN-β-induced transcription of RIG-I, ISG56, IRF7, IFNA and IFNB1 genes. In total, 293 cells (2×105) were transfected with the indicated RNAi plasmids (2 µg each). Twelve hours after transfection, the cells were selected with puromycin (1 µg/ml) for 24 h, then treated with IFN-β or left untreated for 12 h before real-time PCR was performed. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (b) Knockdown of DAPK1 inhibited IFN-β-induced protein expression of RIG-I and ISG56. In total, 293 cells (2×105) were transfected with a DAPK1-RNAi plasmid for 12 h, selected with puromycin (1 µg/ml) for 24 h and then treated with IFN-β or left untreated for the indicated time points. The cell lysates were analyzed by immunoblot with the indicated antibodies. (c) DAPK1 interacts with STAT1 and IKKε, but not with IRF9 or TBK1. In total, 293 cells (1×107) were transfected with the indicated plasmids (6 µg each). Co-immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. DAPK, death-associated protein kinase; IFN, interferon; IRF, interferon regulatory factor.
Because DAPK1 is involved in both virus- and IFN-β-induced signaling, we examined its role in the cellular antiviral response. Knockdown of DAPK1 by RNAi increased VSV and NDV replication (Figure 4a). These results indicated that DAPK1 is important for efficient cellular antiviral responses through the regulation of both virus-induced type I IFN production and type I IFN-induced signaling.
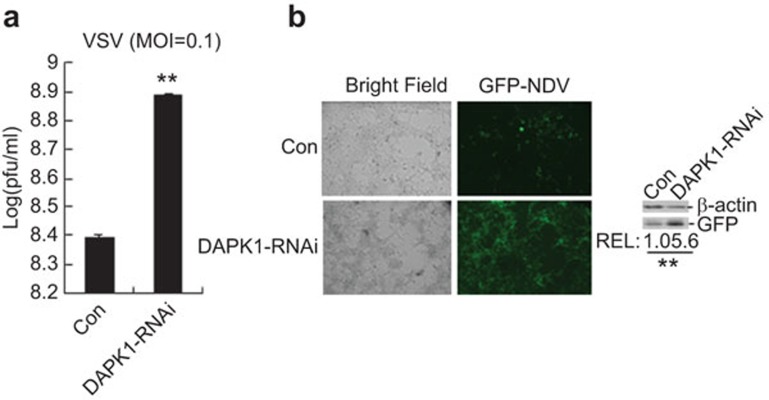
Figure 4.
Roles of DAPK1 in cellular antiviral response. (a) Effects of DAPK1-RNAi on VSV replication. Two hundred and ninety-three cells (1×105) were transfected with the indicated expression plasmids (1 µg each). Twenty-four hours after transfection, the cells were infected with VSV (MOI=0.1). The supernatants were harvested 24 h after infection for standard plaque assays. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. (b) Effects of DAPK1-RNAi on NDV replication. In the left panels, 293 cells were transfected with a DAPK1-RNAi plasmid (1 µg each). Twenty-four hours later, the cells were infected with NDV-GFP (MOI=0.1) for 36 h and then imaged by microscopy. In the right panels, the cell lysates were analyzed by immunoblot with the indicated antibodies. The GFP bands from three independent experiments were quantitated using the Bio-Rad Quantity One Program and normalized to the levels of the control protein β-actin. The graphs show the mean±s.d., n=3. *P<0.05; **P<0.01. DAPK, death-associated protein kinase; MOI, multiplicity of infection; NDV, Newcastle disease virus; VSV, vesicular stomatitis virus.
DAPK1 is induced by SeV infection and IFN-β treatment
When performing the real-time PCR experiments, we repeatedly found that the expression of the DAPK1 gene was increased in cells that were stimulated by SeV or IFN-β (Figure 5a). SeV- or IFN-β-induced expression of DAPK1 was confirmed at the protein level with immunoblot analysis (Figure 5b). Autophosphorylation at Ser308 was demonstrated to inhibit the catalytic activity of DAPK1, and the dephosphorylation of Ser308 was required for DAPK1 activation.39 Interestingly, we found that the level of Ser308-phosphorylated DAPK1 (DAPK1-pS308) was decreased following these stimuli, indicating that DAPK1 was activated after viral infection or IFN-β treatment (Figure 5b). Interestingly, however, we found that both the kinase active mutant DAPK1(S308A) and inactive mutant DAPK1(K42A) could increase SeV-induced activation of ISRE to similar levels as wild-type DAPK1 (Figure 5c), indicating that the kinase activity of DAPK1 is dispensable for mediating virus-induced signaling. Taken together, these data suggest that DAPK1 is induced and activated by SeV infection and IFN-β stimulation, supporting the conclusion that DAPK1 is involved in SeV- and IFN-β-induced signaling.
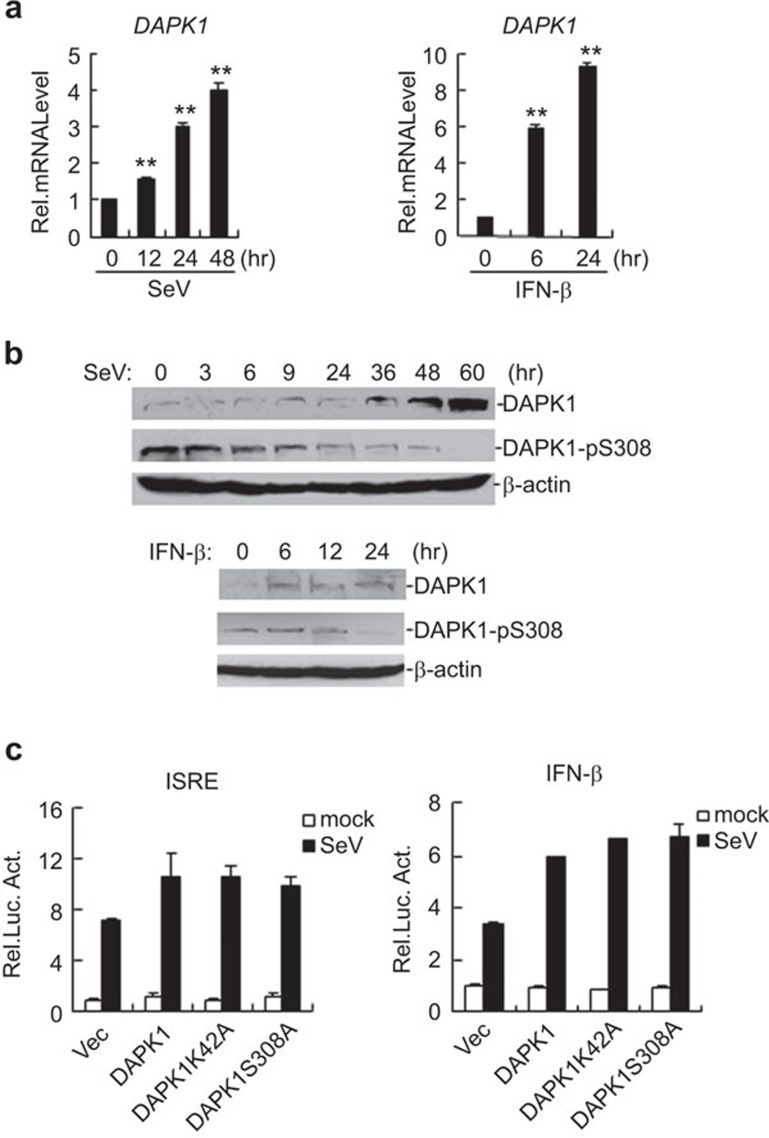
Figure 5.
DAPK1 is induced and activated by SeV and IFN-β. (a) SeV and IFN-β induce the transcription of DAPK1. In total, 293 cells (2×105) were treated with SeV or IFN-β for the indicated time points before real-time PCR was performed. The graphs show the mean±s.d., n=3. (b) Effects of SeV and IFN-β on the expression of total and phosphorylated DAPK1. In total, 293 cells (1×107) were treated with SeV or IFN-β for the indicated time points. The cell lysates were analyzed by immunoblot with the indicated antibodies. (c) Overexpression of DAPK1 mutations enhance SeV-induced ISRE and IFN-β promoter activation to the same level as wild-type DAPK1 in 293 cells. A luciferase assay was performed as in Figure 1c. The graphs show the mean±s.d., n=3. DAPK, death-associated protein kinase; IFN, interferon; ISRE, interferon-stimulated response element; SeV, Sendai virus.
Discussion
IRF7 and IRF3 are master transcription factors of the type I IFN-dependent immune response. In this study, we identified DAPK1 as an IRF7/3-interacting protein. Although DAPK1 interacted with IRF7/3 constitutively in a mammalian overexpression system, the endogenous association was viral infection-dependent. This finding can be explained by the observation that DAPK1 was expressed at low levels under physiological conditions and was induced following viral infection.
In our study, we found that overexpression of DAPK1 increased virus-triggered activation of ISRE and the IFN-β promoter, whereas knockdown of DAPK1 inhibited both virus- and IFN-β-triggered induction of downstream genes, including IFNB1, RIG-I and ISG56. DAPK1 was specifically involved in SeV-induced activation of the IFN-β promoter, but not in TNFα-induced activation of NF-κB (Supplementary Figure 2a and b), which was consistent with an earlier report showing that DAPK1 has no effect on TNFα-induced activation of NF-κB.28 Taken together, these results demonstrate an important role for DAPK1 in the type I IFN-mediated immune response.
Our study showed that DAPK1 expression was induced and DAPK1Ser308 phosporylation was decreased following viral infection, indicating that viral infection activates DAPK1. However, both the DAPK1 inactive (K42A) and active mutants (S308A) could increase the SeV-triggered activation of ISRE, suggesting that the kinase activity of DAPK1 is dispensable for mediating virus-induced type I IFN signaling. We and others have shown that two kinases, RIP1 and GSK3β, mediate viral infection-induced signaling independent of their kinase activity.15,40,41 It is possible that viral infection leads to the induction and activation of DAPK1, which then interacts with IRF3 and IRF7, contributing to the amplification of type I IFN. Interestingly, we also found that the knockdown of DAPK1 inhibited IFN-β-induced expression of downstream genes, such as ISG56, RIG-I, IRF7, IFNA1 and IFNB1. Consistent with these findings, co-immunoprecipitation experiments demonstrated that DAPK1 interacted with STAT1 and IKKε, which are components of the IFN-β-induced signaling pathway. These results suggest that DAPK1 also plays a role in the signaling triggered by type I IFNs. Taken together, our results demonstrate that DAPK1 is an important modulator of virus-induced expression of type I IFNs and other downstream genes and that it plays an important role in the innate antiviral response.
Acknowledgments
We thank the members of our laboratory for their technical help and stimulating discussions. This work was supported by grants from the Ministry of Science and Technology of China (2012CB910201, 2010CB911802) and the National Natural Science Foundation of China (31221061, 31130020, 31101019 and 91029302).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. Sensing and signaling in antiviral innate immunity. Curr Biol. 2010;20:R328–R333. doi: 10.1016/j.cub.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Ermolaeva MA, Michallet MC, Papadopoulou N, Utermöhlen O, Kranidioti K, Kollias G, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9:1037–1046. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Venkataraman T, Fisher PB, Barber GN. Fas-associated death domain-containing protein-mediated antiviral innate immune signaling involves the regulation of Irf7. J Immunol. 2007;178:2429–2439. doi: 10.4049/jimmunol.178.4.2429. [DOI] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Lei CQ, Zhong B, Zhang Y, Zhang J, Wang S, Shu HB, et al. Glycogen synthase kinase 3beta regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity. 2010;33:878–889. doi: 10.1016/j.immuni.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Lu R, Au WC, Yeow WS, Hageman N, Pitha PM. Regulation of the promoter activity of interferon regulatory factor-7 gene. Activation by interferon snd silencing by hypermethylation. J Biol Chem. 2000;275:31805–31812. doi: 10.1074/jbc.M005288200. [DOI] [PubMed] [Google Scholar]
- Litvak V, Ratushny AV, Lampano AE, Schmitz F, Huang AC, Raman A, et al. A FOXO3-IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature. 2012;490:421–425. doi: 10.1038/nature11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RP, Zhang M, Li Y, Diao FC, Chen D, Zhai Z, et al. Differential regulation of IKK alpha-mediated activation of IRF3/7 by NIK. Mol Immunol. 2008;45:1926–1934. doi: 10.1016/j.molimm.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Ning S, Campos AD, Darnay BG, Bentz GL, Pagano JS. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol Cell Biol. 2008;28:6536–6546. doi: 10.1128/MCB.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Hayward GS. The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity. 2010;33:863–877. doi: 10.1016/j.immuni.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao AP, Li S, Zhong B, Li Y, Yan J, Li Q, et al. Virus-triggered ubiquitination of TRAF3/6 by cIAP1/2 is essential for induction of interferon-beta (IFN-beta) and cellular antiviral response. J Biol Chem. 2010;285:9470–9476. doi: 10.1074/jbc.M109.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li C, Xue P, Zhong B, Mao AP, Ran Y, et al. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci USA. 2009;106:7945–7950. doi: 10.1073/pnas.0900818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao F, Li S, Tian Y, Zhang M, Xu LG, Zhang Y, et al. Negative regulation of MDA5- but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase. Proc Natl Acad Sci USA. 2007;104:11706–11711. doi: 10.1073/pnas.0700544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu T, Xu LG, Chen D, Zhai Z, Shu HB. SIKE is an IKK epsilon/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J. 2005;24:4018–4028. doi: 10.1038/sj.emboj.7600863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YT, Fang LW, Lin-Feng MH, Chen RH, Lai MZ. The tumor suppressor death-associated protein kinase targets to TCR-stimulated NF-kappa B activation. J Immunol. 2008;180:3238–3249. doi: 10.4049/jimmunol.180.5.3238. [DOI] [PubMed] [Google Scholar]
- Wang YY, Liu LJ, Zhong B, Liu TT, Li Y, Yang Y, et al. WDR5 is essential for assembly of the VISA-associated signaling complex and virus-triggered IRF3 and NF-kappaB activation. Proc Natl Acad Sci USA. 2010;107:815–820. doi: 10.1073/pnas.0908967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- Kuo JC, Wang WJ, Yao CC, Wu PR, Chen RH. The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway. J Cell Biol. 2006;172:619–631. doi: 10.1083/jcb.200505138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B, Kraus M, Burch L, Stevens C, Craig A, Gordon-Weeks P, et al. DAPK-1 binding to a linear peptide motif in MAP1B stimulates autophagy and membrane blebbing. J Biol Chem. 2008;283:9999–10014. doi: 10.1074/jbc.M706040200. [DOI] [PubMed] [Google Scholar]
- Kang BN, Ahmad AS, Saleem S, Patterson RL, Hester L, Doré S, et al. Death-associated protein kinase-mediated cell death modulated by interaction with DANGER. J Neurosci. 2010;30:93–98. doi: 10.1523/JNEUROSCI.3974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi I, Mestiri S, Bartegi A, Amor NB. TNF-alpha and its inhibitors in cancer. Med Oncol. 2009;27:185–198. doi: 10.1007/s12032-009-9190-3. [DOI] [PubMed] [Google Scholar]
- Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J Exp Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenoever BR, Ng SL, Chua MA, McWhirter SM, García-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, et al. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem. 2001;276:47460–47467. doi: 10.1074/jbc.M105133200. [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.